Method Article
DiI Dye-Filling as a Simple and Inexpensive Tool to Visualize Ciliated Sensory Neurons in C. elegans
In This Article
Summary
DiI dye-filling is a method commonly used in C. elegans to visualize a subset of the ciliated sensory neurons, allowing for the identification of genetic mutations that alter sensory neuron structure or function.
Abstract
C. elegans have long been used as a simple and accessible model to study neuronal structure and the many functions of the nervous system. Of the 302 neurons within the adult hermaphrodite nervous system, 60 are classified as ciliated sensory neurons. These neurons are central to a number of C. elegans behaviors, including but not limited to chemo-, mechano-, and osmosensing, male mating, and dauer formation. For several decades now, members of the C. elegans community have used the red fluorescent lipophilic dye DiI to visualize a subset of the ciliated sensory neurons that are directly exposed to the external environment. This dye enters the ciliated ends of the neurons and distributes in a relatively uniform pattern throughout the dendrites, cell bodies, and axons. This simple and powerful method makes an excellent first-pass tool to identify genetic mutants that impart structural or functional defects in ciliated sensory neurons. Here, we present a streamlined version of this staining method to visualize the eight pairs of amphid and two pairs of phasmid neurons that are environmentally exposed in C. elegans. We discuss tips for using this inexpensive method for imaging cellular dye-filling patterns in anesthetized animals.
Introduction
Caenorhabditis elegans (C. elegans) are easy to manipulate, have fast generation times, and are low-cost to maintain. Due to these and many other advantages, C. elegans have served as a preferred model organism for studying many biological processes, especially the development and function of the nervous system. An entire issue in the Journal of Neurogenetics was recently dedicated to the historical impacts of research on this particular topic1. They are particularly beneficial for studying the function of primary cilia, which are involved in sensing chemical and physical environmental conditions2.
Adult hermaphrodite C. elegans have a total of 302 neurons, 60 of which possess primary cilia at the end of their dendritic processes3. These 60 ciliated neurons are classified as sensory neurons and are involved in many C. elegans behaviors, including but not limited to chemo-, mechano-, and osmosensing, male mating, and dauer formation3,4. There are two subsets of ciliated sensory neurons that are exposed to the external environment, which include sixteen amphid neurons (8 pairs) in the head and four phasmid neurons (2 pairs) in the tail3,5.
For several decades, researchers in the C. elegans community have used lipophilic dyes, such as the red fluorescent 1,1'-dioctadecyl-3,3,3'3'-tetramethylindocarbocyanine perchlorate (DiI), to visualize a number of different tissues in live animals6,7,8,9. When animals are exposed to DiI, the dye easily and quickly intercalates into the membrane of the dendrites, axons, and cell bodies of the 20 externally exposed amphid and phasmid neurons in a relatively uniform distribution. When wild-type animals are exposed to DiI, the dye can be visualized in these neurons by fluorescent imaging during a relatively broad window of time. If there are any morphological or functional abnormalities in the primary cilia, the dye may not properly fill the neurons, and therefore, a signal may appear weaker in some or all cells or may be completely absent6,7,10,11. Any of these outcomes can be informative of structural or functional deficits that may be present in the ciliated sensory neurons of genetic variants.
This manuscript aims to demonstrate the ease with which dye-filling can be used in C. elegans to visualize the structure of ciliated sensory neurons (Figure 1). We applied this technique in wild-type and mutant animals to demonstrate how different genetic backgrounds can show a variety of dye-filling outcomes, often related to the structural or functional integrity of their ciliated sensory neurons. We show staining at 30 min, 24 h, and 48 h post-dye-filling in a variety of different animal ages to aid in determining the optimal time course for live imaging. We also provide examples of difficulties that can arise during staining and imaging and tips to avoid these problem points. Through the use of this method, researchers at institutions of any size can begin to build upon the foundation of ciliated sensory neuron biology in C. elegans. Dye-filling is simple and cost-effective enough to be incorporated into lab activities with undergraduates to permit them the chance to work with C. elegans and fluorescence microscopy with minimal prior technical expertise. In addition, there is a significant conservation of genes involved in primary cilia biology and, more broadly, sensory neuron function between humans and C. elegans12. Continued research on ciliary gene functions and genetic interactions in C. elegans could ultimately provide greater insight into the complexity of human ciliopathies13.
Protocol
1. Preparation of solutions
- Prepare the bleach solution. Combine 2 mL of bleach, 500 µL of 10 M NaOH, and 7.5 mL of dH2O in a 15 mL centrifuge tube.
NOTE: Bleach solution remains stable for up to 1 week when stored at room temperature (RT). - Prepare the M9 buffer. Combine 3 g of KH2PO4, 6 g of Na2HPO4, and 5 g of NaCl in 1 L of sterile water and autoclave to sterilize. Once cooled completely to RT, add 1 mL of sterile 1 M MgSO4.
- Prepare the DiI stock solution. Add 10 mg of DiI to 5 mL of dimethylformamide (DMF) for a final concentration of 2 mg/mL DiI in DMF. DiI is light-sensitive, so always cover the solutions with aluminum foil. DiI in DMF can be stored at -20 °C for many years and does not require thawing between uses.
CAUTION: DMF is both flammable and harmful when in contact with skin or inhaled. Work with this solution under a chemical hood, away from open flames, and wearing proper personal protective equipment (PPE).
2. Isolation of synchronized populations by bleach preparation
NOTE: Gather all of the equipment and solutions necessary for all steps of the process prior to beginning. Bleach preparations are very time-sensitive, so having the necessary materials before starting the process ensures protocol success.
- Wash unstarved, gravid adult animals off plates using 1 mL of M9 buffer. Allow the animals to collect at one edge of the plate by tilting the plate slightly.
NOTE: Typically, aim to collect ~50-100 unstarved, gravid adult animals for a reliable yield of eggs. However, bleach preparation can be performed on nearly any number of gravid adult animals. The number of gravid adult animals available for collection can vary widely depending on the genetic background of the animals. A greater number of animals collected usually yields a greater number of eggs. - Using a glass Pasteur pipette, collect and transfer the animals in the M9 buffer to a labeled 1.5 mL microcentrifuge tube.
- Centrifuge the animals at 350 g for 30 s. This speed is slow enough to collect the live animals at the bottom of the tube without causing any harm.
- Decant most of the excess M9 buffer, leaving the collected worms at the bottom of the tube undisturbed.
- Add 1 mL of the prepared bleach solution and allow the animals to float in the solution until they begin to disintegrate, inverting the tube regularly to encourage tissue breakdown. View the tissue breakdown and release of eggs by periodically checking the tube under a dissecting microscope. This process usually takes between 5 min and 10 min.
- Once the tube contains mostly released eggs with only a few visible animal tissues remaining, centrifuge at maximum speed for a standard tabletop microcentrifuge (typically between 15,000-21,000 g) for 30 s to collect the eggs at the bottom.
- Decant as much of the bleach solution as possible using a glass pipette while careful not to disturb the pelleted eggs. Add 1 mL of M9 buffer to halt the degradation of the eggs by the remaining bleach solution.
NOTE: Steps 2.6 and 2.7 should be carried out quickly. Delays in centrifugation, removal of bleach solution, and washing with M9 buffer can result in low egg yields due to degradation. - Centrifuge the tube again at 21,000 g for 30 s. Decant the supernatant without disturbing the pelleted eggs.
- Wash twice more with 1 mL of M9 buffer, decanting liquid between each wash without disturbing the pelleted eggs.
- After the final wash, decant most of the M9 buffer, then resuspend the eggs in the remaining liquid by flicking the tube or shaking vigorously.
- Using a sterile glass pipette, transfer a drop of the solution containing the eggs to the surface of a fresh NGM plate with E. coli. Aim to place the drop next to the E. coli and add around 100 eggs per plate. These eggs are approximately synchronized in age.
NOTE: In cases where the egg yield is high enough that obtaining 100 eggs per drop is difficult, additional M9 buffer can be added to the tube containing eggs to dilute their concentration.
3. Dye-filling procedure
- Label as many 1.5 mL microcentrifuge tubes as needed for the number of strains being dye-filled. Create foil covers for each tube to protect light sensitive DiI solution, once added.
- Wash animals of a desired developmental stage from the age-synchronized NGM plates using approximately 1 mL of M9 buffer. Ensure that each plate contains approximately 100 animals hatched from the 100 eggs placed on the plate during bleach synchronization. Collect the animals into the properly labeled 1.5 mL microcentrifuge tubes.
NOTE: Dye-filling works well on a wide variety of developmental stages. This study demonstrates dye-filling in animals ranging from larval stage 3 (L3) to adults (48 h post-L3). - Centrifuge animals at 350 g for 30 s to collect them at the bottom of the tube.
- Decant the M9 buffer without disturbing the animals collected at the bottom of the tube.
- Wash twice more with 1 mL of M9 buffer, decanting liquid between each wash without disturbing the collected animals. On the last round of washing, decant to leave 200 µL of M9 buffer remaining in the tube.
- Cover all tubes with aluminum foil to protect from light exposure and add 1 µL of DiI in DMF directly to the M9 buffer in each tube.
NOTE: If changing the amounts of M9 buffer and DiI, make sure to maintain a 1:200 ratio of DiI in the M9 buffer. - Rock or shake all tubes at RT for 2 h.
- Centrifuge tubes at 350 g for 30 s to collect animals at the bottom of the tubes.
- Decant the liquid containing the DiI from the microcentrifuge tubes. Wash with M9 buffer three times, decanting liquid between each wash.
- After decanting from the final wash, transfer animals in a small (~50 µL) volume of M9 buffer to fresh NGM plates with E. coli.
- Allow animals to recover after staining on fresh NGM plates for at least 30 min. Cover the plates with aluminum foil until observation to preserve the DiI stain. Observe animals up to 24 h after dye-filling with no significant loss in fluorescent signal. At or after 48 h, the signal can begin to fade (see Figure 2).
4. Imaging dye-filling
- Prepare a 2% agarose in a sterile water solution. Fully dissolve the agarose using a microwave.
NOTE: Agarose (2%) in water solution can be reused multiple times until repeated water loss increases agarose concentration to a point where the solution becomes too viscous. - Obtain glass slides and 22 mm x 22 mm coverslips to prepare agarose pads. Using a glass pipette, place a small drop of the molten 2% agarose in water on one glass slide and quickly place a cover slip on top of the agarose drop. Once the agarose pad is fully solidified, remove the coverslip, leaving the agarose pad on the glass slide.
NOTE: Avoid the formation of bubbles in agarose pads, as they can obstruct the imaging of animals. - Add 5-10 µL of anesthetic (10 mM levamisole) to the surface of the agarose pad. Transfer 5-20 animals to the anesthetic, then place the cover slip back on top of the agarose pad.
- Observe animals using a stereo, compound, and/or confocal fluorescence microscope, depending on available equipment and screening and imaging needs. DiI has an excitation peak at 550 nm and an emission peak at 564 nm; thus, view it using a tetramethylrhodamine/cyanine dye 3/yellow fluorescent protein (TRITC/Cy3/YFP)-compatible filter cube.
NOTE: Appropriate microscope settings, such as exposure time, gain, and laser power, needed to obtain clear images of dye-filling can vary between different types of microscopes and should be independently determined by users for their unique needs.
Results
Adult N2 worms imaged 24 h after dye-filling demonstrate clear fluorescent signal spread relatively evenly throughout the amphid neurons (Figure 1A,A') and phasmid neurons (Figure 1D,D'). In these animals, the dendritic projections and cell bodies of the amphid neurons can be easily distinguished. There are no clumps of dye in the dendritic projections, nor are there any interruptions in fluorescent signal in any part of the dye-filled neurons. In the phasmid neurons, the cell bodies can also be easily distinguished, though the dendritic projections can often appear fainter than in the amphids. This is likely due to the smaller number of phasmid neurons that stain using DiI3,5. These results collectively indicate that the dye can successfully enter the externally exposed ciliated sensory neurons and then disperse evenly throughout all structures of the cells.
When adults from the YH2125 strain, containing mutations in two genes, bbs-5 and nphp-4, that cause ciliary dysfunction, are imaged 24 h after dye filling, a range of results can be seen. The frequency of each of these results was determined in a previous publication10. In a majority of the animals (~60%), the dye appears to enter only a subset of the amphids (Figure 1B,B'). This result is described as partial dye filling and often also features dye clumping (white arrowheads, Figure 1B), uneven fluorescent signals, and even a complete lack of fluorescent signals in some areas of the amphids. In a small fraction of the animals (~10%), the dye fails to enter any of the amphid neurons, which leads to a complete lack of fluorescent signal and is described as defective dye-filling (Figure 1C,C'). When assessing dye-filling in the phasmid neurons in YH2125 adults, only one of two outcomes is observed. About half of the animals display normal dye-filling that resembles adult N2 animals. The remaining half of the animals show a lack of fluorescent signal indicative of defective dye-filling (Figure 1E,E'). Partial dye-filling of phasmid neurons has not been observed by our lab and, therefore is not included in this figure. The observed frequency of defective phasmid dye-filling is greater than the observed frequency of defective amphid dye-filling, suggesting that it is possible to observe partial or complete amphid dye-filling without any phasmid dye-filling in the same animal10. These results are examples of impaired dye-filling, which may suggest defects in ciliated sensory neuron structure or function. Such results are often followed up with experiments to assess changes in sensory neuron function, such as chemotaxis assays, and changes in sensory neuron structure, such as fluorescent reporter analysis.
Imaging of a cohort of synchronized N2 animals over a series of days following dye-filling helps to identify the best time points for analysis. The fluorescent signal from the dye is strong and evenly distributed in animals just 30 min after staining (Figure 2A). Imaging at 24 h after dye-filling shows very little decrease in fluorescent signal when compared to animals from the first day (Figure 2B). Only after 48 h can a noticeable decrease in fluorescent signal be detected (Figure 2C). Figure 2 also demonstrates that dye-filling is visible over a wide range of ages, including L3 (Figure 2A,A'), L4 (24 h post-L3, Figure 2B,B'), and young adults (48 h post-L3, Figure 2C,C'). A second set of dye-filling experiments confirms that the decreases in fluorescent signal observed during longer periods of recovery are not the result of developmental changes in C. elegans. In these experiments, age-synchronized animals at the L3, L4, and young adult (48 h post-L3) stages were each dye-filled and recovered for 30 min before imaging. A similar level of fluorescent signal was observed after 30 min of recovery in L3 (Figure 3A,A'), L4 (Figure 3B,B'), and adult animals (Figure 3C,C').
It may be preferable to image at 24 h after dye-filling to avoid an undesirable outcome that can be observed at earlier time points, especially at 30 min after staining. Fluorescent signals from the amphids and phasmids can sometimes be obscured or overpowered by fluorescent signals observed in the intestine (L3, Figure 4A,A'; L4, Figure 4B,B'). This signal is present due to ingestion of the dye during the staining process and is more likely to be absent after a 24 h rest period due to excretion.
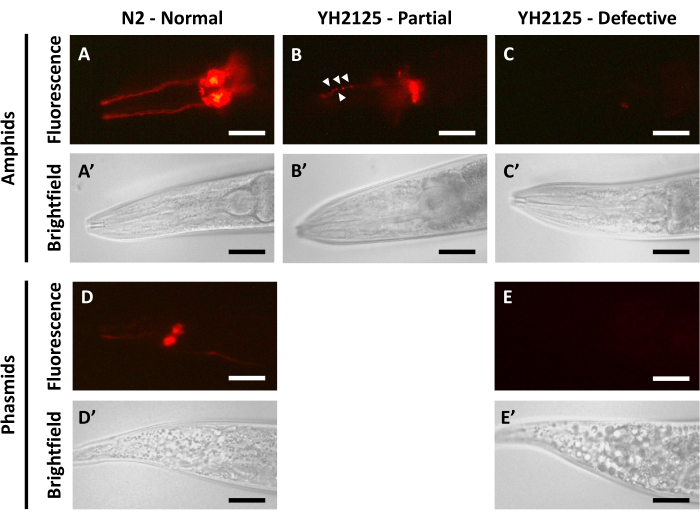
Figure 1: Dye-filling in C. elegans yields varied outcomes depending on genetic background. (A-E) Imaging 24 h after dye-filling in (A,A') N2 amphid neurons, (B,B',C,C') YH2125 amphid neurons, (D,D') N2 phasmid neurons, and (E,E') YH2125 phasmid neurons. White arrowheads (B) indicate clumping of DiI dye in dendrites. DiI fluorescent signal is in the top panels, and bright field imaging is in the bottom panels. The anterior of the animal is to the left in all panels. All animals shown here are at the adult stage (48 h post-L4). Scale bars represent 30 µm (amphids) and 45 µm (phasmids). Please click here to view a larger version of this figure.

Figure 2: Fluorescent signals in synchronously dye-filled animals decrease over time. Imaging of N2 animals dye-filled at L3 stage after (A,A') 30 min, (B,B') 24 h (L4 stage), and (C,C') 48 h (young adult). DiI fluorescent signal is in the top panels, and bright field imaging is in the bottom panels. The anterior of the animal is to the left in all panels. Scale bars represent 30 µm. Please click here to view a larger version of this figure.
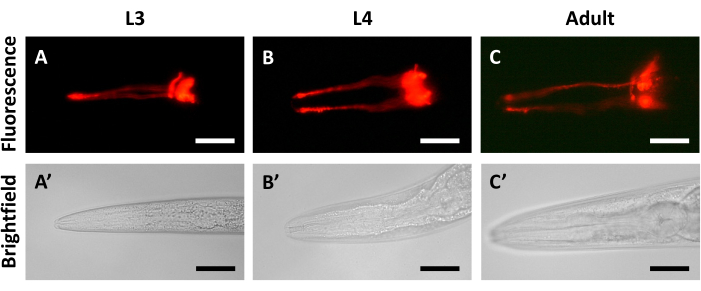
Figure 3: Fluorescent signal intensity remains consistent in different developmental stages. Imaging of N2 animals dye-filled and recovered for 30 min at (A,A') L3 stage, (B,B') L4 stage, and (C,C') young adult stage. DiI fluorescent signal is in the top panels, and bright field imaging is in the bottom panels. The anterior of the animal is to the left in all panels. Scale bars represent 30 µm. Please click here to view a larger version of this figure.
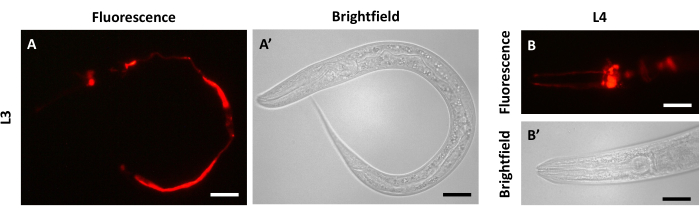
Figure 4: Intestinal fluorescence can be observed in dye-filled animals 30 min after staining. Imaging 30 min after dye-filling of N2 animals at (A) L3 stage and (B) L4 stage. (A) DiI fluorescent signal in the left panel, (A') bright field imaging in the right panel for L3. (B) DiI fluorescent signal in the top panel, (B') bright field imaging in the bottom panel for L4. The whole animal is visible in panels A and A'. The anterior of the animal is to the left in panels B and B'. Scale bars represent 30 µm. Please click here to view a larger version of this figure.
Discussion
xSuccessful dye-filling relies on careful consideration of the developmental stage and genetic background of the animals, as well as the elapsed time until imaging. Some genetic mutations disrupt the structure and/or function of the externally exposed ciliated sensory neurons, resulting in animals that are unable to dye fill properly. Therefore, dye-filling of novel C. elegans mutants can be used as a simple first-pass identifier for defects in sensory neuron structure or function. However, one limitation of this technique is that it cannot be used to determine what type of defects are present in the sensory neurons. Thus, any newly discovered dye-filling defects should be followed up with some combination of behavioral assays to analyze sensory neuron function and transgenic reporters to identify any structural defects that might be present.
When preparing animals for dye-filling, proper synchronization is a critical step in the protocol to ensure the comparison of animals at similar developmental stages. Age synchronization by bleach preparation is a commonly used method in the C. elegans research community. This method allows for the isolation of fertilized embryos from gravid adults, with the eggshells of the embryos protecting them from any detrimental effects of the bleach over a short exposure time14. However, it is especially important to watch animals closely during the bleaching phase (step 2.5), as it is still possible to over-bleach samples, which can cause the degradation of embryos in addition to gravid adults. Synchronization by bleach preparation is the preferred method if working with strains that have any egg-laying defects, which some animals with dysfunctional cilia display10. For an alternative method of isolating roughly synchronized populations in strains that do not display egg-laying defects, 10 gravid young adult animals can be placed on an NGM plate with E. coli for several hours, then removed after sufficient eggs have been laid.
As early as 30 min after dye-filling, imaging can be performed, as described at the end of the dye-filling phase (step 3.11). However, animals swimming in DiI dye can also ingest some of the dye, allowing it to concentrate in the intestine, in addition to localization throughout the neurons. This can lead to unwanted fluorescent signals appearing in the intestine for at least 30 min and up to several hours after dye-filling (Figure 4). Because of this drawback to early imaging, we find that the clearest images of the neurons, without any undesirable background signals, are seen after 24 h of recovery. Within the 24 h window after dye-filling, the fluorescent signal from the dye remains bright and relatively even (Figure 2).
Imaging dye-filling after 48 h of recovery may yield undesirable results. First, the fluorescent signal from DiI fades over time, making it particularly difficult to visualize the dye-filled dendrites (Figure 2). This result does not appear to be caused by developmental changes in the animals (Figure 3), but could be the result of some combination of dye diffusion, dye breakdown, and fluorescence quenching. Second, C. elegans are known to develop intestinal autofluorescence that accumulates with aging15. This autofluorescence is visible through the same filter set (TRITC) as DiI and can cause difficulty distinguishing dye-filled neurons. For this reason, it is also recommended that dye-filling is completed on animals in late larval stages (L3 or L4) or early adulthood, as this window of developmental stages is easiest to image based on the size of the animals and least likely to show autofluorescence due to aging. Selecting the appropriate recovery time for specific experiments and teaching applications is another critical step in this protocol that should be determined by each unique user.
Existing and alternate methods for dye-filling include the use of other lipophilic dyes, such as fluorescein isothiocyanate (FITC) and 3,3́ -dioctadecyloxacarbocyanine perchlorate (DiO)6,7. Dye-filling can also be modified using different staining conditions to reach harder-to-fill targets, such as the six IL2 neurons8, or to visualize additional tissue types, such as cuticular structures9. Different kinds of microscopy can be used to visualize dye-filling, depending on the equipment available. Fluorescent compound microscopy is used in this paper and is an accessible, low-cost option that still provides relatively high-resolution visualization of dye-filling patterns. Fluorescent stereo microscopy is compatible with fast, high-throughput screening of large numbers of live animals and can be used to quantify the frequency of dye-filling defects. Higher-resolution imaging techniques, such as confocal microscopy, can provide even more precise information about dye-filling patterns11.
In conclusion, dye-filling is an inexpensive and simple technique to implement, making it an especially attractive method for use in undergraduate biology classes. In addition, it is a very accessible technique for independent research projects led by undergraduate trainees, as well as those new to the C. elegans field. As a stand-alone lab exercise or as one step in a larger research project with C. elegans, dye-filling is an excellent method for understanding the disruption of the fundamental processes occurring in ciliated sensory neurons.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We would like to thank Nancy Shough and Cameron Brisbine (Southern Oregon University). Work was supported by startup funds from Southern Oregon University for M. LaBonty. Some C. elegans strains were provided by the Caenorhabditis Genetics Center (CGC), which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440).
Materials
| Name | Company | Catalog Number | Comments |
| DiI | Biotium | 60010 | Not water soluble, make 2 mg/mL solution in DMF. Solution is light sensitive, cover with foil. Store at -20 °C. Solution good for many years. |
| Levamisole hydrochloride | Fisher | AC187870100 | 10 mM solution in M9 Buffer. Store at -20 °C. Solution good for many years. |
| M9 Buffer | IPM Scientific | 11006-517 | Available for purchase, but also easy to make in house following recipe in protocol. |
| N2 (C. elegans strain) | CGC | N2 | C. elegans wild isolate |
| YH2125 (C. elegans strain) | n/a | n/a | Strain generated in Yoder Laboratory (Bentley-Ford et al, 2021) |
References
- Alcedo, J., et al. Nature's gift to neuroscience. J Neurogenet. 34 (3-4), 223-224 (2021).
- Anvarian, Z., Mykytyn, K., Mukhopadhyay, S., Pedersen, L. B., Christensen, S. T. Cellular signalling by primary cilia in development, organ function and disease. Nat Rev Nephrol. 15 (4), 199-219 (2019).
- Inglis, P. N., Ou, G., Leroux, M. R., Scholey, J. M. The Sensory Cilia of Caenorhabditis elegans. WormBook. , (2007).
- Bae, Y. -. K., Barr, M. M. Sensory roles of neuronal cilia: cilia development, morphogenesis, and function in C. elegans. Front Biosci. 13 (15), 5959-5974 (2008).
- Altun, Z. F., Hall, D. H. Nervous System, Neuronal Support Cells. WormAtlas. , (2010).
- Perkins, L. A., Hedgecock, E. M., Thomson, J. N., Culotti, J. G. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 117 (2), 456-487 (1986).
- Starich, T. A., et al. Mutations affecting the chemosensory neurons of Caenorhabditis elegans. Genetics. 139 (1), 171-188 (1995).
- Tong, Y. -. G., Bürglin, T. R. Conditions for dye-filling of sensory neurons in Caenorhabditis elegans. J Neurosci Methods. 188 (1), 58-61 (2010).
- Schultz, R. D., Gumienny, T. L. Visualization of Caenorhabditis elegans cuticular structures using the lipophilic vital dye DiI. J Vis Exp. (59), e3362 (2012).
- Bentley-Ford, M. R., et al. Evolutionarily conserved genetic interactions between nphp-4 and bbs-5 mutations exacerbate ciliopathy phenotypes. Genetics. 220 (1), iyab209 (2022).
- Guha, S., Pujol, A., Dalfo, E. Anti-oxidant MitoQ rescue of AWB chemosensory neuron impairment in a C. elegans model of X-linked Adrenoleukodystrophy. MicroPubl Biol. 2021, (2021).
- Chen, N., et al. Identification of ciliary and ciliopathy genes in Caenorhabditis elegans through comparative genomics. Genome Biol. 7 (12), R126-R212 (2006).
- Reiter, J. F., Leroux, M. R. Genes and molecular pathways underpinning ciliopathies. Nat Rev Mol Cell Biol. 18 (9), 533-547 (2017).
- Porta-de-la-Riva, M., Fontrodona, L., Villanueva, A., Céron, J. Basic Caenorhabditis elegans methods: synchronization and observation. J Vis Exp. 64, e4019 (2012).
- Pincus, Z., Mazer, T. C., Slack, F. J. Autofluorescence as a measure of senescence in C. elegans: look to red, not blue or green. Aging (Albany NY). 8 (5), 889-898 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved