Method Article
Ultrasound Assessment of Aortic and Carotid Artery Intima-media Thickness in Children and Adolescents
En este artículo
Resumen
The present article describes the methodological considerations for the noninvasive assessment of abdominal aortic and carotid intima-media thickness using B-mode ultrasonography. This technique is commonly used in the developmental origins of health and disease research as a surrogate for early arterial changes.
Resumen
Carotid intima-media thickness (IMT), measured using high-resolution B-mode ultrasonography, is a widely utilized surrogate marker of subclinical atherosclerosis, the pathophysiological process underlying most clinical cardiovascular disease events. Atherosclerosis is a gradual disease that originates early in life, thus, there has been increased interest in measuring carotid IMT in childhood and adolescence to assess structural change in the arterial vasculature in response to adverse exposures. However, the timing of atherosclerosis varies across the vascular tree. Primordial atherosclerotic lesions are present in the abdominal aorta as early as infancy, compared to mid-adolescence for the common carotid. Measurement of IMT at either site is susceptible to several technical challenges that need to be considered, especially in younger children. In this paper, we provide a detailed stepwise method for high-quality assessment of IMT of the abdominal aorta and common carotid artery in the young. We also provide insight into the appropriateness of either site when exploring the associations between early-life exposures and later-life cardiovascular disease.
Introducción
The Developmental Origins of Health and Disease (DoHAD) hypothesis proposes a link between environmental exposures during critical periods of development - from conception to 2 years of age - and later-life susceptibility to cardiometabolic diseases1. Several observational studies have shown that exposures in the perinatal period, such as low birth weight and pre-term birth, are associated with longer-term cardiovascular disease (CVD) risk2. Atherosclerosis, the gradual thickening of the two innermost layers of the arterial wall, is a precursor to most clinical CVD events3. This thickening can be measured non-invasively at the sub-clinical stage using high-resolution Brightness mode (B-mode) ultrasound, a technique referred to as intima-media thickness (IMT).
In the 1980s, carotid IMT measured ultrasonography was validated against direct histology and since then has become a hallmark non-invasive method to identify early arterial changes4. Assessment of carotid IMT is popular within DoHAD research as it allows us to explore the association between environmental exposures and adaptations in the vasculature early in life and the potential monitoring of these adaptations over time. Carotid IMT is increased in children with exposure to early-life risk factors such as fetal growth restriction5, and excessive weight gain in the first two years of life6, in addition to traditional CVD risk factors such as obesity7, smoking exposure, and dyslipidemia8. While IMT of the carotid bifurcation, internal, and common carotid have been studied with risk factors and are all predictive of later-life cardiovascular events9,10, far-wall IMT of the common carotid (cIMT) artery is the only site to have been validated against direct histology3 and the focus of the present manuscript.
Importantly, studies exploring the natural progression of atherosclerosis indicate that the abdominal aorta is the first of the large elastic arteries to present with primordial atherosclerotic lesions known as fatty streaks, particularly the distal far wall of the vessel11,12. Comparatively, the common carotid presents with fatty streaks in mid-adolescence. Thus, measurement of abdominal aortic IMT (aIMT) may facilitate earlier detection of changes in vascular structure. In the Muscatine Offspring Study of 635 people aged 11 - 34 in the United States, aIMT was found to have stronger associations with conventional CVD risk factors in adolescents (11-17 years), while cIMT had stronger associations in older subjects (18-34)13. In high-risk children compared to controls, IMTs of both vessels increased, but the effect was greater in the aorta compared to the carotid, accounting for luminal diameter14. These results and natural history studies collectively suggest prioritizing the measurement of aIMT in younger populations compared to cIMT. Although this is not without its limitations, the measurement of aIMT tends to be more variable15, the methodology until recently lacked standardization3, and there are concerns about its utility in individuals with greater central adiposity.
When focusing specifically on exposures in the first 1,000 days of life, two recent systematic reviews and meta-analyses provide meaningful insights into the sensitivity of each technique. In studies with apparently healthy subjects aged 0 to 18, Epure et al.9 assessed the associations between clinical conditions within the first 1,000 days of life and cIMT. They found being born small-for-gestational age (SGA), with or without fetal growth restriction, was significantly associated with increased cIMT in children and adolescents (16 studies, 2,570 participants, pooled standardized mean difference 0.40 [95% CI: 0.15-0.64], p= 0.001, I2 = 83%) compared to those born appropriate for gestational age. In a near-identical meta-analysis with aIMT instead as the outcome measure, Varley et al.10 reported significantly increased aIMT for those born SGA compared to controls and the magnitude of the effect was greater than that for cIMT (14 studies, 592 participants, pooled standardized mean difference 1.52 [95% CI: 0.98-2.06], p < 0.001, I2 = 97%). Moreover, they found associations with other risk factors that Epure et al.9 did not, such as exposure to pre-eclampsia and being born large-for-gestational age, perhaps owing to the greater sensitivity of aIMT than cIMT.
Importantly, both reviews identified a lack of standardization in methodology and an absence of tailored advice for measuring children and adolescents as a limitation for in-depth cross-study comparisons and inconclusive results for other exposures. Accordingly, the present manuscript aims to provide a detailed protocol for each measurement in the young. The rationale and justification for these protocols have been presented in greater detail previously3. We discuss common methodological challenges and provide practical recommendations to overcome them.
The below protocol assumes a basic understanding of an ultrasound machine and its components and the manipulations that can be performed with an ultrasound transducer16,17,18. It is also strongly recommended that the examiner, participant, and machine are positioned appropriately to increase the efficiency of testing and minimize strain, consistent with modern best practices in sonography. Suggestions are provided below. All assessments should be performed in a quiet, temperature-controlled room with dimmed lighting for the comfort of the participant and the ascertainment of imaging. Ask participants to fast for at least eight hours before testing to reduce gas in the bowel15, clear fluids are allowed, although at younger ages this may not be possible. However, avoid assessing straight after a meal. Conducting measurements in the morning within the first two hours of awakening has been previously reported as the best timeframe for visualization of the abdominal aorta3,15, this may also reduce any inconvenience associated with fasting. This protocol has been adapted from guidelines outlined by the American Society of Echocardiography Carotid Intima-Media Thickness Task Force19, the Mannheim Carotid Intima-Media Thickness and Plaque Consensus18, and the Association for European Paediatric Cardiology AECP20 for the measurement of cIMT and recently published recommendations for the measurement of aIMT3. We strongly recommend also reviewing a recent point-of-care ultrasound protocol to assist with understanding the anatomy of the abdominal aorta and surrounding structures21.
Protocolo
All research was performed in compliance with the Sydney Local Health District Human Research Ethics Committee (Protocol Nos. X16-0065 and X15-0041). All ultrasound images are free of identifying information. Images used to illustrate transducer placement were performed on individuals with their consent or with consent from their parent or guardian for those unable to provide consent.
1. Common carotid intima-media thickness
- Ask the participant to lie down on the bed without a pillow. This will help keep the neck as straight as possible.
- Ask the examiner to be positioned at the head of the participant and the bed elevated to allow the examiner's elbows to rest on the bed to stabilize the scanning arm.
- Place the machine in front of the examiner, opposite the scanning side, to manipulate the machine with the examiner's non-scanning hand without overextension. Allow sufficient space so that the ultrasound can be moved to the opposite side when examining the other side of the neck.
- Acquire an electrocardiogram (ECG) simultaneously. A 3-lead ECG is sufficient; apply the appropriate leads as per manufacturer instructions and the age of the participant. Place gel on the transducer. In neonates and infants, the use of single-use sterile gel packets is recommended for infection control purposes. Warming the gel beforehand may also help prevent discomfort.
- Using a linear transducer with a minimum frequency of 7 MHz, set the device to a depth of 3-4 cm, a frame rate of 25 Hz, and a dynamic range of 55-65 dB. Retrospective capture is recommended, as children can abruptly move.
NOTE: To assist with standardization of the measurement across participants and examiners and to increase efficiency in testing, the use of a preset is recommended. Most ultrasound machines have this functionality. The above settings are recommended and may vary according to the machine being used; the effects of ultrasound settings are discussed below. - To increase reproducibility, acquire a minimum of three insonation angles per side. Use an instrument like Meijer's Carotid Arc to standardize the angles. The angles collected here are left 210°, 240°, and 270° and right 150°, 120°, and 90°, which correspond to the anterior, lateral, and posterior views of each vessel19.
NOTE: Collecting multiple angles is recommended as thickening is typically eccentric; however, it will increase the examination and analysis burden. - Ask the participant to extend their neck and tilt their head to the left at an angle of approximately 45°; a rolled towel or pillow can be wedged underneath the participant's head for comfort and to help maintain the lateral rotation19.
- Position the transducer in the transverse scanning plane at the base of the neck with the indicator at 9 o'clock and scan upwards towards the head. Ensure the position of the indicator matches what is on the screen. Identify the common carotid artery, a pulsatile anechoic circle in the center of the screen. The jugular vein can also be seen directly atop the common carotid; it has a thinner wall and is collapsible with moderate pressure, while the carotid maintains its circular shape (Figure 1).
- While moving up the neck, observe the common carotid artery enlarge and then bifurcate into the internal and external carotid arteries. Position the transducer at the point of enlargement, also referred to as the bulb, and turn clockwise into the longitudinal view. Ensure the indicator position is now facing towards the head.
- Adjust gain settings to obtain symmetrical brightness for the near- and far-wall (wall closest to and wall furthest from the ultrasound beam, respectively; see Figure 2 and Figure 3) and minimal intraluminal artifact.
NOTE: Imaging artifacts from inappropriate gain settings, such as washed-out borders, can affect the interpretation of the IMT. The IMT has a distinct double-line pattern: the intima and adventitia are hyperechoic (bright), while the media is hypoechoic (darker). - Obtain a digital loop with a minimum of three cardiac cycles of the common carotid artery 10 mm proximal to the bulb (Figure 2) . For optimal imaging, ensure the vessel is perpendicular to the probe beam. This can be achieved by subtly tilting the transducer on its short axis and differential pressure along the long axis of the transducer, also known as rocking or the heel-toe movement16,17.
- Repeat the process for the remaining two angles and adequately label each digital loop to allow for easy re-identification of the angle.
- Repeat steps 1.7 - 1.11 on the right side of the neck. Conclude the exam. Clean off any remaining gel and remove ECG stickers. Considerations for removing ECG stickers in newborns and infants are discussed in Supplementary Table 1.
2. Aortic intima-media thickness
- Ask the participant to lie down on the bed in a supine position with the abdomen exposed. Ask the participants to bend their knees with their feet flat on the bed; this relaxes the abdominal muscles and can improve imaging.
- Acquire an ECG simultaneously by following step 1.4. Place the machine adjacent to the participant and within easy reach of the examiner's scanning hand. Place gel on the transducer.
NOTE: Neonates and infants have a high breathing rate, which can strongly influence the interpretation of vessel diameter during the cardiac cycle. Hence, an ECG is essential to measure IMT at end-diastole. - Use a linear transducer with a minimum frequency of 7 MHz, depth to be adjusted to keep the aorta in view, a frame rate of 25 Hz, and a dynamic range of 55-65 dB. Use an appropriate zoom to maintain the aorta in the center of the screen. Use lower frequencies for those with higher body mass. Retrospective capture is recommended as per step 1.5.
- Identify the aorta by placing the transducer in the transverse plane directly below the xiphisternum with the indicator in the 9 o'clock position (facing the sonographer). The aorta will appear as a pulsatile anechoic circle to the right of the screen. Surrounding structures include the inferior vena cava (IVC) to the left of the aorta, the liver directly above, and the anechoic vertebral body directly below.
- Turn the probe clockwise until the aorta appears in the longitudinal view. Ensure the indicator position is facing towards the head and matches what is on the screen. In the longitudinal view, surrounding structures will include the liver and pancreas directly above and the vertebrae at the bottom of the screen.
- Slowly move the probe downwards until the coeliac artery (CA) and superior mesenteric artery (SMA) branches are identified. Caudal to this branching is the proximal abdominal aorta, and distal to this branching is the mid to distal abdominal aorta.
- Continue scanning until a straight segment without branching is identified. The aorta becomes more anterior as it moves distally, so adjust the zoom settings to ensure the aorta is centered on the screen. Ensure the vessel is perpendicular to the ultrasound beam and adjust gain settings as needed (Figure 5).
- Obtain a digital loop with a minimum of three cardiac cycles. Obtain digital loops of different straight non-branching segments scanning from the proximal to the distal abdominal aorta (just before the left and right iliac arteries) and multiples for each segment. These can be labeled as proximal, middle, and distal aIMT.
NOTE: Digital loops of different segments will not always be possible. Gas in the bowel can commonly obstruct image acquisition and limit the ultrasonic window to just proximal to the CA and SMA branching. - Conclude the exam. Clean off any remaining gel and remove ECG stickers.
3. Off-line intima-media analysis using semi-automated edge-detection software
- Using a semi-automated edge detection software can lower inter-operator variability and increase reproducibility. This analysis is offline; thus, export the images in native Digital Imaging and Communications in Medicine (DICOM) format without digital compression.
- Keep the analysis blinded to reduce potential operator bias. Practically, if there are more than two research staff members, the researcher conducting off-line image analysis should be different from the researcher who collected the images. If there is only one researcher, blinding could be achieved through deferred analysis of deidentified images.
- Carotid IMT
- Select a minimum 10 mm region of interest (ROI) proximal to the bulb. The bulb can be defined as the point where the two parallel walls of the common carotid start to diverge; this is not always symmetrical and heterogeneous across individuals.
NOTE: The recommended distance between the ROI and the bifurcation varies between children and adults and is the primary difference between them. In children, the ROI should ideally be a 10 mm region just proximal to the bulb. This is different from the recommendation for the adult population, which suggests measuring the IMT from a 10 mm straight segment at least 5 mm below the bulb19,20. - The software used automatically detects the intima-lumen and media-lumen borders for both the near and far walls. To do this, train the software on an operator-selected frame with clearly defined IMT and ask the operator to adjust the software's border detection if needed.
- Analyze the digital loop and report the vessel diameter, average, maximum, and minimum near- and far-wall IMT for each frame. The software also produces a trace of vessel diameter over time, which can be used to identify end-diastolic frames in the absence of an ECG (Figure 6).
NOTE: Most semi-automated software relies on similar calculations of the IMT values. Within an ROI, multiple paired data points are drawn between the intima and media borders. Mean IMT is the average value of all points within an ROI. Maximum and minimum IMT are the single largest and smallest data points in the ROI, respectively. Due to the focal nature of plaque formation, the average maximum IMT may better indicate subclinical atherosclerosis compared to the average mean IMT3. Ideally, both methods of segmental thickness should be reported. - Select diameter and far-wall IMT measurements from three consecutive cardiac cycles at end-diastole (on or near the R-wave of the ECG) and calculate the average. As the selection of end-diastolic frames is operator-dependent, record details such as loop and frame number to facilitate cross-checking of work. We recommend minimum operator intervention. Report the mean of each vessel separately, as IMT in the left common carotid can be higher than the right18.
NOTE: Manual border adjustment is possible for difficult scans but should only be done for the selected end-diastolic frames to avoid increasing the analysis burden. If collecting the recommended three angles per side, three measurements per side will be recorded, which can be further averaged to produce overall left and right IMT.
- Select a minimum 10 mm region of interest (ROI) proximal to the bulb. The bulb can be defined as the point where the two parallel walls of the common carotid start to diverge; this is not always symmetrical and heterogeneous across individuals.
- Abdominal aortic IMT
- Select a minimum 5 mm region in a straight, non-branching segment of the vessel. Apply steps 3.2.2-3.2.3. Select diameter and far-wall IMT measurements from three consecutive cardiac cycles at end-diastole and average . Repeat if multiple digital loops have been collected and average results from all ROIs.
- Include an expert to assess the reliability and reproducibility of both image acquisition and off-line analysis for all imaging studies. Report the outcomes of these in publications. We recommend repeats of 10% of scans. The recommended acceptable intra and inter-observer coefficient of variation is less than 6 %, or the mean difference in raw IMT measurements should be less than 0.055 mm20.
Resultados
In this section, we represent results from prior studies to highlight key aspects of cIMT and aIMT measurement. Figure 1 and Figure 2 focus on cIMT, demonstrating both transverse and longitudinal views in young, healthy subjects and a detailed visualization of the IMT complex. Figure 3 and Figure 6 further emphasize best practices based on the positioning of the bulb, image settings, as well as semi-automated analysis. The subsequent section on aIMT, demonstrated by Figure 4 and Figure 5, discusses probe positioning strategies and the impact of ultrasound settings on measurement accuracy.
Carotid intima-media thickness
Figure 1 shows the right common carotid artery of a healthy young adult test subject in the transverse view with the jugular vein adjacent. Figure 2A displays the right common carotid artery of a healthy seven-year-old male in the longitudinal view and a close-up of the intima-media complex in Figure 2B, showcasing the distinct double-line pattern. Careful visual inspection of the images at acquisition can markedly reduce the rate of missing data. Figure 3 provides examples of ideal, acceptable, and unacceptable images for cIMT measurement, which can be used as a guide. Figure 6 is a screenshot of the semi-automated software analyzing the common carotid artery of a healthy young adult female test subject. This is an ideal image with the bulb visible to the left of the screen, and the region of interest is positioned within 10 mm of the bulb as per the guidelines for assessment in the young. The mean right far-wall cIMT in this participant was 0.54 mm, and the lumen diameter was 6.24 mm.
Aortic intima-media thickness
Figure 4 shows the positioning of the ultrasound probe in the transverse and longitudinal view when measuring aIMT with a 3-year-old example subject. Internally, the start of the abdominal aorta is defined as when the thoracic aorta crosses the diaphragm, which can be approximated externally as the end of the xiphisternum. Positioning the probe here allows us to visualize the proximal abdominal aorta before the CA and SMA branches. In this figure, we also show the left-recumbent position to displace gas when imaging the abdominal aorta. Compared to when the participant is supine, this position will increase the depth of the vessel and reduce the resolution of the IMT. Thus, we recommend flagging measurements performed in this position for sensitivity analysis. Figure 5 provides an example of an acceptable and unacceptable image for aIMT measurement. Inappropriate gain can result in intraluminal artifacts of the distorted intima-media borders, e.g., excessive gain causes artificial thickening by washing out the hyperechoic intima. Thus, it is important to optimize gain settings during acquisition.
Comparison of carotid and aortic intima-media thickness and age
Lastly, Figure 7 presents representative percentiles of mean and maximum aIMT and cIMT from male and female healthy subjects aged 2 to 20 years (mean age 11.2 years [SD 5.1]). Their baseline characteristics are presented in Table 1. As reported in the literature for older subjects15, aIMT is greater than cIMT at any age and increases with age at a greater rate than cIMT. The overall mean aIMT in this cohort was 0.54 mm (SD 0.08), and cIMT was 0.48 mm (SD 0.04).
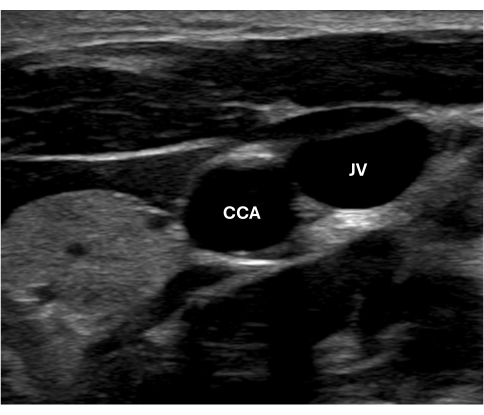
Figure 1: Image of the anechoic common carotid artery in transverse view. Abbreviations: CCA = Common carotid artery; JV = Jugular vein. Please click here to view a larger version of this figure.
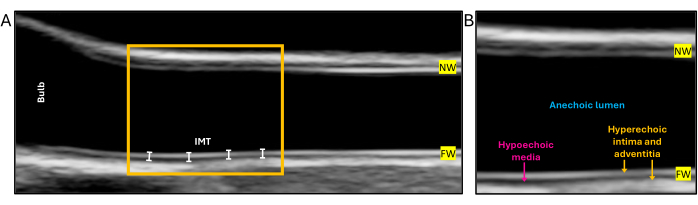
Figure 2: Longitudinal view of the common carotid artery in a healthy 7-year-old male at end-diastole and a close-up of the intima-media complex in the same subject. (A) An ideal capture of the common carotid artery for assessment of IMT. The yellow box indicates the region of interest where measurement of intima-media thickness is recommended in children within 10 mm of the carotid bulb. (B) A close-up of the IMT displays the distinct double-line pattern and the echogenicity features observed in the ultrasound. Abbreviations: IMT = intima-media thickness, NW = near-wall, FW = far wall. Please click here to view a larger version of this figure.

Figure 3: Longitudinal view of the common carotid artery, left or right, of healthy subjects aged 6.5 - 12.5 years at end-diastole. (A) An ideal capture of the common carotid artery at end-diastole for assessment of intima-media thickness (IMT). The vessel is perpendicular to the ultrasound beam, the bulb is visible to the left of the screen, and the IMT is visible and continuous. (B) The gain settings are too high, resulting in an intraluminal artifact and a washed-out near wall. The start of the bulb is also not clear. Nonetheless, the IMT is visible and continuous and should be measured. (C) The gain settings are too low, resulting in a non-continuous IMT, artificial enlargement of the hypoechoic media, and reduced visibility of the bulb. The bulb serves as a landmark to facilitate consistent measurement of IMT within and across individuals and must be visible across all scans. (D) The vessel is not horizontal, and the gain settings are too low, resulting in artificial thinning of the IMT near the bulb. Images (C) and (D) should be excluded. Please click here to view a larger version of this figure.

Figure 4: Positioning of ultrasound transducer for the imaging of abdominal aortic intima-media thickness (IMT) in a 3-year-old male. The red dot indicates the positioning of the transducer indicator; the green line represents the approximate positioning of the abdominal aorta; the red line indicates the limit of the xiphisternum; and the blue line indicates the limit of the lower ribs for reference. (A, B) The participant is supine. In (A), the transducer is positioned in the transverse view directly below the xiphisternum with the indicator in the 9 o'clock position, and in (B), the transducer has been rotated clockwise to the longitudinal view with the indicator in the 12 o'clock position. (C) The participant is in the left recumbent position with the transducer in the longitudinal view. In this positioning of the probe, the proximal abdominal aorta should be visible. Abbreviations: C = caudal; D = distal; R = right; L = left. Please click here to view a larger version of this figure.

Figure 5: Abdominal aortic intima-media thickness (IMT) in the same newborn subject. (A) The gain settings are too high, resulting in noise in the lumen, wash-out of the media-adventitia border, and false borders. (B) Gain settings have been optimized to reduce said artifacts and visualize the IMT complex of the far wall. Abbreviations: NW = near-wall; FW = far-wall. Please click here to view a larger version of this figure.

Figure 6: Example of common carotid intima-media thickness (IMT) measured by a semi-automated edge-detection software of a healthy young adult female. A region of interest (ROI) is placed within 10 mm of the carotid bifurcation, estimated as the first point at which the two parallel walls of the vessel begin to diverge. To the right of the image is the results window, populated with the trace of vessel diameter over time as well as vessel diameter and mean near and far-wall IMT per frame. Please click here to view a larger version of this figure.
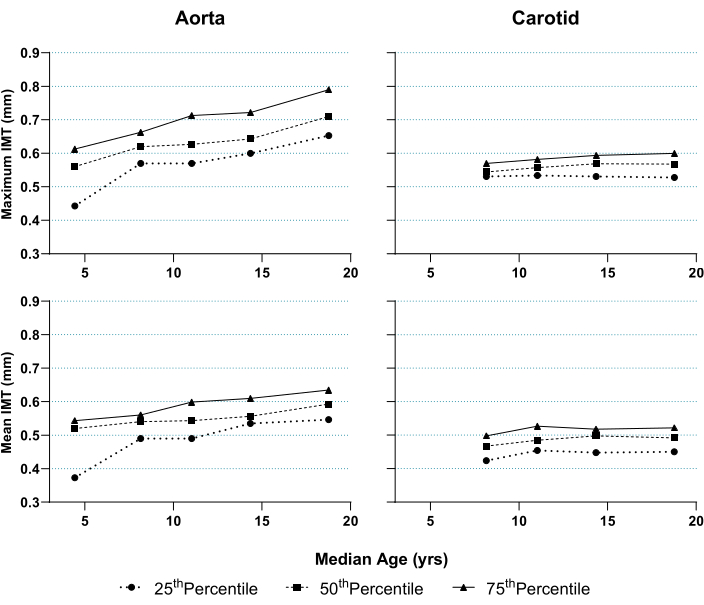
Figure 7: Percentiles of mean and maximum abdominal aortic intima-media thickness (IMT) and common carotid artery IMT according to median age in healthy subjects. Both were measured as per the method described in this paper. Subjects were divided into five equally balanced age groups with near-equal sex distribution. Median age and IMT percentiles per group were calculated and graphed. Aortic IMT was assessed across subjects aged 2-20 (n = 80); carotid IMT was assessed in the subgroup aged 6.5-20 (n = 75). Please click here to view a larger version of this figure.
| Variable | Aortic intima-media thickness (N = 80) | Carotid intima-media thickness (N = 75) | ||
| Demographics | ||||
| Age (years) | 11.2 (5.0) | 13.1 (4.2) | ||
| Sex (% Female) | 50 | 49.3 | ||
| Weight (kg) | 38.7 (18.2) | 45.5 (17.1) | ||
| Weight z-score | 0.0 (0.9) | 0.1 (0.9) | ||
| Height (cm) | 143.0 (24.0) | 152.9 (17.2) | ||
| Height z-score | 0.04 (2.8) | 0.03 (2.9) | ||
| BMI (kg/m2) | 17.7 (3.1) | 18.8 (3.3) | ||
| BMI z-score | -0.2 (0.9) | -0.1 (0.9) | ||
| Intima media thickness | ||||
| Mean (mm) | 0.54 (0.08) | 0.48 (0.05) | ||
| Maximum (mm) | 0.63 (0.10) | 0.56 (0.04) | ||
| Diameter (mm) | 9.2 (2.1) | 6.0 (0.4) | ||
| Values are mean (SD) or [n (%.)]. BMI = body mass index. | ||||
Table 1: Baseline characteristics for the cohort in this study.
Supplementary Table 1: Summary of consideration(s) and proposed intervention(s) to improve compliance, cooperation, and reduce missingness in data during measurement of abdominal aortic and common carotid artery intima-media thickness (IMT) in children and adolescents. Please click here to download this File.
Discusión
The present manuscript provides guidance on the acquisition and analysis of ultrasound images to measure aIMT and cIMT, specifically in younger populations (ages 0-18). Both techniques have demonstrated utility in exploring the influence of early life exposures on atherosclerosis but are susceptible to technical challenges, which we discuss below.
Critical steps in protocol implementation
Ultrasound system and settings: Acquisition of high-quality B-mode images is essential. Thus, the operator must have significant expertise in measurement and knowledge of ultrasonography. Depending on the setting, this may require a specific training and accreditation pathway or dedicated training with an experienced research team. Significant investment is needed to establish the technique in a facility (e.g., ultrasound and dedicated analysis software), and there may be ongoing costs (e.g., software updates and operator training).
The quality of the images is also machine-dependent; larger mainframe ultrasounds should be prioritized as they produce higher-quality images compared to smaller portable machines. Individual instrument settings must also be considered, especially with significant advances in ultrasound technology, although few studies have thoroughly tested their impact on IMT measurement. Bhagirath et al.22 compared cIMT measurements collected with either a standard 8 MHz or a high frequency 14 MHz transducer and found a minimal difference (within 0.05 mm of each other in 95% of subjects). Comparatively, in a study using phantom arteries, small changes in dynamic range were shown to affect wall thickness significantly23. Additionally, in their review, Mitchell et al.24 tested four separate ultrasound systems produced in the mid-1990s till the mid-2010s and found that mean cIMT varied due to improvements in acquisition speed and programming. Until normative data is developed with contemporary technology and seminal validation studies are repeated8, we recommend utilizing the above-recommended settings where possible. These are based on expert opinions and facilitate comparisons with earlier studies. Importantly, machine and ultrasound settings must be kept consistent within a study.
Participant characteristics: Participant tolerability and compliance can affect the feasibility of IMT assessment. In our experience, the most challenging cohort is children younger than seven years of age. In this cohort, image acquisition often cannot be attempted, might be stopped prematurely, or is inadequate for analysis (e.g., excessive movement). Thus, it is essential to have both an understanding and a plan to manage age-specific behaviors in addition to significant operator expertise. Feasibility studies to inform practical recommendations for infants and young children are scarce; Zhao et al.25 found similar success rates in healthy 2-year-olds for measuring cIMT (72%) and aIMT (67%), utilizing distractions such as reading or watching cartoons. Mivelaz et al.26 demonstrated good feasibility (79%) and reproducibility for measuring cIMT in 1-year-old infants. These rates fall below the 10% threshold for missing data proposed by Skilton et al.3. While arbitrary, this threshold has been informed by rates of missing data in the existing literature for aIMT and similar values have been reported for cIMT27. Consequently, recommendations for enhancing compliance and cooperation in this cohort and those older are adapted from advice for healthcare practitioners performing non-invasive medical procedures. These are summarized in Supplementary Table 1. Another participant characteristic that may impact results is higher body mass. In a cross-sectional study of healthy volunteers aged 11-34 years, BMI was a significant predictor for missing aIMT data. Similarly, higher BMI is associated with data incompleteness for cIMT in adults28. In this cohort, lower-frequency transducers or a curved linear array transducer may reduce missing data, but the validity of this has yet to be tested.
Measurement site: There are some challenges specific to the vessel being measured. Firstly, while post-mortem studies indicate the distal far wall of the abdominal aorta has the greatest predisposition for the formation of an atheroma, measuring that site is not always possible. Skilton et al.3 note that the presence of interstitial gas can mean that only the proximal abdominal aorta is visualized. Potential solutions to displace gas are discussed in Supplementary Table 1. Due to this common issue, we recommend pre-specifying the site of measurement and reporting any deviations of the protocol in publications to better enable cross-study comparisons. When measuring cIMT, a common issue is the number of angles. Current consensuses18,19,20,29 recommend averaging IMT from multiple insonation angles, which can be burdensome for both the participant and examiner. However, multiple angles facilitate better reproducibility in longitudinal studies, particularly for average maximum IMT30. As we do not recommend cIMT in very young children, who are most likely to find it intolerable, we recommend attempting at least three measurements per side.
Limitations of the techniques
There are limitations associated with both methods. Firstly, both measurements are operator-dependent at the time of acquisition and analysis. To minimize the bias, we recommend inter and intra-observer reliability testing, firstly during examiner training and then regularly throughout data collection and analysis. These metrics should be reported in publications. Where possible, the analysis should be blinded, and we have provided practical suggestions to achieve this. We also strongly recommend the use of continuous semi-automated software as it is shown to reduce bias, increase analysis efficiency, and is comparable to manual caliper measurements, which can be machine-dependent31. Secondly, some amount of intimal thickening is expected due to the physiological growth of both vessels with age. Thus, corrections for body size of the absolute measurements should be included in statistical analysis and have been described elsewhere3,32. The statistical plan should also include sensitivity testing, excluding scans flagged at the time of measurement or analysis as being at risk of bias, e.g., extensive manual adjustment.
Importantly, there is a paucity of age, sex, and race-specific normative values and pathological cut-offs for both aIMT and cIMT. Studies that have attempted this have done so in too few people, with a narrow age range, or have not reported methodology adequately20. The AEPC has recommended classifying cIMT values above the 75th percentile, adjusted for age and sex, as abnormal using the available literature20. However, we are not aware of any studies that have used this classification as an inclusion criterion to prescribe treatment. Despite the absence of normative data, these measurements have the potential to test the efficacy of putative interventions. Indeed, cIMT has demonstrated utility as the primary endpoint for trials in children with pharmacological33 and lifestyle intervention34, and aIMT has been tested as an exploratory outcome in sub-studies of lifestyle intervention trials35,36. Establishing normative data on the young should be the focus of future research. In the interim, cohort studies should include appropriately matched controls.
Significance compared to alternative methods
An alternative non-invasive modality to assess vascular health is pulse wave velocity (PWV). This test assesses the elastic and functional properties of the vessel, i.e., arterial stiffness, an intermediatory biomarker of CVD37,38. The utility of PWV to detect pathological changes in arterial stiffness in pre-pubertal children remains uncertain. In their meta-analysis, Varley et al.39 found limited evidence supporting the effect of early-life exposures on arterial stiffness in healthy subjects aged 0-18 years. They identified only a single study of children and adolescents in which PWV was significantly higher in those exposed to maternal diabetes compared to controls (42 participants, mean difference 0.17 m/s [95% CI: 0.14-0.20], p < 0.001)39. This appears to be consistent with the literature, which shows that arterial compliance is greatest in childhood, and age-related changes to arterial stiffness do not appear until puberty40,41,42. We found similar results in our cohort of healthy participants aged 2 to 20, whereby there was an age-related increase in cfPWV after approximately ten years of age43. As such, we caution against the use of PWV outside of at-risk groups in children younger than ten years of age.
Importance and potential applications of the method in Developmental Origins of Disease research
Adaptive changes in the vasculature in the first 1,000 days of life can predispose individuals to an increased risk of later-life CVD1. As CVD endpoints are unlikely to occur in children and adolescents, the application of an age-appropriate modality to measure early arterial changes during this critical period and beyond allows us to achieve the following: (1) retrospectively identify and characterize risk factors that can inform potential prevention strategies; (2) assess the effectiveness of prevention strategies, especially during the first 1,000 days of life; (3) identify those at an increased risk for later-life CVD who may benefit from targeted intervention.
Compared to other modalities, IMT remains a cost-effective, accessible, and acceptable option for identifying early structural changes in arterial vasculature in younger populations. There are few head-to-head comparisons of the common carotid and abdominal aortic IMT in children and several technical challenges of cIMT measurement in this population. In contrast, aIMT is better tolerated in this age range and performs as well as cIMT in assessing subclinical atherosclerosis. Therefore, we recommend aIMT in infants and younger children and caution against the use of cIMT to assess early arterial changes until mid-adolescence. A lack of standardization and homogeneity in IMT assessment, especially in children and adolescents, has been identified as a crucial limitation of the technique for either site, and we hope this protocol addresses this limitation, improves data collection and reproducibility, facilitates meaningful comparisons between studies, and improves the clinical validity of IMT measurement.
Divulgaciones
The authors have nothing to disclose.
Agradecimientos
The authors would like to thank all participants in our studies.
Materiales
| Name | Company | Catalog Number | Comments |
| 12-3 MHz Broadband linear array transducer | Phillips | L12-3 | |
| Meijer's Carotid Arc | Meijer | - | |
| Semi-automated edge detection analysis software | Medical Imaging Applications | Carotid Analyzer 5 | |
| Ultrasound | Phillips | Epiq 7 | |
| Ultrasound transmission gel | Parker | 01-08 |
Referencias
- Barker, D. J. Fetal origins of coronary heart disease. BMJ. 311 (6998), 171-174 (1995).
- Belbasis, L., Savvidou, M. D., Kanu, C., Evangelou, E., Tzoulaki, I. Birth weight in relation to health and disease in later life: an umbrella review of systematic reviews and meta-analyses. BMC Med. 14 (1), 147 (2016).
- Skilton, M. R., et al. Natural history of atherosclerosis and abdominal aortic intima-media thickness: Rationale, evidence, and best practice for detection of atherosclerosis in the young. J Clin Med. 8 (8), 1201 (2019).
- Poli, A., et al. Ultrasonographic measurement of the common carotid artery wall thickness in hypercholesterolemic patients. A new model for the quantitation and follow-up of preclinical atherosclerosis in living human subjects. Atherosclerosis. 70 (3), 253-261 (1988).
- Skilton, M. R., et al. Weight gain in infancy and vascular risk factors in later childhood. Pediatrics. 131, e1821-e1828 (2013).
- Bots, M. L., Hoes, A. W., Koudstaal, P. J., Hofman, A., Grobbee, D. E. Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation. 96 (5), 1432-1437 (1997).
- Iglesias del Sol, A., Bots, M. L., Grobbee, D. E., Hofman, A., Witteman, J. C. Carotid intima-media thickness at different sites: relation to incident myocardial infarction; The Rotterdam Study. Eur Heart J. 23 (12), 934-940 (2002).
- Pignoli, P., Tremoli, E., Poli, A., Oreste, P., Paoletti, R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 74 (6), 1399-1406 (1986).
- Epure, A. M., et al. Risk factors during first 1,000 days of life for carotid intima-media thickness in infants, children, and adolescents: A systematic review with meta-analyses. PLoS Med. 17 (11), e1003414 (2020).
- Varley, B. J., Nasir, R. F., Skilton, M. R., Craig, M. E., Gow, M. L. Early life determinants of vascular structure in fetuses, infants, children, and adolescents: A systematic review and meta-analysis. J Pediatr. 252, 101-110.e9 (2022).
- Napoli, C., et al. Fatty streak formation occurs in human fetal aortas and is greatly enhanced by maternal hypercholesterolemia. Intimal accumulation of low density lipoprotein and its oxidation precede monocyte recruitment into early atherosclerotic lesions. J Clin Invest. 100 (11), 2680-2690 (1997).
- Solberg, L. A., Eggen, D. A. Localization and sequence of development of atherosclerotic lesions in the carotid and vertebral arteries. Circulation. 43 (5), 711-724 (1971).
- Dawson, J. D., Sonka, M., Blecha, M. B., Lin, W., Davis, P. H. Risk factors associated with aortic and carotid intima-media thickness in adolescents and young adults: the Muscatine Offspring Study. J Am Coll Cardiol. 53 (24), 2273-2279 (2009).
- Jarvisalo, M. J., et al. Increased aortic intima-media thickness: a marker of preclinical atherosclerosis in high-risk children. Circulation. 104 (24), 2943-2947 (2001).
- Davis, P. H., Dawson, J. D., Blecha, M. B., Mastbergen, R. K., Sonka, M. Measurement of aortic intimal-medial thickness in adolescents and young adults. Ultrasound Med Biol. 36 (4), 560-565 (2010).
- Bahner, D. P., et al. Language of transducer manipulation: Codifying terms for effective teaching. J Ultrasound Med. 35 (1), 183-188 (2016).
- End, B., et al. Language of transducer manipulation 2.0: continuing to codify terms for effective teaching. Ultrasound J. 13 (1), 44 (2021).
- Touboul, P. J., et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis. 34 (4), 290-296 (2012).
- Stein, J. H., et al. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 21 (2), 93-111 (2008).
- Dalla Pozza, R., et al. Intima media thickness measurement in children: A statement from the Association for European Paediatric Cardiology (AEPC) Working Group on Cardiovascular Prevention endorsed by the Association for European Paediatric Cardiology. Atherosclerosis. 238 (2), 380-387 (2015).
- Hartrich, M., Eilbert, W. An Approach to Point-Of-Care Ultrasound Evaluation of the Abdominal Aorta. J Vis Exp. (199), e65487 (2023).
- Bhagirath, K. M., Lester, S. J., Humphries, J., Hentz, J. G., Hurst, R. T. Carotid intima-media thickness measurements are not affected by the ultrasound frequency. Echocardiography. 29 (3), 354-357 (2012).
- Potter, K., Reed, C. J., Green, D. J., Hankey, G. J., Arnolda, L. F. Ultrasound settings significantly alter arterial lumen and wall thickness measurements. Cardiovasc Ultrasound. 6, 6 (2008).
- Mitchell, C., Korcarz, C. E., Zagzebski, J. A., Stein, J. H. Effects of ultrasound technology advances on measurement of carotid intima-media thickness: A review. Vasc Med. 26 (1), 81-85 (2021).
- Zhao, B., Johnston, F. H., Dalton, M., Negishi, K. Feasibility and normal ranges of arterial intima-media thickness and stiffness in 2-year-old children: A pilot study. Pediatr Cardiol. 40 (5), 914-920 (2019).
- Mivelaz, Y., et al. Feasibility and reliability of carotid intima-media thickness measurements in nonsedated infants. J Hypertens. 34 (11), 2227-2232 (2016).
- Drole Torkar, A., Plesnik, E., Groselj, U., Battelino, T., Kotnik, P. Carotid intima-media thickness in healthy children and adolescents: Normative data and systematic literature review. Front Cardiovasc Med. 7, 59776 (2020).
- Peters, S. A., et al. Extensive or restricted ultrasound protocols to measure carotid intima-media thickness: analysis of completeness rates and impact on observed rates of change over time. J Am Soc Echocardiogr. 25 (1), 91-100 (2012).
- Urbina, E. M., et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 54 (5), 919-950 (2009).
- Dogan, S., et al. Ultrasound protocols to measure carotid intima-media thickness in trials; comparison of reproducibility, rate of progression, and effect of intervention in subjects with familial hypercholesterolemia and subjects with mixed dyslipidemia. Ann Med. 42 (6), 447-464 (2010).
- McCloskey, K., et al. Reproducibility of aortic intima-media thickness in infants using edge-detection software and manual caliper measurements. Cardiovasc Ultrasound. 12, 18 (2014).
- McCloskey, K., et al. Early-life markers of atherosclerosis using aortic and carotid intima-media thickness: An assessment of methods to account for child size. J Vasc Ultrasound. 39 (3), 119-126 (2018).
- Wiegman, A., et al. Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA. 292 (3), 331-337 (2004).
- Woo, K. S., et al. Effects of diet and exercise on obesity-related vascular dysfunction in children. Circulation. 109 (16), 1981-1986 (2004).
- Pahkala, K., et al. Ideal cardiovascular health in adolescence: effect of lifestyle intervention and association with vascular intima-media thickness and elasticity (the Special Turku Coronary Risk Factor Intervention Project for Children [STRIP] study). Circulation. 127 (21), 2088-2096 (2013).
- Kizirian, N. V., et al. Effects of a low-glycemic index diet during pregnancy on offspring growth, body composition, and vascular health: a pilot randomized controlled trial. Am J Clin Nutr. 103 (4), 1073-1082 (2016).
- Boutouyrie, P., et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 39 (1), 10-15 (2002).
- Laurent, S., et al. Aortic stiffness is an independent predictor of fatal stroke in essential hypertension. Stroke. 34 (5), 1203-1206 (2003).
- Varley, B. J., Nasir, R. F., Craig, M. E., Gow, M. L. Early life determinants of arterial stiffness in neonates, infants, children and adolescents: A systematic review and meta-analysis. Atherosclerosis. 355, 1-7 (2022).
- Climie, R. E., et al. Vascular ageing in youth: A call to action. Heart Lung Circ. 30 (11), 1613-1626 (2021).
- Hidvegi, E. V., et al. Reference values of aortic pulse wave velocity in a large healthy population aged between 3 and 18 years. J Hypertens. 30 (12), 2314-2321 (2012).
- Hidvegi, E. V., et al. Updated and revised normal values of aortic pulse wave velocity in children and adolescents aged 3-18 years. J Hum Hypertens. 35 (7), 604-612 (2021).
- Nasir, R., Cai, T. Y., Meroni, A., Skilton, M. P.32 Non-invasive measures of arteriosclerosis across childhood and adolescence: Insights into the natural history of disease. Artery Res. 26 (S1), S55-S55 (2020).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoThis article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados