Method Article
Urethroplasty with Pedicled Tunica Vaginalis for the Treatment of Long-segment Anterior Urethral Stricture Caused by Lichen Sclerosus of Glans Penis
In This Article
Summary
This article introduces a surgical method using a pedicled tunica vaginalis to treat long-segment urethral stricture resulting from lichen sclerosus. The tunica vaginalis is an excellent substitute for the urethra, leading to quick postoperative recovery for patients.
Abstract
Urethroplasty for the management of long-segment urethral strictures associated with lichen sclerosus presents considerable clinical challenges. Oral mucosal grafts are commonly employed but are vulnerable to posttransplantation infection and recurrent stricture formation. Furthermore, the necessity for anesthesia and oral graft harvesting restricts their application in primary healthcare settings.
The single layer of flattened epithelium of the tunica vaginalis can serve as a potential alternative to oral mucosa. Animal experiments have demonstrated that the tunica vaginalis can readily form a tight connection with the multilayered urothelium of the urethra. Utilizing the tunica vaginalis as a scaffold for urethral re-epithelialization may help reduce the risk of recurrence of urethral stricture after surgery. Over a 19 year period, pedicled tunica vaginalis urethroplasty has been used for successfully treating 86 cases.
The surgical procedure involves dorsally incising the urethral stricture segment, then covering it with a pedicled tunica vaginalis patch followed by suturing. Postoperatively, the pedicled tunica vaginalis graft exhibits good vascularization and take rate, facilitating urethral re-epithelialization. The surgical procedure is conducted in a sterile environment to mitigate the potential for infectious complications. Moreover, the operation can be executed under spinal anesthesia, which facilitates its implementation in primary healthcare settings.
Introduction
Lichen sclerosus (LS) of the penis is a chronic inflammatory dermatological condition mediated by lymphocytes, and it is considered an acquired dermatosis1. Hallmark symptoms of LS of the penis include chronic inflammation of the glans penis, abnormal dryness in the appearance of the affected skin, and endarteritis in the subcutaneous arteries supplying the glans penis2. Most patients with LS of the penis have a history of phimosis, with lesions primarily occurring on the foreskin, glans penis, external urethral meatus, or anterior urethra. Some patients may be asymptomatic throughout the disease course, while others can experience foreskin and glans adhesions leading to phimosis and voiding difficulties due to urethral lesions3. The peak incidence of LS occurs in the age groups of 30-49 years and 8-10 years4. Clinical manifestations of LS are variable. In the early stages of the disease, symptoms can be inconspicuous. Initial changes may include milky-white plaques, recurrent ulcers, or purulent discharge on the inner foreskin and glans, accompanied by itching, stinging, and burning sensations.
As LS progresses, decreased elasticity of skin and mucosa develops due to atrophic changes of the foreskin and glans, which can negatively impact sexual function. As the LS further progresses, the external urethral meatus may become stenotic owing to LS invasion, and even long segment anterior urethral stricture may form with the proximal extension of lesions. However, LS involvement of the anterior urethra generally stops with the urethra bulbar and is rarely seen in the membranous and prostatic segments1. Severe urethral stricture can result in increased postvoid residual urine, upper tract hydronephrosis, or renal damage. In urethroplasty for long-segment anterior urethral stricture caused by LS, grafts are usually required to be implanted in the urethra.
Long-segment anterior urethral strictures, defined as strictures exceeding 2 cm in length within the anterior urethra, pose a significant clinical challenge in management and often necessitate the utilization of grafts or substitutes to repair the urethral defect-a procedure known as urethroplasty5. Among the commonly employed urethral substitutes are bladder mucosa6, oral mucosa7, colonic mucosa8, preputial graft9, and tunica vaginalis10, each possessing unique advantages and limitations.
The tunica vaginalis, an extension of the peritoneum, has exhibited promising outcomes in both preclinical animal models and clinical trials as a substitute material for urethral reconstruction11,12,13,14. Its mesothelial nature confers an intrinsic ability to mitigate scar tissue formation. Experimental evidence from animal studies15 has indicated that the single layer of flattened epithelial cells comprising the tunica vaginalis readily establishes a tight junctional interface with the multilayered epithelial cells of the urethral mucosa.
Here, we utilized the technique of transplanting a pedicled tunica vaginalis patch to address long-segment anterior urethral strictures caused by lichen sclerosus of the glans penis (Figure 1). From August 2004 to April 2023, 86 cases were successfully completed, yielding satisfactory postoperative outcomes. This surgical approach offers a novel and effective treatment modality for patients with long-segment anterior urethral strictures caused by lichen sclerosus of the glans penis.
It overcomes the limitations associated with other surgical techniques, boasting a high success rate, convenient material sourcing, simplicity of execution, minimal invasiveness, and a reduced incidence of complications13.
However, long-segment anterior urethral stricture caused by lichen sclerosus of the glans penis is not very commonly employed worldwide, and the literature on the subject is not abundant16. Yet it warrants implementation in medical institutions at all levels, particularly at grassroots facilities, as an ideal method for treating long-segment anterior urethral strictures.
Protocol
The study cohort comprised 86 male patients aged between 20 and 66 years, with a mean age of 40. Each patient received a single-stage tunica vaginalis graft, with 70 cases involving a unilateral tunica vaginalis graft and 16 cases involving a bilateral tunica vaginalis graft. The patients provided informed consent to use and publish their data.
1. Preoperative preparation
Ensure that patients with concurrent urethral infection undergo preoperative cystostomy first. Examine urinary bacterial cultures according to the drug sensitivity results and then, use antibiotics to which the bacteria are sensitive to treat them for 2-3 weeks before surgery. Perform the surgery after the urethral infections are sufficiently under control.
- Ensure that patients with concomitant urethroperineal fistulas undergo cystostomies first. Perform the surgery 3 to 5 months after the healing of the fistulas (Figure 2).
- Ensure that patients with concurrent unilateral epididymitis undergo anti-infective treatment first and perform the surgery 3 weeks after inflammation has been controlled.
- Ensure that patients with concurrent bilateral epididymitis undergo cystostomy first and perform the surgery 3 months after epididymitis is under control.
- Ensure that all patients undergo retrograde urethrography and uroflowmetry examinations.
2. Surgical positioning of patients
NOTE: The surgical positioning must vary according to the location of the urethral stricture in each patient.
- Place the patient in supine position if the patient's urethral stricture is from the urethral meatus to the junction of the penis and scrotum (Figure 3A).
- Place the patient in supine or lithotomy position if the patient's urethral stricture is from the urethral meatus to the bulbar urethra (Figure 3B).
- Place the patient in lithotomy position if the patient's urethral stricture is from the urethral meatus to the proximal end of the bulbar urethra (Figure 3C).
3. Surgical procedure
- Exposure of the urethral stricture segment (Figure 4A).
- Perform the procedure under combined spinal-epidural anesthesia.
- Measure and mark a position approximately 0.5 cm proximal to the coronal sulcus (the indented margin between the glans penis and the penile shaft) as the starting point.
- Perform a circumferential incision of the foreskin at the marked position.
- After the incision, gradually retract the foreskin proximally towards the base of the penis. Carefully dissect and release any adhesions of the inner foreskin during this process until the penile Buck's fascia is fully exposed.
- Incision of the urethral stricture segment (Figure 4B,C).
- Make a ventral longitudinal incision along the stenotic urethral segment, extending approximately 0.5-1.0 cm into the adjacent normal urethral tissue.
NOTE: Make sure the incision is precise in length, without causing unnecessary trauma to the surrounding healthy tissues. - Following the incision, examine the incised site to confirm that the area of urethral stricture has been adequately exposed.
- Make a ventral longitudinal incision along the stenotic urethral segment, extending approximately 0.5-1.0 cm into the adjacent normal urethral tissue.
- Harvest a pedicled rectangular tunica vaginalis (Figure 4D).
- Perform a longitudinal incision through the scrotal wall. Through this incision, sequentially incise the layers of skin and the underlying fascia to gradually expose the parietal layer of the tunica vaginalis.
- Make an arcuate incision on the tunica vaginalis wall, in proximity to the epididymal margin. Through this incision, open the tunica vaginalis cavity.
- Harvest a rectangular flap of approximately 1 cm x 10 cm within the tunica vaginalis cavity through excision along the course of the epididymis. During the excision, carefully preserve the blood vessels, fascia, and any other attachments to the rectangular flap (pedicled tunica vaginalis).
- Utilizing hemostatic forceps, gently create a tunnel through blunt dissection in the subcutaneous loose connective tissue between the scrotum and the base of the penis. Ensure that the tunnel is adequately sized to allow the passage of the pedicled tunica vaginalis flap without undue compression or restriction.
- Carefully feed the pedicled tunica vaginalis flap through the tunnel without torsion, and subsequently mobilize it to the site of the incised ventral urethra.
- Urethral reconstruction by sutures (Figure 4E, F).
- Retain the incised urethral stricture segment as a urethral plate. Orient the smooth serosal surface of the mobilized tunica vaginalis flap to face the urethral plate.
- Use 5-0 absorbable sutures to place continuous running locked sutures separately, approximating the edge of the tunical flap to both sides of the urethral incision. Continually inspect the patency of the urethral lumen and the watertight closure at the suture line throughout the suturing process.
- Final stage of the operative procedure (Figure 4G, H).
- Carefully insert a Foley catheter, sized 24 French (F24), into the urethra.
- Meticulously reposition the foreskin over the glans penis.
- Close the scrotal tissue in layers using fine, absorbable sutures.
4. Special cases
- Case 1: Bilateral tunica vaginalis grafts during urethroplasty (Figure 5).
- Position the patient in lithotomy (due to the urethral stricture extending from the urethral meatus to the proximal bulbar urethra).
- Following the incision from the urethral meatus to the penoscrotal junction, make a perineal incision and continue the incision through the stricture in the bulbar urethra. Harvest rectangular grafts from both testicles.
- Proceed with the ensuing procedure in accordance with the surgical steps outlined above in section 3.
- Case 2: Preserving glans appearance during urethroplasty (Figure 6).
- Given the patient's well-developed glans, perform a subcoronal ventral urethrotomy to preserve the natural contour of the glans.
- Proceed with the ensuing procedure in accordance with the surgical steps outlined above in section 3.
5. Postoperative care
- Upon completion of the surgery, ensure that the catheter is retained in the urethra for 3 weeks. While ensuring the patency of the catheter, promptly cleanse any secretions at the urethral opening.
- Postoperatively, start the patients on a liquid diet initially, transitioning to a normal diet within 3-5 days. During the postoperative recovery phase, advise the patients to to reduce the frequency of bowel movements to aid in the healing process.
- Make sure all patients undergo retrograde urethrograms at 3 weeks post surgery, followed by uroflowmetry 1 week after catheter removal.
- Calculate the mean ± standard deviation (X± S) of the patient's preoperative and postoperative maximum urinary flow rates and perform statistical analysis using software of choice. Perform intergroup comparisons using t-tests, with a P-value < 0.05 considered statistically significant.
Results
See Supplemental Table S1 for patients' clinical history and preoperative measures. Preoperatively, all 86 patients underwent retrograde urethrography and uroflowmetry examinations. Each patient received a single-stage tunica vaginalis graft, with 70 cases involving a unilateral tunica vaginalis graft and 16 cases involving a bilateral tunica vaginalis graft. Postoperative follow-up ranged from 6 to 48 months, with a mean of 24 months. Among these individuals, 84 exhibited unimpeded urination without complications. Retrograde urethrograms revealed a well-dilated urethral lumen. See Table 1 for patients' preoperative and postoperative maximum flow rate information. The preoperative mean maximum flow rate was (2.8 ± 0.5) mL/s, while the postoperative mean maximum flow rate was (18.8 ± 2.1) mL/s, indicating a significant improvement in the patients' urinary flow rates after surgery (P < 0.05, Table 2). Retrograde urethrograms demonstrated a markedly dilated urethral caliber postoperatively compared to the preoperative state (Figure 7). The reconstructed phalluses exhibited no deformities and were fully functional for sexual intercourse.
Two cases of urethral stricture were observed after urethroplasty. In the first case, a patient with epididymitis and hydrocele underwent urethroplasty utilizing the tunica vaginalis from the affected testicle after a 3 week course of anti-infective treatment. However, a long-segment urethral stricture developed 2 years postoperatively, necessitating a subsequent dorsal urethrotomy.
In the second case, a patient developed a stricture at the distal bulbous urethra. Within a year following the surgery, a stricture occurred at the anastomosis between the reconstructed and proximal urethra. This patient underwent holmium laser incision for treatment. In the second postoperative year, a recurrent urethral stricture occurred, and the patient underwent a cold knife urethrotomy, resulting in unobstructed urination at the 9 month follow-up.

Figure 1: Urethral strictures caused by penile Lichen Sclerosus. Please click here to view a larger version of this figure.

Figure 2: Urethral perineal fistula complications. (A) Urethral perineal fistula. (B) Long-segment anterior urethral stricture. Please click here to view a larger version of this figure.
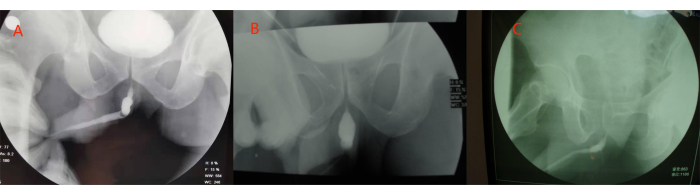
Figure 3: Three types of long-segment anterior urethral stricture. (A) Stricture from the urethral meatus to junction of penis and scrotum. (B) Stricture from the urethral meatus to the bulbar urethra. (C) Stricture from the urethral meatus to the proximal end of the bulbar urethra. Please click here to view a larger version of this figure.
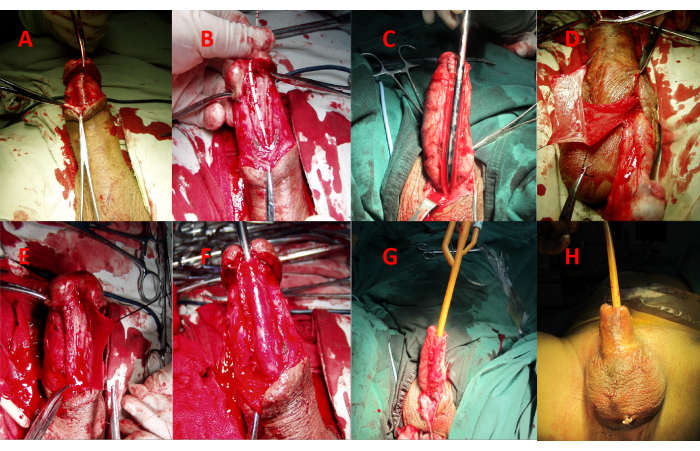
Figure 4: Surgical procedure. (A) Exposure of the urethral stricture segment. (B) Make a ventral longitudinal incision along the urethral stricture segment. (C) Extend the incision approximately 0.5-1.0 cm into the adjacent normal urethral tissue. (D) Harvest a pedicled rectangular tunica vaginalis. (E) Use 5-0 absorbable suture to place continuous running locked sutures, approximating the edge of the tunical flap to one side of the urethral plate. (F) Suture the other side of the urethral plate. (G) Insert an F24 catheter into the urethra. (H) Reposition the prepuce and close the scrotum. Please click here to view a larger version of this figure.

Figure 5: Bilateral tunica vaginalis grafts during urethroplasty. (A)Harvest two rectangular grafts from two testicles. (B) Suture one piece of rectangular graft to the urethral plate. (C) Suture the other piece of rectangular graft to the urethral plate. Please click here to view a larger version of this figure.
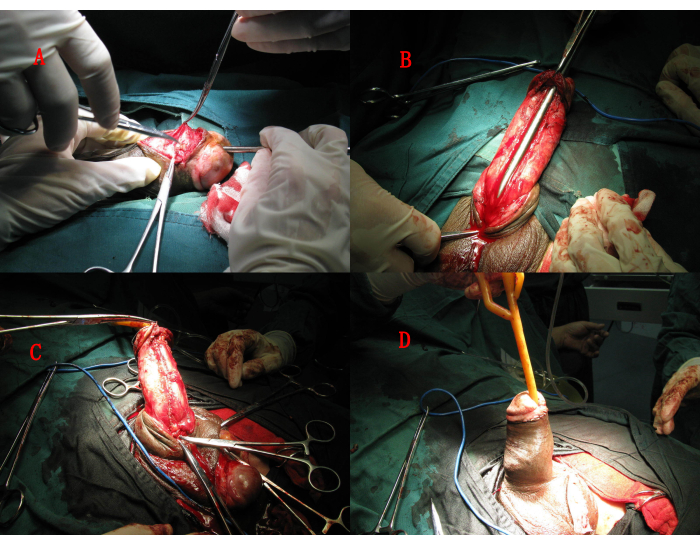
Figure 6: Preserving glans appearance during urethroplasty. (A) Perform a subcoronal ventral urethrotomy to preserve the natural contour of the glans. (B) Exposure of the urethral stricture segment, make a ventral longitudinal incision along the urethral stricture segment. (C) Suture the rectangular graft to the urethral plate. (D) Insert an F24 catheter into the urethra. Reposition the prepuce and close the scrotum. Please click here to view a larger version of this figure.
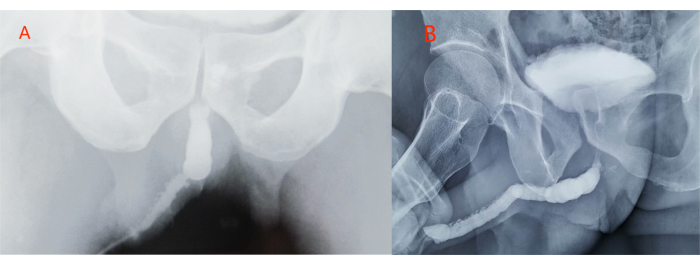
Figure 7: Retrograde urethrography depicting the augmented urethral caliber after surgical management. (A) Preoperative state; (B) postoperative state. Please click here to view a larger version of this figure.
Table 1: Preoperative and postoperative maximum flow rate information for all patients. Please click here to download this Table.
Table 2: Comparison of preoperative and postoperative maximum urinary flow rates. The postoperative maximum flow rate was significantly higher than that before surgery (P < 0.05). Please click here to download this Table.
Supplemental Table S1: Clinical history and preoperative measures. Please click here to download this File.
Discussion
Numerous urological researchers have investigated a diverse range of graft materials for urethral reconstruction, including genital and extragenital flaps or free grafts17. These materials include preputial graft, bladder mucosa, oral mucosa, tunica vaginalis, and colonic mucosa.
Oral mucosal grafts have achieved high success rates for the treatment of anterior urethral strictures caused by LS, with Xu et al. reporting a success rate of 88.9%18. However, harvesting oral mucosa grafts relies on nasal intubation and general anesthesia performed by experienced surgeons and anesthesiologists. Wood et al.19 evaluated the morbidity of oral mucosa urethroplasty in 57 patients. Of these patients, 83% developed postoperative pain, 68% experienced perioral numbness that persisted after 6 months, 67% initially had difficulty with mouth opening, and 2% developed a mucous retention cyst. Moreover, as a no-blood-support graft, oral mucosa is prone to infection and ischemic necrosis after transplantation. All these factors pose challenges for the widespread adoption of such surgery in primary healthcare settings.
Preputial grafts are commonly utilized as materials in urethral reconstruction. However, they bear the potential risk of contracture and hair growth within the urethra20. The bladder mucosa has been associated with numerous complications, including meatal stenosis, prolapse, and fistula formation6. The colonic mucosa, deemed suitable for extensive urethral strictures, has been reported to yield favorable outcomes8. Nevertheless, harvesting this material necessitates a highly invasive procedure and carries the potential complications inherent to abdominal surgery.
The present study employed the pedicled tunica vaginalis urethroplasty technique for the treatment of long-segment anterior urethral strictures caused by LS, thereby providing a novel and efficacious therapeutic modality for patients afflicted with this condition. The tunica vaginalis, a novel serosal graft material utilized for the repair of anterior urethral defects, possesses a reliable anatomical and experimental foundation. Hua et al.15 reported the application of tunica vaginalis urethroplasty in a rabbit model of urethral stricture, with post-operative imaging and histological studies confirming the advantages of this material in this context. Numerous investigations have substantiated the survival of tunica vaginalis grafts and verified the transformation of this tissue into urinary epithelium upon implantation within the urinary tract11,12,13,14. The tunica vaginalis, a single layer of flattened epithelial cells, possesses the ability to establish intimate connections with the multilayered epithelial cells of the urethral mucosa. This physiological process effectively impedes the formation of scar tissue, thus ensuring optimal functionality and structural integrity of the affected regions.
The proposed surgical approach addresses the shortcomings of alternative techniques, exhibiting a high success rate, convenient graft harvesting, simplicity of execution, minimal trauma, and a reduced incidence of complications. Currently, this surgical modality remains predominantly confined to the realm of animal experimentation, with limited clinical application by urological surgeons. However, it merits broader implementation in medical institutions at all levels, particularly in primary healthcare settings, as an ideal therapeutic approach for long-segment anterior urethral strictures.
In this study, pedicled tunica vaginalis urethroplasty was employed to treat long-segment anterior urethral strictures resulting from LS. Over the past 19 years, 86 cases have been successfully treated using this approach. During the surgical procedure, the incised urethral stricture was covered by the patient's own pedicled tunica vaginalis graft and subsequently sutured. The new urethral tissue would then undergo re-epithelialization, utilizing the graft as a scaffold. The selection of a pedicled tunica vaginalis graft not only reduces the risk of infection but also ensures an adequate blood supply and a favorable take rate following transplantation. The tunica vaginalis, approximately 10 cm in length, can be retrieved from a single testis. Theoretically, the total surface area of bilateral tunica vaginalis grafts can meet the mucosal requirement for urethroplasty of the entire anterior urethra. This technique can be performed under combined spinal-epidural anesthesia, which reduces surgical complexity and facilitates its broader adoption in primary healthcare settings.
Tunica vaginalis urethroplasty has the following advantages for long anterior urethral stricture caused by LS: The tunica vaginalis is smooth and hairless, less prone to stone formation21, richly vascularized with preserving the pedicle maintaining the blood supply and further improving graft survival, in proximity to the urethra facilitating graft harvesting and simplifying the procedure, providing ample mucosal tissue for grafting especially beneficial for patients with concomitant hydrocele, able to form a tight connection between its single layer of flattened epithelium and the multilayered urothelial cells less prone for erosion again after surgery as shown by animal experiments15, and as a peritoneal interstitial tissue, can effectively prevent scar formation.
To achieve optimal clinical outcomes, the following points should be noted: topical corticosteroid cream should be applied regularly on the affected area 1-3 months pre- and post-operatively22. Comprehensive preoperative urine culture and appropriate management of potential urinary tract infections based on antibiotic sensitivity testing are necessary. The tunica vaginalis graft should be avoided from the affected side in patients with epididymitis. For patients with bilateral epididymitis, surgery should be performed 3 months after inflammation is controlled. In patients with urethrocutaneous fistulas, cystostomy should be performed first, and surgery should be undertaken 3-6 months after fistula healing. During urethrotomy, the urethral plate formed by the stricture segment should be preserved. The incised urethral stricture segment was retained as a urethral plate23, ensuring better blood supply for the tunica vaginalis graft. An F24 catheter should be left in the urethra to maintain patency. Postoperative medications, including Solifenacin Succinate 5-10 mg daily (for bladder spasms) and Estriol 1 mg three times daily for 2 weeks (to prevent erections), should be prescribed24. Overtight pressure dressing should be avoided. The first dressing change is preferably on the third day after operation. Postoperative urethral care should be reinforced, and urethral secretions should be promptly removed.
The present study has limitations. A comparative analysis evaluating the therapeutic efficacy of urethral reconstruction using the tunica vaginalis graft against other transplantable materials has not been conducted. Addressing this gap will be a primary focus for future research endeavors.
In conclusion, the pedicled tunica vaginalis patch serves as an excellent urethral substitute for tunica vaginalis urethroplasty in cases of LS-induced anterior urethral stricture. This surgical technique offers advantages such as a high success rate, convenient graft harvesting, a simple procedure, minimal trauma, and rare complications. Moreover, it can be easily implemented in primary healthcare settings and is an ideal approach for treating long-segment anterior urethral strictures.
Disclosures
None of the authors have any conflicts of interest to declare.
Acknowledgements
In the writing process of this manuscript, the authors utilized the Claude language model developed by Anthropic AI as an assistive tool for grammar checking and correction. Claude provided valuable feedback and suggestions, but the final content was thoroughly reviewed and verified by the authors to ensure accuracy and originality. The authors take full responsibility for the content and views expressed in this manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| 2-0/T non-absorbable suture | Ethicon Inc | SA845G | Sterile, radiation sterilization, disposable |
| 22F silicone catheter | UROVISION | G2219043 | A disposable Foley Catheter |
| 3-0 non-absorbable suture | Ethicon Inc | SA84G | Sterile, radiation sterilization, disposable |
| 5-0 absorbable suture | Ethicon Inc | VCP1433 | Sterile, radiation sterilization, disposable |
| Digital X-ray Radiography Fluoroscopy System | Beijing Shimadzu Madical Equipment Co.,Ltd | ZS-200 | Retrograde urethrograms use |
| Iohexol Injection | STARRY PHARMACEUTICAL | H20203258 | Sterile, disposable,Retrograde urethrograms use |
| SPSS 20.0 | statistical software | ||
| Urodynamic Analyzer | WBL MEDICAL | Nidoc 970A | Maximum flow rate detection |
References
- Pugliese, J. M., Morey, A. F., Peterson, A. C. Lichen sclerosus: Review of the literature and current recommendations for management. J Urol. 178 (6), 2268-2276 (2007).
- Powell, J. J., Wojnarowska, F. Lichen sclerosus. Lancet. 353 (9166), 1777-1783 (1999).
- Fergus, K. B., et al. Pathophysiology, clinical manifestation, and treatment of Lichen sclerosus: A systematic review. J Urol. 135 (1), 11-19 (2020).
- Das, S., Tunuguntla, H. S. Balanitis xerotica obliterans a review. World J Urol. 18 (6), 382-387 (2000).
- Peterson, A. C., Webster, G. D. Management of urethral stricture disease: developing options for surgical intervention. BJU Int. 94 (1), 971-976 (2004).
- Kinkead, T. M., Borzi, P. A., Duffy, P. G., Ransley, P. G. Long-term followup of bladder mucosa graft for male urethral reconstruction. J Urol. 151 (4), 1056-1058 (1994).
- Riordan, A. O., Narahari, R., Kumar, V., Picard, R. Outcome of dorsal buccal graft urethroplasty for recurrent bulbar urethral strictures. BJU Int. 102 (9), 1148-1151 (2008).
- Xu, Y. M., et al. Urethral reconstruction using colonic mucosa graft for complex strictures. J Urol. 182 (3), 1040-1043 (2009).
- Hendren, W. H., Horton, C. E. Experience with 1-stage repair of hypospadias and chordee using free graft of prepuce. J Urol. 140 (11), 1259-1264 (1988).
- Mathur, R. K., Sharma, A. K., Odiya, S. Tunica albuginea urethroplasty for panurethral strictures. J Urol. 14 (9), 120-124 (2009).
- Liu, J. S., et al. Long-term outcomes of urethroplasty with abdominal wall skin grafts. J Urol. 85 (1), 258-262 (2015).
- Calado, A. A., et al. The tunica vaginalis dorsal graft urethroplasty: experimental study in rabbits. J Urol. 174 (2), 765-770 (2005).
- Theodorescu, D., et al. Urethral replacement with vascularized tunica vaginalis: Defining the optimal form of use. J Urol. 159 (5), 1708-1711 (1998).
- Talja, M., Kivisaari, L., Makinen, J., Lehtonen, T. Free tunica vaginalis patch in urethroplasty. An experimental study. Eur Urol. 13 (4), 259-263 (1987).
- Hua, X. L., Chen, J., Xu, Y. j., Li, B. Combined dorsal plus ventral double tunica vaginalis graft urethroplasty: An experimental study in rabbits. J Urol. 126 (4), 209-216 (2019).
- Martins, F. E., Kulkarni, S. B., Joshi, P., Warner, J., Martins, N. Management of long-segment and panurethral stricture disease. Adv Urol. 2015 (1), 853914-853928 (2015).
- Wang, W. L., Liu, R. Y., Wu, D. P., Yan, L. P. Non-tube urethroplasty with tunica vaginalis flap grafting for treating extensive stricture of the anterior urethra. J Clin Urol. 24 (9), 672-674 (2009).
- Xu, Y. M., et al. Outcome of 1-stage urethroplasty using oral mucosal grafts for the treatment of urethral strictures associated with genital lichen sclerosus. J Urol. 83 (1), 232-236 (2014).
- Wood, D. N., Allen, S. E., Andrich, D. E., Greenwell, T. J., Mundy, A. R. The morbidity of buccal mucosal graft harvest for urethroplasty and the effect of nonclosure of the graft harvest site on postoperative pain. J Urol. 172 (2), 580-583 (2004).
- Kim, B. S., et al. Nontransected ventral onlay-augmented urethroplasty using autologous saphenous vein graft in a rabbit model of urethral stricture. J Urol. 83 (1), 225-231 (2014).
- Li, X. G., Jin, T. X. Urethroplasty with pedunculated preputial flap and testicular tunica vaginalis for long anterior urethal stricture: A case report and review of the literature. Zhonghua Nan Ke Xue. 18 (2), 168-171 (2012).
- Akel, R., Fuller, C. Updates in lichen sclerosus: British Association of Dermatologists guidelines for the management of lichen sclerosus 2018. Br J Dermatol. 178 (4), 823-824 (2018).
- Zhang, X. D. . Smith's General Urology. 607, (2005).
- Hui, P. Y., et al. Clinical study of oral mucosal graft urethroplasty for the treatment of lichen sclerosus-related long segment anterior urethral stricture. Journal of China Medical University. 51 (3), 221-225 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved