Method Article
Lymphatic and Blood Network Analysis During Obesity
* Estos autores han contribuido por igual
En este artículo
Resumen
Obesity is a growing global public health issue. It has been previously associated with lymphatic dysfunction, suggesting a vital crosstalk between the adipose tissue and the lymphatic system. Here, we propose an accessible methodology allowing the distinct labeling of blood and lymphatic vasculatures within the subcutaneous adipose tissue.
Resumen
Lymphatic collecting vessels and lymph nodes are inevitably embedded in adipose tissue. The physiological significance of this observation remains still not elucidated. However, obesity is characterized by impaired lymphatic function and increased vessel permeability. Inversely, lymphatic dysfunction induces obesity in mice, suggesting a significant interplay between lymphatic vessels and the adipose tissue. Therefore, understanding factors leading to lymphatic dysfunction might open new therapeutic windows to prevent obesity and associated comorbidities. The first step in this process requires a precise and detailed visualization of the lymphatic network in healthy and inflamed adipose tissue. Here, we describe a rapid, inexpensive, and efficient method that allows to label and analyze lymphatic and blood vessels. This approach takes advantage of the skin-draining brachial lymph node localization within the subcutaneous adipose tissue. The lymphatic arborization of this tissue can be revealed by injecting fluorochrome-conjugated lectins subcutaneously. Moreover, the in vivo labeling approach provides a way to evaluate lymphatic vessel density and functions. Coupled to blood vessel, adipocyte and immune cell staining, the protocol allows for high-resolution mapping of the subcutaneous adipose tissue by 3D imaging.
Introducción
The lymphatic circulatory system plays a crucial role in the maintenance of tissue homeostasis and the induction of efficient immune responses. Lymphatic vessels run parallel to blood vessels and carry interstitial fluid, metabolites, and immune cells to the local draining lymph node (LN) and finally towards the venous circulation1. Dysfunctional lymphatic drainage has been observed during infection, inflammation and metabolic diseases2,3,4,5. The lymphatic vasculature is composed of small size vessels named lymphatic capillaries. Lymphatic capillaries are formed by a single layer of thin lymphatic endothelial cells (LECs) characterized by open junctions (“button-like” junctions) facilitating interstitial fluid, metabolites, and immune cells, mainly dendritic cells (DCs) and T cells, entry into the lymphatic capillary lumen5. Lymphatic capillaries merge into larger vessels named lymphatic collecting vessels. Lymphatic collectors are characterized by a layer of LECs surrounded by a muscle layer providing autonomous contractile tonus and maintaining fluid flow5. Moreover, collecting vessels possess valves assuring a unidirectional lymph flow.
The LECs of collectors and capillaries express a specific set of markers that distinguish them from blood endothelial cells (BECs). Among those factors, Prox1 is a transcription factor guiding LECs generation and is highly expressed in LECs while absent in BECs. The critical involvement of Prox1 in LECs biology was illustrated by the generation and analysis of Prox1-deficient mice6. Prox1 heterozygous mice have a defective lymphatic vasculature development characterized by reduced lymphatic vessel density and increased vascular permeability6. LECs highly express VEGFR3, Podoplanin and CCL215. Those markers are not found on BECs and allow to separately analyze the network of lymphatic and blood vessels. Lyve1 is selectively expressed by lymphatic capillaries while absent on collecting vessels5.
Three types of adipose tissue have been described based on their mitochondrial content and subsequent color. Mitochondria-rich thermogenic brown adipose tissue plays a key role during cold exposure and is located in the interscapular region in mice7,8. White and beige adipocytes have lower mitochondrial density and are mainly involved in energy storing in the form of lipid droplets. White and beige adipocytes are located in visceral and subcutaneous depots9.
Clinical observations established a link between obesity and lymphatic dysfunction10. Obesity induces morphological changes of adipose tissue lymphatic vasculature and leads to an impaired lymph transport11. Data obtained in pre-clinical models revealed that High Fat Diet (HFD) induces a lymphatic remodeling and obese mice have smaller lymph nodes and fewer number of lymphatic vessels12. Nevertheless, the precise molecular mechanisms governing this phenotype remain to be elucidated. LECs involvement during obesity is further supported by observations in genetic models with impaired lymphatic vessel development. As discussed earlier, Prox1 heterozygous mice (Prox1+/-) present an ill-functioning lymphatic system, and coincidently develop excessive visceral adipose tissue accumulation in comparison to Prox1 sufficient animals6. Interestingly, this adipose tissue phenotype is rescued by restoration of lymphatic function13. Together, these results have brought to light strong inter-connections between lymphatic vessels and the adipose tissue, which need further investigation.
In the context of inflammation, a hallmark of obesity, the altered expression of LEC and BEC markers compromises the analysis of these cells via classical antibody staining14,15. Genetic models to label specifically LECs and BECs have been developed and allow to palliate this problem16,17,18,19. However, the usage of genetic reporter lines requires multiple steps of breeding and considerably increases the length and cost of a project. Thus, we propose to use fluorochrome-conjugated lectin injections to investigate blood and lymphatic circulatory systems in the subcutaneous adipose tissue, a simple and relatively non-expensive approach. Lectin conjugated to various fluorochromes are commercially available and can be injected intravenously to label blood vessels, or subcutaneously to label the skin-draining lymphatic vessels embedded in the subcutaneous adipose tissue. This approach relies on the use of separate fluorochrome-lectin conjugates for each injection and allows the distinct labeling of each vasculature. This method is also compatible with the use of genetic models to label the lymphatic or blood vasculature network. Importantly, it provides multiple readouts to analyze the overall health status of the subcutaneous adipose tissue and the blood and lymphatics vasculatures perfusing it. This procedure could be easily applied to analyze the lymphatic and blood vasculature networks during acute and chronic skin diseases including psoriasis and infections.
Protocolo
All animal experimentation was performed in accordance with local ethical committees.
NOTE: Prox1-cre-ERT2 (Prox1tm3(cre/ERT2)Gco/J, Jax #022075) and Rosa26-LSL-tdTomato (B6.Cg-Gt(ROSA)26Sortm9(CAG-tdTomato)Hze/J, Ai9, Jax #007914) were obtained from The Jackson Laboratory and crossed to obtain the inducible lymphatic reporter mouse line Prox1-cre-ERT2::tdTomato. Mice were backcrossed to C57BL/6 background for 10 generations. Six-week-old Prox1-cre-ERT2::tdTomato male mice received tamoxifen diet for 3 weeks. Tamoxifen diet from Envigo Teklad (diet no. TD.130857; 500 mg/kg) was used. Experiments were performed in 12-14-week-old mice. This protocol is applicable to mice of any age, sex or strain.
1. Material preparation
- Sterilize scissors, forceps and dissecting pins using 70% ethanol.
- Prepare two 1 mL syringes with 25 to 27 gauge needles. For subcutaneous injection we recommend using a micro-syringe to inject 10 μL in the upper footpad.
- In a plastic 1.5 mL tube, prepare 200 µL of DyLight 488-conjugated lectin diluted in sterile PBS at a final concentration of 100 μg/mL.
- In another plastic, 1.5 mL tube prepare 100 µL of DyLight 649-conjugated lectin diluted in sterile PBS at a final concentration of 100 μg/mL.
- Prepare a 6-well plate containing PBS and keep it on ice.
- Prepare a solution of 4% paraformaldehyde and 30% sucrose.
2. Labeling of subcutaneous adipose tissue blood and lymphatic vessels.
- Anesthetize the mouse by inhalation of 5% isoflurane. Assess anesthesia depth by firmly pinching the animal’s paw to ensure that the animal is not harmed during the procedure. Anesthesia should be maintained until the end of the lectin injection steps or until euthanasia.
- Inject 100 µL of DyLight 488-conjugated lectin intravenously in the tail vein to label the blood vasculature. Wait 15 minutes before proceeding to tissue harvesting.
- Using a different syringe, inject 10 µL of DyLight 649-conjugated lectin subcutaneously on the upper footpad of the animal to label the draining lymphatic vessels. Wait 15 minutes before proceeding to tissue harvesting.
- Euthanize the mouse by cervical dislocation or exposure to CO2 15 minutes post-injection.
3. Harvesting of subcutaneous adipose tissue
- Lay the mouse on its back on a dissection board.
- Sterilize the fur of the mouse using 70% ethanol.
- Lift the skin of the flank using forceps and make a transversal incision to expose the brachial fat pad.
- Gently pull on the skin to dissociate it from the brachial adipose tissue depot, thus revealing the entire subcutaneous brachial adipose tissue containing the lymph node and the lymphatic collector vessel.
- Using forceps and scissors, gently remove subcutaneous fat depots and transfer them to a dish containing cold PBS. For best results, we recommend removing the fat depots as one single piece.
- From here on out, protect the tissue from light.
4. Tissue fixation
- Submerge the harvested tissue in a solution containing 4% paraformaldehyde and 30% sucrose.
- Incubate the tissue in this solution at least overnight before preparing it for imaging.
5. Staining and imaging
- For optimal results, perform tissue clearing and 3D confocal acquisition as described by Gilleron and colleagues20. We recommend using a light sheet microscope for analysis of large tissues. Additional staining can be performed during the clearing process.
- To achieve best tissue mapping, use of the following antibodies:
For adipocytes: Perilipin
For macrophages: CD68 and CD11b staining.
For dendritic cells: MHC II and CD11b.
For B cells: B220 (CD45R) staining.
For T cells: CD3 staining.
NOTE: This method is compatible with the use of genetic models to label immune cells. - For the analysis of the lymphatic collector vessel by intravital 2-photon microscopy, inject the lectin subcutaneously in the lower footpad and visualizing the popliteal lymphatic collecting vessel.
Resultados
To perform topological analysis of brachial adipose tissue blood and lymphatic vessel networks, we subcutaneously injected Alexa Fluor 649-conjugated lectin, and intravenously injected Alexa Fluor 488-conjugated lectin. The brachial adipose tissue was carefully excised, fixed, submitted to clearing protocol, and analyzed by whole-mount staining. A schematic representation of the procedure is included in Figure 1A. Blood vessels are labeled in green and lymphatics are in red. The brachial adipose tissue contains a single lymphatic collector vessel that enters the brachial lymph node. We previously published the results obtained via this method16. Furthermore, we obtained similar data using genetic lymphatic labeling (Prox1creERT2 x TdTomatofl/fl mice)16 (Figure 1B). The blood vasculature was efficiently labelled (in blue) following i.v. lectin injection. Of interest, subcutaneous lectin injection labelled the lymphatic collector vessel (in red) (Figure 1B). To ensure that lymphatic and blood vessels were labelled specifically, we took advantage of Prox1creERT2 x TdTomatofl/fl mice. Subcutaneous lectin administration specifically labelled only the lymphatic vessels, as illustrated by the co-localization with the Prox1-reporter (Figure 1B). We also detected a second lymphatic collector vessel that stained positive for Prox1 but not for lectin injected subcutaneously (Figure 1B). This might be the efferent collector vessel leaving the brachial lymph node. The absence of lectin staining could be explained by: 1) a kinetic issue, assuming that 15 minutes are not sufficient for the lectin to exit from the draining lymph node; 2) insufficient amount of lectin injected, providing only a labelling in the first afferent collector and the brachial lymph node. Those points require further investigation and might be used to optimize the current protocol described in this manuscript. MHC II staining (in green) revealed the presence of many antigen-presenting cells, likely dendritic cells, in the subcutaneous brachial adipose tissue (Figure 1C). The same observation was made in Prox1-reporter mice crossed to CD11cYFP-reporter mice (Figure 1D).
To further confirm that the lectin labeling method specifically stains lymphatic vessels, we performed podoplanin staining in the brachial adipose tissue extracted from CD11c-YFP reporter mice. Podoplanin staining efficiently labels lymphatic endothelial cells. Again, we obtained similar results to our lectin-injection method (Figure 1E). Thus, we believe that the protocol is well-suited for the analysis of lymphatic and blood vessels structure in healthy and inflamed adipose tissue. This procedure is highly and easily reproducible.
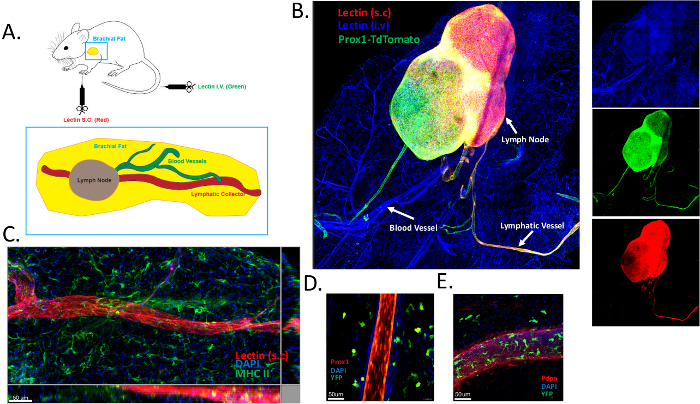
Figure 1: Labeling of blood and lymphatic vasculatures in the subcutaneous adipose tissue. (A) Schematic representation of brachial subcutaneous adipose tissue containing the brachial lymph node. DyLight 649–conjugated lectin (red) was injected subcutaneously (s.c) to visualize the draining lymphatic vasculature, and DyLight 488–conjugated lectin was administered intravenously (i.v) in order to map the blood vasculature. (B) Representative image illustrating blood (in blue) and lymphatic (red and green) labelling using the protocol described in panel A. Prox1-TdTomato (in green) mice were used in this experiment to ensure that lectins separately bound to blood and lymphatic endothelial cells. (C) Identification of the lymphatic collecting vessel in brachial subcutaneous adipose tissue using lectin administration. Antigen-presenting cells were labeled using MHC-II staining (green). (D,E) Identification of lymphatic vessels in the brachial subcutaneous adipose tissue of CD11cYFP reporter mice crossed to Prox1cre x TdTomatofl/fl mice (D) or stained for podoplanin (Pdpn) (E). Please click here to view a larger version of this figure.
Discusión
This approach provides efficient and robust labeling of the blood and lymphatic vasculatures of the subcutaneous adipose tissue. The separate analysis of blood and lymphatic endothelial networks might unravel pathological mechanisms affecting one or both of the circulatory systems during obesity or other pathological conditions. This protocol aims to analyze the architecture of the vascular systems, their interaction with stromal and immune cells, and their functionality during health and disease.
The method is facilitated by the presence of the brachial skin-draining lymph node, which is embedded in subcutaneous adipose tissue. As shown in Figure 1, the method provides similar results to genetic reporter labeling or antibody staining, while also being exempt of the caveats we discussed earlier. It not only provides a practical way of separately labeling blood and lymphatic vasculatures, but also provides multiple readouts on the functionality of the latter and on the overall health of the tissue. Notably, the appearance of the dye in the interstitial tissue space could indicate an increased vascular permeability. Moreover, the presence of the dye in the draining lymph node may be analyzed in a time-course fashion as a relative measure of the lymph flow rate.
Another parameter to take into account is the association of leukocytes with blood and lymphatic vessels. Immune cells, and particularly macrophages, dendritic cells and B cells, are known to play a key role in obesity21. Notably, we previously reported on the role played by dendritic cells in the control of lymphatic permeability16. We therefore highly suggest coupling labeling of blood and lymphatic vasculatures with labeling of subcutaneous adipose tissue immune cells to determine their localization relative to both vasculatures. Cell-type specific labeling can be achieved by traditional antibody staining or by the use of reporter mice (e.g., CD11cYFP, CX3CR1GFP, Zbtb46GFP, LysMCre x tdTomatofl/fl) in which case the fluorochromes used for lectin labeling must be adapted. Changes in adipocyte number or morphology can be appreciated using perilipin staining, which also provides a great way of mapping the tissue structure.
Lastly, although the methodology is described here to fit a 3D confocal imaging approach, we have previously adapted this protocol for intravital imaging16. This alternative approach has the benefit of providing additional information on immune cell motility within and around lymphatic collecting vessels, as well as vessel contractility. We therefore suggest lectin labeling of blood and lymphatic vasculatures as a versatile approach to study adipose tissue biology in health and disease.
Divulgaciones
The authors have no disclosure and conflict of interest to declare.
Agradecimientos
SI is supported by Institut National de la Sante et de la Recherche Medicale (INSERM) and Agence Nationale de la Recherche (ANR-17-CE14-0017-01 and ANR-19-ECVD-0005-01). AG is supported by the French government, through the UCAJedi Investments in the Future projects managed by the National Research Agency (ANR) with the reference number ANR-15-IDEX-01. RSC is supported by FA-2020-01-IBD-1 from the Lawrence C. Pakula, MD IBD Education & Innovation Fund”.
Materiales
| Name | Company | Catalog Number | Comments |
| Lectin DyLight 649 | Vector Labs | DL-1178-1 | Described in protocol |
| Lectin DyLight 488 | Vector Labs | DL-1174 | Described in protocol |
| Paraformaldehyde | VWR Chemicals | 9713.1000 | |
| Sucrose | Euromedex | CAS Number 57-50-1 | |
| Anti-Podoplanin | AngioBio | 11-033 | Dilution : 1/50 |
| Lectin DyLight 594 | Vector Labs | DL-1177 | Described in protocol |
| Anti-MHCII (Clone M5/114.15.2) | Biolegend | 107618 | Dilution : 1/100 |
| Anti-CD11b (Clone M1/70) | Biolegend | 101218 | Dilution : 1/100 |
| Anti-CD68 (Clone FA.11) | Biolegend | 137004 | Dilution : 1/100 |
| Anti-B220 (Clone RA3-6B2) | Biolegend | 103225 | Dilution : 1/100 |
| Anti-Perilipin (Clone PERI 112.17) | Progen | 651156 | Dilution : 1/50 |
| Anti-CD3 (Clone 17A2) | Biolegend | 100210 | Dilution : 1/100 |
Referencias
- Baluk, P., et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. Journal of Experimental Medicine. 204 (10), 2349-2362 (2007).
- Fonseca, D. M., et al. Microbiota-Dependent Sequelae of Acute Infection Compromise Tissue-Specific Immunity. Cell. 163 (2), 354-366 (2015).
- Thomas, S. N., et al. Impaired humoral immunity and tolerance in K14-VEGFR-3-Ig mice that lack dermal lymphatic drainage. The Journal of Immunology. 189 (5), 2181-2190 (2012).
- Kuan, E. L., et al. Collecting lymphatic vessel permeability facilitates adipose tissue inflammation and distribution of antigen to lymph node-homing adipose tissue dendritic cells. The Journal of Immunology. 194 (11), 5200-5210 (2015).
- Randolph, G. J., Ivanov, S., Zinselmeyer, B. H., Scallan, J. P. The Lymphatic System: Integral Roles in Immunity. Annual Review of Immunology. 35, 31-52 (2017).
- Harvey, N. L., et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nature Genetics. 37 (10), 1072-1081 (2005).
- Kajimura, S., Spiegelman, B. M., Seale, P. Brown and Beige Fat: Physiological Roles beyond Heat Generation. Cell Metabolism. 22 (4), 546-559 (2015).
- Wu, J., Cohen, P., Spiegelman, B. M. Adaptive thermogenesis in adipocytes: is beige the new brown. Genes and Development. 27 (3), 234-250 (2013).
- Cinti, S. The adipose organ. Prostaglandins, Leukotrienes & Essential Fatty Acids. 73 (1), 9-15 (2005).
- Kataru, R. P., et al. Regulation of Lymphatic Function in Obesity. Frontiers in Physiology. 11, 459 (2020).
- Escobedo, N., Oliver, G. The Lymphatic Vasculature: Its Role in Adipose Metabolism and Obesity. Cell Metabolism. 26 (4), 598-609 (2017).
- Weitman, E. S., et al. Obesity impairs lymphatic fluid transport and dendritic cell migration to lymph nodes. PLoS One. 8 (8), 70703 (2013).
- Escobedo, N., et al. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight. 1 (2), (2016).
- Commerford, C. D., et al. Mechanisms of Tumor-Induced Lymphovascular Niche Formation in Draining Lymph Nodes. Cell Reports. 25 (13), 3554-3563 (2018).
- Gregory, J. L., et al. Infection Programs Sustained Lymphoid Stromal Cell Responses and Shapes Lymph Node Remodeling upon Secondary Challenge. Cell Reports. 18 (2), 406-418 (2017).
- Ivanov, S., et al. CCR7 and IRF4-dependent dendritic cells regulate lymphatic collecting vessel permeability. Journal of Clinical Investigation. 126 (4), 1581-1591 (2016).
- Zhong, W., et al. Prox1-GFP/Flt1-DsRed transgenic mice: an animal model for simultaneous live imaging of angiogenesis and lymphangiogenesis. Angiogenesis. 20 (4), 581-598 (2017).
- Choi, I., et al. Visualization of lymphatic vessels by Prox1-promoter directed GFP reporter in a bacterial artificial chromosome-based transgenic mouse. Blood. 117 (1), 362-365 (2011).
- Pham, T. H., et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. Journal of Experimental Medicine. 207 (1), 17-27 (2010).
- Gilleron, J., et al. Exploring Adipose Tissue Structure by Methylsalicylate Clearing and 3D Imaging. Journal of Visualized Experiments. (162), e61640 (2020).
- Ivanov, S., Merlin, J., Lee, M. K. S., Murphy, A. J., Guinamard, R. R. Biology and function of adipose tissue macrophages, dendritic cells and B cells. Atherosclerosis. 271, 102-110 (2018).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoThis article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados