Method Article
Laser Capture Microdissection on Surgical Tissues to Identify Aberrant Gene Expression in Impaired Wound Healing in Type 2 Diabetes
* These authors contributed equally
In This Article
Summary
This technique provides a guide to inflicting ex vivo wounds, performing laser capture microdissection and quantifying changes in gene expression related to poor wound healing processes in diabetes using clinically relevant human tissue.
Abstract
The global prevalence Type 2 diabetes mellitus (T2DM) is escalating at a rapid rate. Patients with T2DM suffer from a multitude of complications and one of these is impaired wound healing. This can lead to the development of non-healing sores or foot ulcers and ultimately to amputation. In healthy individuals, wound healing follows a controlled and overlapping sequence of events encompassing inflammation, proliferation, and remodelling. In T2DM, one or more of these steps becomes dysfunctional. Current models to study impaired wound healing in T2DM include in vitro scratch wound assays, skin equivalents, or animal models to examine molecular mechanisms underpinning wound healing and/or potential therapeutic options. However, these do not fully recapitulate the complex wound healing process in T2DM patients, and ex vivo human skin tests are problematic due to the ethics of taking punch biopsies from patients where it is known they will heal poorly. Here, a technique is described whereby expression profiles of the specific cells involved in the (dys)functional wound healing response in T2DM patients can be examined using surplus tissue discarded following amputation or elective cosmetic surgery. In this protocol samples of donated skin are collected, wounded, cultured ex vivo in the air liquid interface, fixed at different time points and sectioned. Specific cell types involved in wound healing (e.g., epidermal keratinocytes, dermal fibroblasts (papillary and reticular), the vasculature) are isolated using laser capture microdissection and differences in gene expression analyzed by sequencing or microarray, with genes of interest further validated by qPCR. This protocol can be used to identify inherent differences in gene expression between both poorly healing and intact skin, in patients with or without diabetes, using tissue ordinarily discarded following surgery. It will yield greater understanding of the molecular mechanisms contributing to T2DM chronic wounds and lower limb loss.
Introduction
The incidence of type 2 diabetes is growing globally, driven by an obesity epidemic and physical inactivity. Poor wound healing is common in these patients and up to 25% of patients will develop a chronic non-healing wound1. The mechanisms underpinning this are complex and incompletely understood, limiting the discovery rate for new therapeutics. One of the contributing factors to this is the lack of a suitable model for studying wound healing in type 2 diabetes patients. Thus, the purpose of this method is to provide a physiologically relevant ex vivo model for examining wound healing in those at risk of chronic wounds, allowing for transcriptomic analysis to progress the identification of new therapeutic targets.
There are multiple models currently available to study wound healing, and each have their strengths and weaknesses. In vivo animal models, such as the db/db mouse2 or streptozotocin-induced diabetic rats3 are widely used; however, wounds in rodent models heal via contraction, which is very different to the mechanism employed in the human body, and have limited success in translation to clinical trials4,5. The benefits of using human tissue are well recognized4, but are complicated by the ethics of inflicting experimental wounds on individuals who are already known to sustain an impaired wound healing response. Consequently, studies on human subjects with diabetes is more commonly directed towards the inflammatory response rather than experimenting on excised tissue6. Organ-on-a-chip7 and artificial skin models8 are also available. These have the benefit of being able to analyze human cell contributions but give little indication of inter-patient variability. Thus, clinically relevant models to study the progress of wound healing in vulnerable patient populations could accelerate mechanistic understanding and drug discovery in this area.
The ex vivo wounding protocol described below is adapted from Stojadinovic and Tomic-Canic, 20139. It is suitable for examining transcriptional data from controlled wounding of human tissue samples ex vivo and can be applied to clinical samples from patients with poor wound healing (e.g., type 2 diabetes, elderly individuals), in order to advance knowledge on how wound healing is impacted in these conditions and potentially how it can be restored.
Protocol
This protocol relies on the provision of human surgical tissue. Ethical approval and informed patient consent were obtained prior to experimentation, and the study conformed with the principles outlined in the Declaration of Helsinki.
1. Collection of tissue and ex vivo wounding
- Collect surgical tissue following limb amputation/surgery into a sterile container containing Dulbecco's Modified Eagle Medium (DMEM) with 5% penicillin-streptomycin-fungizone, 2 mM L-glutamine and 10% fetal bovine serum (FBS; complete growth medium).
NOTE: Tissue collected in this manner can be stored at 4 °C for up to 24 h prior to experimentation. To ensure viability of tissue for ex vivo experiments, it is recommended to process the tissue as soon as possible or to maintain a fixed time gap between collection and wounding to standardize experiments across multiple tissue donations. If the tissue is to be stored for any amount of time, an RNA stabilization solution should be added to the sample to maintain RNA integrity. - Using sterile forceps, transfer the tissue to a 60 mm Petri dish filled with sterile phosphate buffered saline (PBS) supplemented with 5% penicillin-streptomycin-fungizone. Trim the fat off the dermis using a sterile scalpel and/or surgical scissors and discard. Transfer the tissue to a fresh petri dish filled with sterile PBS.
- Attach two sterile blades together such that there is a 1 mm gap between the two blades. Score two parallel linear lines, ~1 mm apart, across the length of the epidermis and the upper part of the papillary dermis10,11.
- Remove the epidermis between the score lines using sterile forceps and surgical scissors to create a linear wound.
- Use a scalpel to cut the tissue into small rectangles (maximum size of 1 cm2) encompassing the wound with intact epidermis on either side.
NOTE: To maintain tissue and subsequent RNA integrity, steps 1.2-1.5 should be conducted as quickly as possible. - Take an image of the wounded tissue using a microdissection microscope at 4x magnification ensuring the field of view captures the entirety of the wound and the surrounding intact epidermal tissue.
- Using sterile forceps, place the tissue rectangles on a tissue culture insert in a 6-well plate and gently pipette 2 mL of complete growth medium into the well. This will ensure that the sample is cultured at the liquid-air interface.
- Incubate at 37 °C in 5% CO2 in air for up to 120 h.
NOTE: The tissue should be imaged on a microdissection microscope at the same magnification as in step 1.6 at the end of the incubation. Images can also be taken every 24 h if necessary.
2. Tissue fixation and cryosectioning
- Snap freeze the tissue in liquid nitrogen. Place a small amount of cryostat-compatible cutting medium onto a cryostat chuck and embed the tissue within it. Orient the tissue perpendicular to the chuck so that the cutting face will cut through all the layers of the skin including the wounded area. Store at -80 °C.
NOTE: The protocol can be paused here. - Clean the cryostat at room temperature with 70% ethanol and RNase decontamination spray. Set the cryostat temperature to -28 °C.
- Check that the orientation of chuck and cryostat blade can produce sections that encompass the full thickness of the tissue including the wounded tissue and underlying dermis.
- Using the cryostat, cut up to ten 7 µm sections from each wound and place on to RNA-free microdissection slides.
- Store the slides in the original box that the microdissections slides were provided in at -80 °C to ensure an RNase-free environment.
NOTE: The protocol can be paused here but be aware RNA degradation will increase the longer the storage time is.
3. Laser capture microdissection
- Place the microdissection slides with the tissue section in dry ice and go to the laser capture microdissection microscope room.
- Air-dry the 1st slide and quickly proceed to stain the sections with hematoxylin using an RNase-free hematoxylin and eosin staining kit immediately prior to performing laser capture microdissection.
- Visualize the wounded tissue using a laser capture microdissection microscope at 10x magnification and take a picture of the area of interest that will be laser captured.
- Trace around that area (for example, epithelial tongue, granulation tissue, microvessels) and collect into microdissection 0.5 mL diffuser isolation caps using the software instructions for the particular microscope that is being used.
- Re-visualize and image the laser captured area of interest using the laser capture microdissection microscope at 10x magnification to demonstrate the location and complete excision of the dissected tissue.
NOTE: Perform laser capture for each sample (up to 10 sections) for a maximum time of 1 h to minimize RNA degradation. - Add RNA storage buffer to the tube containing the microdissected tissue according to manufacturer's instructions, and store in dry ice until it can be transferred to a -80°C freezer.
NOTE: The protocol can be temporarily paused here.
4. Quantification of differential gene expression
- Centrifuge the sample tubes at full speed for 1 min.
- Isolate RNA from the microdissected tissue following the manufacturer's instructions.
- Elute the RNA in a final volume of 12 µL of RNase free water and amplify the RNA using an RNA amplification kit and thermal cycler, according to manufacturer's instructions. Two rounds of amplification are recommended to ensure sufficient RNA for further analysis. Use the amplification conditions in Table 1.
| First amplification round | Second amplication round | ||||
| First strand synthesis | First strand synthesis | ||||
| Step | Temperature | Time | Step | Temperature | Time |
| 1 | 65 °C | 5 min | 1 | 65 °C | 5 min |
| 2 | 4 °C | hold | 2 | 4 °C | hold |
| 3 | 42 °C | 45 min | 3 | 25 °C | 10 min |
| 4 | 4 °C | hold | 4 | 37 °C | 45 min |
| 5 | 37 °C | 20 min | 5 | 4 °C | hold |
| 6 | 95 °C | 5 min | |||
| 7 | 4 °C | hold | Second strand synthesis | ||
| Step | Temperature | Time | |||
| Second strand synthesis | 1 | 95 °C | 2 min | ||
| Step | Temperature | Time | 2 | 4 °C | hold |
| 1 | 95 °C | 2 min | 3 | 37 °C | 15 |
| 2 | 4 °C | hold | 4 | 70 °C | 5 min |
| 3 | 25 °C | 5 min | 5 | 4 °C | hold |
| 4 | 37 °C | 10 min | |||
| 5 | 70 °C | 5 min | In vitro transcription | ||
| 6 | 4 °C | hold | Step | Temperature | Time |
| 1 | 42 °C | 6 h | |||
| In vitro transcription | 2 | 4 °C | hold | ||
| Step | Temperature | Time | 3 | 37 °C | 15 min |
| 1 | 42 °C | 3 h | 4 | 4 °C | hold |
| 2 | 4 °C | hold | |||
| 3 | 37 °C | 15 min | |||
| 4 | 4 °C | hold | |||
Table 1: Amplification conditions.
- Quantify the concentration and purity of the amplified RNA. Absorbance ratios of A260/230 (purity) and A260/280 (contaminants) > 1.8 are suitable for further analysis.
NOTE: Purified amplified RNA can be used for focused gene expression studies (steps 4.5-4.6) or can be sent away for microarray analysis. - Synthesize cDNA using high-capacity cDNA reverse transcription kit according to manufacturer's instructions. Representative conditions are as follows:
- 25 °C for 10 min
- 37 °C for 2 h
- 85 °C for 5 min
- 4 °C hold
- Perform quantitative PCR using 0.5 µL of cDNA and 1 µM primers (of the gene of interest) according to manufacturer's instructions. Representative conditions and primer sequences are as follows.
- Use the following conditions for quantitative PCR conditions: 95 °C for 10 minutes; 40 cycles of 95°C for 15 s and 62°C for 1 min; 95 °C for 5 min; and 4°C hold.
- Use the following primer sequences:
GAPDH
Forward 5'-TATAAATTGAGCCCGCAGCC-3'
Reverse 5'-CGACCAAATCCGTTGACTCC-3'
KRT17
Forward 5'-AGGGAGAGGATGCCCACCTG-3'
Reverse 5'-GCGGGAGGAGATGACCTTGC-3'
5. Data interpretation
- Calculate the rate of wound healing using the images of the ex vivo tissue generated in steps 1.6-1.8 using ImageJ. There are multiple recent publications highlighting different variations on how to automatically analyze wound areas in ImageJ12,13,14.
- Quantify gene expression by using the ΔCT method, comparing the threshold cycles for the gene of interest compared to that of the housekeeper (e.g., glyceraldehyde-3-phosphate; GAPDH). To do this, note the CT value for the housekeeper and for the gene of interest.
- Calculate the difference between the two CT values to normalize the amount of mRNA present and control for differences in RNA extraction concentrations between different isolations:
ΔCT = CT (gene of interest) - CT (housekeeper) - Calculate the magnitude of difference between the CT values to give the relative quantification of the gene of interest, expressed as a percentage of the housekeeper:
Relative quantification = (2-ΔCT) x 100 - Examine differences between different patient donors/disease states by comparing the relative quantifications of the genes of interest for each sample.
NOTE: A schematic of the entire technique can be found in Figure 1.
- Calculate the difference between the two CT values to normalize the amount of mRNA present and control for differences in RNA extraction concentrations between different isolations:
Results
Following the protocol, a 48 h timepoint was chosen to generate representative results. The creation of the initial wound in surplus tissue from elective cosmetic surgery can be seen in Figure 2A where the excised wound is clearly visible. Haematoxylin and eosin staining confirms that this has generated a full thickness wound (Figure 2B). After 48 h, partial closure of the wound is visible under the light microscope (Figure 2C). Histological staining reveals the epithelial tongue that is progressing to heal the wound (Figure 2D), demonstrating that the ex vivo wound healing model is a valid proxy for in vivo wound healing.
After sectioning and staining with haematoxylin and eosin, the healing wound was visualized on the laser capture microdissection system and the wound area selected (Figure 3A). This area was completely excised using this method as can be seen in Figure 3B. RNA quality and purity was reasonable (analyzed by RNA integrity number (RIN); Figure 3C) - poor quality amplified RNA would have many peaks and troughs indicating multiple degradation products. Isolation of small tissue sections may yield RNA that is of a very low concentration that makes valid interpretation of qPCR difficult. Figure 3D demonstrates the variation in RNA concentration to be expected using this technique, with a range of 2.00 to 6.15 ng/mL. Importantly, even dilute samples were able to give robust CT values for both housekeeper (GAPDH) and skin-specific genes of interest (keratin 17; KRT17; Figure 3E), confirming the suitability of the technique for comparative transcriptomic studies.
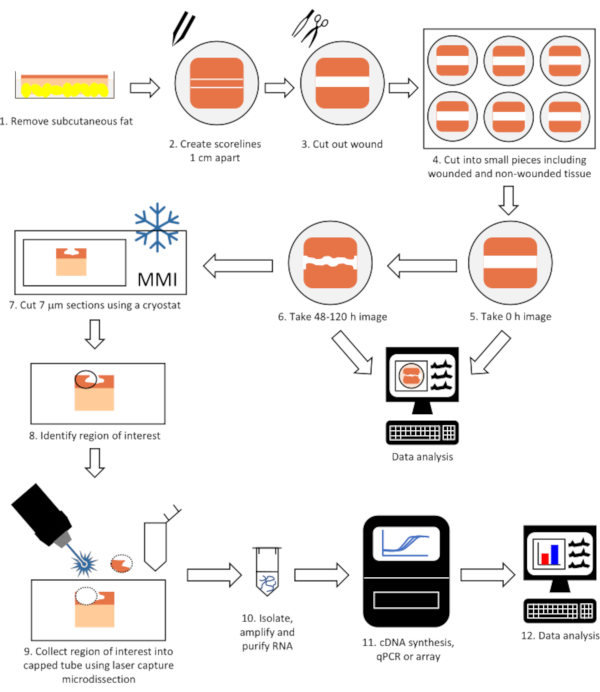
Figure 1: Flow diagram of the complete technique to perform gene expression analysis on laser microdissected tissue from wounded skin. Tissue is wounded, allowed to heal in a tissue incubator and imaged (steps 1-6) before being cut into 7 μm sections using a cryostat (step 7). The region of interest (e.g. epithelial tongue) is identified and collected using laser capture microdissection (steps 8-9) and RNA isolated, purified and gene expression determined (steps 10-12). Please click here to view a larger version of this figure.
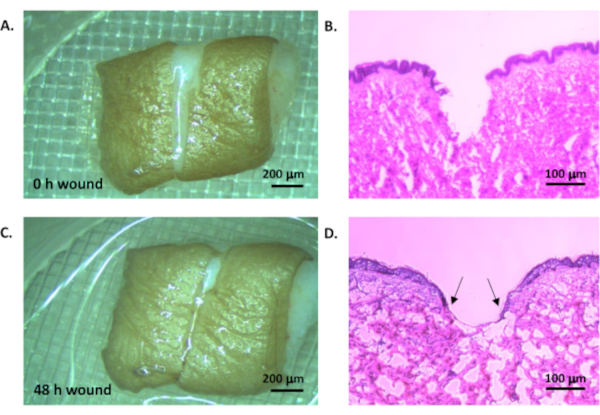
Figure 2: Ex vivo wound model. Human tissue was wounded by creating two parallel cuts and removing the tissue between to leave a uniform wounded area (A, light microscope, scale bar = 200 µm; B, haemoatoxylin and eosin staining, scale bar = 100 µm). The tissue was cultured in a standard tissue culture incubator at 37°C in 5% CO2 in air for 48 h (C, light microscope, scale bar = 200 µm; D, haematoxylin and eosin staining, scale bar = 100 µm). Arrow heads indicate epithelial tongue. Please click here to view a larger version of this figure.

Figure 3: Laser capture microdissection and gene expression. A. The region of interest (in this case, healed tissue) was identified using haematoxylin staining and collected using laser capture microdissection. B. The same region imaged after microdissection. Scale bars = 50 µm. C. Representative electropherogram of RNA that was isolated, amplified and quantified from the laser microdissected tissue. D. RNA concentration from collected tissue (n=24 samples). E. Reproducible detection of GAPDH and KRT17 expression using qPCR (n=13 samples, mean ± SEM). Please click here to view a larger version of this figure.
Discussion
As the incidence of chronic disorders such as type 2 diabetes increases globally, the need for techniques that can facilitate pathophysiologically relevant studies becomes more urgent. The protocol described above provides a standardized method for examining transcriptomic data from ex vivo healing wounds utilizing human tissue.
This protocol is dependent on the provision of surplus clinical tissue for which ethical permission has been granted from the relevant authority, and from patients who have given informed consent. Commonly this will be from patients undergoing amputation or elective cosmetic surgeries. The clinical demographics of donors needs to be carefully considered, however one of the strengths of this method is the opportunity to perform studies on tissues from multiple patient donors from healthy control individuals (for example, those who are undergoing amputation following an accident or who are having elective cosmetic procedures) and those with chronic diseases such as type 2 diabetes. Furthermore, as the method can be paused at numerous points (see protocol), ex vivo wounds can be prepped as and when surgeries take place and then stored for side-by-side RNA analysis once enough tissue samples have been secured.
The most critical step in this protocol is laser capture microdissection (protocol section 3). Whilst there are many publications that utilize this technique for transcriptomic analysis, the current report is the first to provide a thorough experimental protocol to apply this to studies on human tissue from patients with an impaired wound healing response. It is essential that laser capture is performed as quickly as possible and for one hour as an absolute maximum. This is to minimize RNA degradation as the frozen sections return to room temperature and can be monitored during the amplified RNA quality check in protocol section 4.
While this technique adds value in allowing consideration of interpatient variability in human wound healing mechanisms, it does have some limitations. The impact of inflammatory cells cannot be assessed as the ex vivo model has no functioning circulation. If this is the focus of a study, then in vivo models (both animal2,3 and human6) would be of more benefit. Furthermore, the provision of clinically relevant tissue can be a limiting factor. In these instances, artificial skin8 or skin-on-a-chip7 models may be more accessible. Regardless, one of the major strengths of the protocol is that it can study transcriptional level events from wounded human tissue, from multiple donors and from different patient groups. Ordinarily, studies of this kind would be conducted using in vitro models which, whilst providing valuable insight, do not replicate the complexities of wounded tissue15. Altogether, this technique is a useful adjunct to the currently existing suite of in vitro, in vivo and ex vivo models for wound healing in diabetes.
In summary, this workflow can be used to study wound healing mechanisms not only in type 2 diabetes but also other clinically vulnerable groups, for example the elderly or those with connective tissue disorders. The continued development of proteomic and metabolomic assays with enhanced sensitivity16,17,18 will expand the applications of this technique even further.
Disclosures
The authors have nothing to disclose.
Acknowledgements
ICP was supported by the European Commission 7th Framework Programme for Research and Technical Development - Marie Curie Innovative Training Networks (ITN), Grant agreement no.: 607886. RW was supported by Aveda, Hair Innovation & Technology, USA. RB, SS were supported by the Centre of Skin Sciences, University of Bradford.
Materials
| Name | Company | Catalog Number | Comments |
| Arcturus RiboAmp PLUS kit | ThermoFisher Scientific | KIT0521 | RNA amplification kit |
| Diffuser Caps 0.5mL | MMI | K10028161 | Laser capture microdissection caps; 50 pack |
| Dulbecco’s Modified Eagle Medium (DMEM) | Sigma-Aldrich | D6046 | With 1000 mg/L glucose, L-glutamine, and sodium bicarbonate, liquid, sterile-filtered, suitable for cell culture |
| Foetal Bovine Serum | Thermo Fisher Scientific | 10270106 | Cell culture supplement |
| H&E Staining Kit Plus | MMI | K10028305 | Rnase-free haematoxylin and eosin staining kit |
| High capacity cDNA reverse transcription kit | Applied Biosystems | 4368814 | Reverse transcription kit |
| L-glutamine | Thermo Fisher Scientific | 25030149 | Cell culture supplement |
| MembraneSlides | MMI | K10028153 | Laer capture microdissection slides; 5 per box |
| Netwell Mesh Insert | Corning | 3479 | Cell culture insert |
| Penicillin-Streptomycin-Fungizone | Thermo Fisher Scientific | 15070-063 | Cell culture supplement |
| 15290-026 | |||
| OCT | Tissue-Tek Sakura | 4583 | Cryostat-compatible cutting medium |
| PBS | Thermo Fisher Scientific | 10209252 | Five tablets per 100ml sterile water and then autoclaved for cell culture use |
| RNeasy Micro Kit | Qiagen | 74004 | RNA extraction kit |
| RNase Away | Sigma-Aldrich | 83931 | RNase spray |
| Sterile blades | Scientific Laboratory Supplies | INS4974 | Tissue dissection implements |
| Support Slide | MMI | K10028159 | Laser capture microdissection support slide, RNase-free |
| Surgical scissors | Scientific Laboratory Supplies | INS4860 | Tissue dissection implements |
| Surgical forceps | Scientific Laboratory Supplies | INS2026 | Tissue dissection implements |
| SYBR Green Supermix | Applied Biosystems | 4344463 | Quantitative PCR mastermix |
References
- Gianino, E., Miller, C., Gilmore, J. Smart Wound Dressings for Diabetic Chronic Wounds. Bioengineering. 5 (3), (2018).
- Song, M., et al. Cryptotanshinone enhances wound healing in type 2 diabetes with modulatory effects on inflammation, angiogenesis and extracellular matrix remodelling. Pharmaceutical Biology. 58 (1), 845-853 (2020).
- Liu, J., et al. Involvement of miRNA203 in the proliferation of epidermal stem cells during the process of DM chronic wound healing through Wnt signal pathways. Stem Cell Research and Therapy. 11 (1), 348 (2020).
- Pastar, I., Wong, L. L., Egger, A. N., Tomic-Canic, M. Descriptive vs mechanistic scientific approach to study wound healing and its inhibition: Is there a value of translational research involving human subjects. Experimental Dermatology. 27 (5), 551-562 (2018).
- Zomer, H. D., Trentin, A. G. Skin wound healing in humans and mice: Challenges in translational research. Journal of Dermatological Science. 90 (1), 3-12 (2018).
- Mirza, R. E., Fang, M. M., Weinheimer-Haus, E. M., Ennis, W. J., Koh, T. J. Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes. 63 (3), 1103-1114 (2014).
- Ataç, B., et al. Skin and hair on-a-chip: in vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip. 13 (18), 3555-3561 (2013).
- Yun, Y., Jung, Y. J., Choi, Y. J., Choi, J. S., Cho, Y. W. Artificial skin models for animal-free testing. Journal of Pharmaceutical Investigation. 48, 215-223 (2018).
- Stojadinovic, O., Tomic-Canic, M. Human ex vivo wound healing model. Methods in Molecular Biology. 1037, 255-264 (2013).
- Castellano-Pellicena, I., et al. Does blue light restore human epidermal barrier function via activation of Opsin during cutaneous wound healing. Lasers in Surgery and Medicine. 51 (4), 370-382 (2019).
- Rizzo, A. E., Beckett, L. A., Baier, B. S., Isseroff, R. R. The linear excisional wound: an improved model for human ex vivo wound epithelialization studies. Skin Research and Technology. 18 (1), 125-132 (2012).
- Castellano-Pellicena, I., Thornton, M. J. Isolation of epidermal keratinocytes from human skin: The scratch-wound assay for assessment of epidermal keratinocyte migration. Methods in Molecular Biology. 2154, 1-12 (2020).
- Suarez-Arnedo, A., et al. An imaje J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE. 15 (7), 0232565 (2020).
- Venter, C., Niesler, C. U. Rapid quantification of cellular proliferation and migration using ImageJ. Biotechniques. 66 (2), (2019).
- Al-Rikabi, A. H. A., Riches-Suman, K., Tobin, D. J., Thornton, M. J. A proinflammatory environment induces changes in the diabetic phenotype of human dermal fibroblasts derived from diabetic and nondiabetic donors: Implications for wound healing. Scientific Reports. , (2020).
- Chen, A., et al. Towards single-cell ionomics: a novel micro-scaled method for multi-element analysis of nanogram-sized biological samples. Plant Methods. 16, 31 (2020).
- Lutz, B. M., Peng, J. Deep profiling of the aggregated proteome in Alzheimer's Disease: from pathology to disease mechanisms. Proteomes. 6 (4), 46 (2018).
- Rinschen, M. M. Single glomerular proteomics: A novel tool for translational glomerular cell biology. Methods in Cell Biology. 154, 1-14 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved