Method Article
Evaluating the Mechanism of a Traditional Herbal Syrup for Anemia Treatment Using Liver Metabolomics
In diesem Artikel
Zusammenfassung
This study primarily introduces the application of liver metabolomics in investigating the effectiveness of Shi-Liu-Bu-Xue Syrup in treating anemia.
Zusammenfassung
As a well-known Uyghur medicine, Shi-Liu-Bu-Xue Syrup (SLBXS) has been widely used to treat anemia in China for over 20 years. However, the underlying mechanisms of its effectiveness in treating anemia remain unclear. In this study, liver metabolomics was primarily employed to determine the potential regulatory mechanisms of SLBXS in treating anemia. Liver metabolomics profiling was conducted to characterize the mechanism of action of SLBXS in an acetylphenylhydrazine-induced mouse model of anemia. SLBXS was shown to decrease liver index, white blood cell count, and platelet count, while increasing red blood cell count, hemoglobin, and hematocrit levels. Core targets were selected for verification using Western blotting. SLBXS demonstrated a significant therapeutic effect on anemia primarily by regulating galactose metabolism and the HIF-1 signaling pathway, as indicated by the downregulation of HIF-1α, NOS3, VEGFA, and GLA proteins in the liver tissues of anemic mice. This study clarifies the potential regulatory mechanisms of hepatic metabolism by SLBXS administration in treating anemia.
Einleitung
Anemia is a pressing and prevalent global health issue, affecting 25% of the world's population and people of all ages, particularly adolescents and pregnant women1,2,3. It is associated with an increased risk of preterm labor and maternal mortality and can lead to physical developmental disorders and impaired cardiovascular performance4. This condition may also negatively impact the health status of adolescents, resulting in infections and heart failure5. Current treatments primarily include blood transfusion, iron supplementation, and erythropoietin therapy. However, these treatments have disadvantages and adverse side effects, such as anaphylaxis, gastrointestinal upset, iron overload, and hives1. Therefore, identifying effective drugs with fewer side effects for treating anemia is crucial.
Traditional Chinese medicine, including Uyghur medicine, offers several advantages, such as multi-ingredient formulations, multi-target effects, multi-link interactions, and fewer side effects in preventing and treating multifactorial diseases. Shi-Liu-Bu-Xue Syrup (SLBXS) is a notable traditional agent in Uyghur medicine used for blood tonics and blood production. It is recognized as a blood-regulating drug that can reduce liver heat and has been included in the guidelines for the clinical use of minority medicines for treating anemia. It is also licensed by the Chinese State Food and Drug Administration (Z20026094)6,7,8. Over the past two decades, SLBXS has been extensively used in China to treat anemia-related conditions. However, its potential mechanisms for treating anemia remain unknown and require further investigation. Metabolomics, which examines the dynamic metabolic responses of biological systems to disease, drug interventions, or environmental conditions9, is increasingly used to elucidate the mechanisms of action of traditional Chinese medicine by evaluating changes in metabolic biomarkers in biological samples following external stimuli9,10.
Accordingly, a liver metabolomics approach was adopted in this study to determine the underlying therapeutic mechanisms of SLBXS in treating anemia. First, an acetylphenylhydrazine (APH)-induced mouse model of anemia was established. Next, the metabolic pathways of endogenous metabolites were investigated using liver metabolomics with gas chromatography-mass spectrometry (GC-MS) and multivariate data methods following SLBXS administration. Finally, key targets were analyzed experimentally to elucidate the anti-anemic effects and molecular mechanisms of SLBXS.
Protokoll
All experimental procedures were approved by the Laboratory Animal Ethics Committee of the Hubei University of Chinese Medicine (HBUCMS201912015). Male C57BL/6 mice (weight 20-22 g) were housed in a specific pathogen-free room with a relative humidity of 50%-60% and a temperature of 22 °C ± 2 °C, subjected to a 12 h light/12 h dark cycle, and provided with free access to food and water. Before the experiment began, all mice were allowed one week to acclimate to the environment. Mice were randomly assigned to one of the following four groups (n = 12): control, model, Fu-Fang-E-Jiao Syrup (FFEJS, a positive drug, administered intragastrically at 7.8 mL/kg), and SLBXS (administered intragastrically at 11.7 mL/kg). Mice in the control and model groups received equal volumes of saline. Mice in all groups were given intragastric administration of the corresponding drugs once daily for 2 weeks. Details of the drugs, reagents, and equipment used in this study are listed in the Table of Materials.
1. Establishment of anemia model in mice
- Weigh 2 g of acetylphenylhydrazine (APH) using an electronic balance and transfer it into a 150 mL beaker. Add 100 mL of saline and stir with a glass rod until the APH is fully dissolved.
- Establish the mouse model of anemia by subcutaneously injecting 2% APH as prepared in step 1.1 on the 1st, 4th, and 7th days at dosages of 200 mg/kg, 100 mg/kg, and 100 mg/kg, respectively11.
NOTE: Starting on the first day, mice in the FFEJS (7.8 mL/kg) and SLBXS (11.7 mL/kg) groups were given intragastric administration once daily for 2 weeks. Mice in the control and model groups received equal volumes of saline once daily for 2 weeks.
2. Determining the liver index
- At the end of the experiment, weigh each mouse using an electronic scale.
- Anesthetize the mice by inhaling 2% isoflurane. Squeeze the eyeball of the mice to make it hyperemic and protruding. Quickly remove the eyeball with forceps and collect the blood in heparinized sample tubes.
- Secure the anesthetized mice from step 2.2 to a surgical manipulation plate.
- Make a complete incision along the midline of the abdomen with a scalpel. Carefully dissect and isolate the intact liver tissue12, then measure its weight with an electronic balance.
NOTE: The liver index of each mouse is calculated using the following formula: Liver index = liver weight/body weight.
3. Hematologic analysis
- Gently shake the tube containing the heparinized blood sample from step 2.2 to prevent blood clotting.
- Place the blood sample below the injection needle to ensure that it is fully immersed in the needle.
- Click the Auto Detect button to measure red blood cell count (RBC), hematocrit (HCT), white blood cell count (WBC), hemoglobin (HGB) levels, and platelet count (PLT) using a fully automatic hemocyte analyzer.
4. Liver metabolomics study
- Liver sample preparation
- Homogenize liver tissue samples (50 mg) from step 2.4 with 1 mL of pre-chilled methanol. Centrifuge at 18,759 x g for 10 min at 4 °C to remove the precipitate.
- Transfer 200 µL of the supernatant to a sample vial using a pipette and vacuum-dry in a freeze-drier at 35 °C for 2 h.
- React the dried samples with 40 µL of a 40 mg/mL solution of methoxyamine hydrochloride in pyridine for 90 min at 30 °C. Then, add 80 µL of N-Methyl-N-(trimethylsilyl)trifluoroacetamide (MSTFA) with 1% Trimethylchlorosilane (TMCS) and incubate for 60 min at 37 °C.
- Add 10 µL of n-hexane to the vial to terminate the derivatization reaction.
- Liver metabolic analysis
- Analyze the derivatized samples (1 µL) using a GC-MS system. Separate the derivatives using a DB-5MS capillary column (30 m × 0.25 mm × 0.25 µm).
NOTE: The oven temperature program conditions were set as follows: 60 °C for 1 min; increase to 325 °C at a rate of 10 °C/min and maintain for 10 min. The injector, ion source, and MS temperatures were set to 250 °C, 230 °C, and 150 °C, respectively. Helium (99.999%) was used as the carrier gas at a flow rate of 1.1 mL/min, and the split ratio was set to 10:1. An electron beam energy of 70 eV and a solvent delay time of 5.9 min were used.
- Analyze the derivatized samples (1 µL) using a GC-MS system. Separate the derivatives using a DB-5MS capillary column (30 m × 0.25 mm × 0.25 µm).
- Data processing and analysis
- Acquire and convert raw GC-MS data using the compatible MassHunter software.
- Conduct spectral analysis using the Automated Mass Spectral Deconvolution and Identification System (AMDIS) tool12.
- Identify all metabolites using the NIST and HMDB databases (see Table of Materials).
- Import the data into the MetaboAnalyst tool for partial least squares-discriminant analysis (PLS-DA), t-tests, pathway analysis, and network analysis12.
5. Western blot analysis
- Extract the total proteins from mouse liver tissue
- Add 50 mg of liver tissue from step 2.4 and 250 µL of cell lysate to a 1 mL glass homogenizer and grind on ice for 5 min.
- Transfer the liver tissue homogenate from step 5.1.1 to a 1.5 mL microcentrifuge tube using a pipettor, and centrifuge at 18,759 x g for 10 min at 4 °C. Then transfer the supernatant to a new 1.5 mL tube using a pipettor.
- Determine protein concentrations and pre-process protein samples
- Add 2 µL of supernatant from step 5.1.2, 18 µL of PBS, and 180 µL of BCA working solution to a 96-well microtiter plate8.
- Oscillate the plate on an oscillator for 30 s, leave it for 30 min at 37 °C, and determine the absorbance at 562 nm using a microplate reader.
- Separate the total proteins using SDS-PAGE, transfer to polyvinylidene fluoride membranes, and block with 5% nonfat milk8.
- Incubate the membranes from step 5.3 with primary antibodies against HIF-1α (1:1000), VEGFA (1:1000), GLA (1:1000), NOS3 (1:1000), and β-actin (1:5000) overnight at 4 °C.
- Place the membranes from step 5.4 in an antibody incubation box, add 10 mL of TBST, and horizontally shake at 111 x g at room temperature to wash off unbound primary antibodies three times for 5 min each.
- Add 200 µL of goat anti-rabbit IgG (H + L)-HRP (1:1000) to each membrane from step 5.5 and incubate for 2 h at room temperature. Then, repeat step 5.5 to wash off unbound secondary antibody (goat anti-rabbit IgG (H + L)-HRP).
- Add 200 µL of ultrahigh sensitivity ECL chemiluminescent solution to the surface of each membrane from step 5.6 and immediately visualize protein bands using an automatic chemiluminescence imaging analysis system.
6. Statistical analysis
- Analyze the data using statistical and graphing software with one-way ANOVA followed by Tukey's test.
- Present the results as mean ± standard deviation (SD) and consider a P-value < 0.05 as statistically significant.
Ergebnisse
To confirm the successful establishment of the mouse model of anemia and analyze the effect of SLBXS on anemia, the liver index and hematological parameters were first investigated. Figure 1 illustrates that the model group exhibited a significant decrease (P < 0.01) in red blood cell count (RBC), hemoglobin (HGB), and hematocrit (HCT) compared to the control group. Conversely, the liver index, white blood cell count (WBC), and platelet count (PLT) in the model group were notably higher (P < 0.05 or P < 0.01). These results confirm that the anemia model was successfully established. The liver index, WBC, and PLT were significantly lower in the SLBXS group compared to the model group (P < 0.05 or P < 0.01), whereas the decreased RBC, HGB, and HCT in the model group were markedly elevated after treatment with SLBXS (P < 0.05 or P < 0.01). There was no significant difference between the parameters in the SLBXS and Fu-Fang-E-Jiao Syrup (FFEJS) groups. These results indicate that SLBXS can improve the symptoms of anemia.
Typical total ion chromatograms (TIC) of liver samples from the control, model, SLBXS, and FFEJS groups are presented in Figure 2. Marked differences are evident in the metabolite profiles among the control, model, SLBXS, and FFEJS groups.
Partial least squares discriminant analysis (PLS-DA) is a supervised statistical method used for discriminant analysis, which models the relationship between metabolite expression and sample class to predict the sample class. The PLS-DA scores plot (Figure 3A) shows a distinct separation among the control, model, SLBXS, and FFEJS groups. The score plot between the control and model groups is shown in Figure 3B, where the model group is significantly separated from the control group. This indicates that anemia disrupts normal metabolism in mice, confirming that the anemia model was successfully developed. Figure 3C displays the PLS-DA score plot based on the model and SLBXS groups. The variable importance in projection (VIP) value was calculated to assess how strongly each metabolite's expression pattern influences the classification of sample groups and to help identify key marker metabolites. A VIP value >1.0 is typically used as the screening criterion (Figure 3D). Cross-validation was then employed to validate the established PLS-DA model. As shown in Figure 3E, the model exhibited good R² (0.95) and Q2 (0.86) values, indicating that the PLS-DA model was reliable with minimal risk of overfitting. Metabolites with VIP > 1 and a P < 0.05 were classified as differential metabolites. A total of 29 differential metabolites were identified (see Table 1). The results suggest that SLBXS could upregulate metabolites such as xylose, tetracosane, alpha-tocopherol, and D-glucose while downregulating metabolites like porphine. Pathway enrichment analysis of these 29 differential metabolites, conducted using MetaboAnalyst, revealed that they were related to 15 pathways. Among these, galactose metabolism and lactose degradation (P < 0.05) were identified as the most pertinent metabolic pathways involved in SLBXS's treatment of anemia (Figure 3F and Figure 4).
Figure 5 demonstrates that the protein expression levels of HIF-1α, VEGFA, GLA, and NOS3 were significantly elevated in the model group compared to the control group (P < 0.01). SLBXS significantly reversed the upregulation of HIF-1α, VEGFA, GLA, and NOS3 expression observed in the model group (P < 0.01).
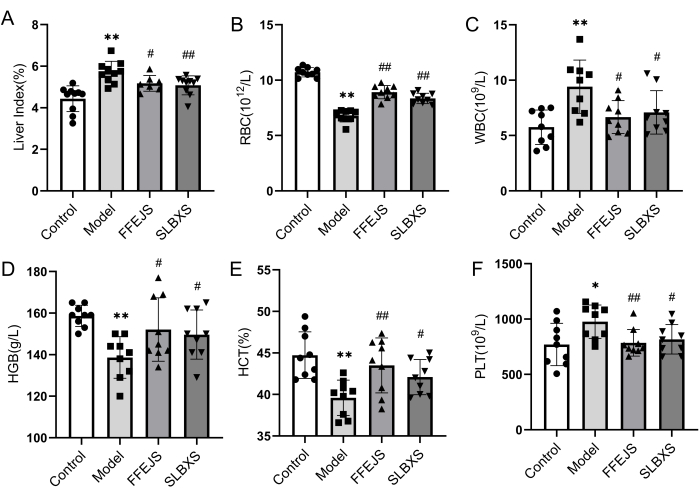
Figure 1: Effects of SLBXS on the liver index and peripheral blood routine of mice with anemia after 14 days of administration. (A) SLBXS decreased the liver index. (B) SLBXS increased RBC levels. (C) SLBXS decreased WBC levels. (D) SLBXS increased HGB levels. (E) SLBXS increased HCT levels. (F) SLBXS decreased PLT levels. Control vs Model, *P < 0.05, **P < 0.01; Model vs FFEJS or SLBXS, #P < 0.05, ##P < 0.01. SLBXS, Shi-Liu-Bu-Xue Syrup; RBC, red blood cell; WBC, white blood cell; HGB, hemoglobin; HCT, hematocrit; PLT, platelet. Please click here to view a larger version of this figure.

Figure 2: Analyzing the metabolic differences of liver samples from different groups of mice in the GC-MS system. TIC of liver samples obtained from the control (A), model (B), SLBXS (C), and FFEJS groups (D). TIC, total ion chromatograms; SLBXS, Shi-Liu-Bu-Xue Syrup; FFEJS, Fu-Fang-E-Jiao Syrup. Please click here to view a larger version of this figure.
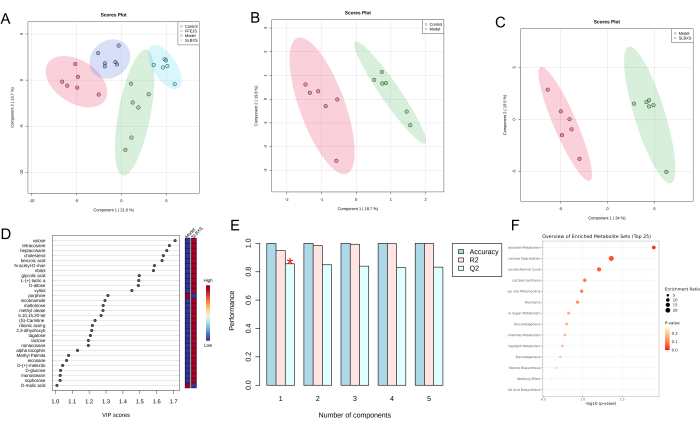
Figure 3: Data analysis of metabolic differences in liver samples from different groups of mice. Liver PLS-DA score plots of the control, model, SLBXS, and FFEJS groups (A); PLS-DA score plots of the control and model groups (B); PLS-DA score plots (C), VIP score plots (D), and cross-validation (E) between the model and SLBXS groups using metabolomics analysis; pathway enrichment analysis of differential metabolites (F). SLBXS, Shi-Liu-Bu-Xue Syrup; FFEJS, Fu-Fang-E-Jiao Syrup. Please click here to view a larger version of this figure.
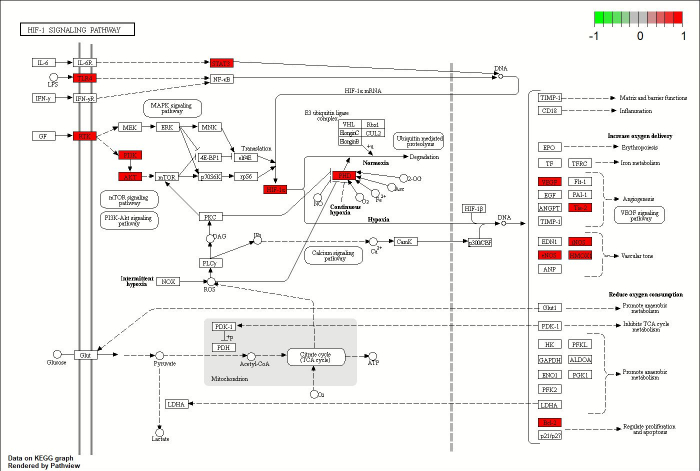
Figure 4: The regulatory network of SLBXS for treating anemia. Please click here to view a larger version of this figure.

Figure 5: Determining HIF-1α, VEGFA, GLA, and NOS3 protein levels in mice liver by Western blotting. (A) Representative different protein bands. (B) Statistical analysis of the expression levels of different proteins. Data are presented as the mean SD (n = 3), **P < 0.01 vs. the control group; ##P < 0.01 vs. the model group. Please click here to view a larger version of this figure.
Table 1: Summary of the differential metabolites. Please click here to download this Table.
Diskussion
Anemia is a common condition affecting many people worldwide, particularly in developing countries1. In China, patients frequently use traditional Chinese medicine, including Uyghur medicine, to alleviate the signs and symptoms of anemia. SLBXS is a Uyghur medicine that has been used in clinical practice for many years; however, its exact mechanism of action against anemia remains poorly understood13. In this study, a mouse model of acetylphenylhydrazine (APH)-induced anemia was established to elucidate the underlying mechanisms of SLBXS in treating anemia through metabolomics analysis.
Metabolomics is a valuable tool for diagnosing diseases, discovering potential biomarkers, and exploring the pathogenesis of diseases through qualitative and quantitative analysis of small molecule metabolites in biological samples. In this study, acetylphenylhydrazine (APH) was used to construct a model of anemia. APH is a strong oxidizing agent that causes slow and progressive oxidative damage to erythrocytes, particularly by interfering with glucose-6-phosphate dehydrogenase, promoting the denaturation of hemoglobin (HGB) and the formation of Heinz bodies. It also directly destroys membrane proteins and lipids of erythrocytes, leading to membrane lysis, rupture, and disintegration of erythrocytes, resulting in hemolytic anemia14,15. The liver, being the largest digestive gland in the human body, plays a crucial role in energy storage and metabolic regulation, maintaining the balance between metabolism and catabolism16. In this study, the enlarged livers of model mice were significantly relieved after 14 days of SLBXS administration. Further studies are needed to determine whether the liver is a likely target organ for SLBXS and to elucidate the related mechanisms. Consequently, gas chromatography-mass spectrometry (GC-MS) was used to detect metabolites in the livers of mice with anemia. Metabolomics analysis identified twenty-nine biomarkers and two key metabolic pathways: galactose metabolism and lactose degradation.
Many active components, including tannins, flavonoids, alkaloids, and organic acids, have been reported in pomegranate17,18. These ingredients exert various pharmacological effects, including antioxidant, anti-inflammatory, antibacterial, and antitumor activities. Evidence suggests that pomegranate juice is more potent than red wine and green tea in terms of antioxidant activity due to its higher polyphenol content19,20. These compounds exhibit their antioxidant effects through several mechanisms, including scavenging or neutralizing free radicals, metal chelation, affecting cellular signaling pathways, and regulating gene expression19,20. Additionally, pomegranate fruit extract is rich in flavonoids, which enhance antioxidant effects by reducing malondialdehyde and hydrogen peroxide levels and increasing the activities of catalase, superoxide dismutase, glutathione peroxidase, and glutathione reductase in the liver21. Healthy white rabbits treated with pomegranate fruit extract showed significant changes in erythrocyte count, hemoglobin (HGB) levels, and mean erythrocyte HGB concentrations, as well as a significant prolongation in bleeding and prothrombin time, marked elevation in protein C (anticoagulant protein) and thrombin-antithrombin compound levels, and a dose-dependent reduction in platelet aggregation and fibrinogen concentration. Based on the results of hematological and coagulation tests, it can be speculated that pomegranate may have anti-anemic and cardioprotective effects22. In Indian traditional medicine, plant materials such as pomegranate juice are used as a food supplement to treat iron deficiency anemia, possibly related to the promotion of iron absorption23.
To further elucidate the mechanism of SLBXS in treating anemia, a comprehensive metabolomics analysis was performed. The metabolites involved in the interaction network were alpha-tocopherol, D-xylitol, niacinamide, D-glucose, and lactose, while the targets were HIF1A, VEGFA, NOS3, and GLA. Alpha-tocopherol (Vitamin E) has antioxidant properties that inhibit premature red blood cell lysis by preventing the oxidation of polyunsaturated fatty acids in the red blood cell membrane24. It may, therefore, act as a potential erythropoietic agent by reducing premature erythrocyte lysis and decreasing the fragility of red blood cells25. Xylitol, at non-toxic doses, can alleviate APH-induced hemolysis. Its antihemolytic effect may be attributed to its role in producing NADPH, which protects against oxidative damage due to the absence of mitochondria in red blood cells. This helps maintain glutathione levels and protects hemoglobin, functional proteins, and other structural components from peroxidative damage26,27. Nicotinamide is a precursor for the synthesis of coenzyme I (NAD) and coenzyme II (NADP) in humans28. NAD is an important cofactor for many enzymes involved in energy metabolism29. D-glucose, lactose, and GLA are key components of galactose metabolism. As an important oxygen-sensitive transcription factor, HIF-1α is critical in the hypoxia adaptation pathway and is considered the primary regulator of homeostatic transcriptional responses in cells and tissues30,31. HIF-1α is rapidly degraded by prolyl hydroxylase (PHD) under normoxic conditions, but under hypoxic conditions, PHD is inhibited, leading to the accumulation of HIF-1α32. HIF-1α activates genes that control cellular oxygen homeostasis, including those involved in red blood cell production, oxygen consumption, vasculogenesis, and mitochondrial metabolism33. VEGFA is a cytokine that primarily acts on vascular endothelial cells and is expressed in many specialized cells during ischemia and hypoxia34. NOS3 (eNOS) is mainly found in the endothelium of coronary vessels and the luminal surface of the heart, participating in arginine and proline metabolism and catalyzing nitric oxide (NO) production. NO acts as a potent vasodilator by activating soluble guanylate cyclase in smooth muscle cells35. Additionally, NO interacts with reactive oxygen species produced by erythrocytes to generate reactive nitrogen oxide species, thereby reducing oxidative damage36.
Lastly, the results of metabolomics were validated using Western blotting. The findings showed that the levels of HIF-1α, VEGFA, GLA, and NOS3 decreased after SLBXS treatment compared with those in the model group. HIF-1α, VEGFA, and NOS3 are important targets in the HIF-1 signaling pathway, while GLA is a key target in galactose metabolism. These results suggest that SLBXS may influence both the HIF-1 signaling pathway and the galactose metabolism pathway, demonstrating a combined effect in treating anemia. Numerous studies have highlighted the significant roles of the HIF-1 and galactose metabolism pathways in the treatment of anemia and related diseases. Anemia reduces the oxygen-carrying capacity of the blood, leading to tissue hypoxia. HIF-1α accumulates in cells under hypoxic conditions, and high levels of HIF-1α enhance the expression of EPO and VEGF, contributing to a stable hematopoietic microenvironment, facilitating nutrient and oxygen transport, and protecting cells from hypoxic damage37,38. Activation of the HIF-1 pathway has been shown to stimulate erythropoiesis, increase hemoglobin levels, correct anemic conditions, and improve iron homeostasis39. The improvement in hypoxia after SLBXS treatment explains the reduced protein expression in the HIF-1 pathway observed in the SLBXS group. GLA (α-galactosidase A) is an exo-glycosidase that targets galacto-oligosaccharides such as stachyose, raffinose, and melibiose, as well as branched polysaccharides like galactomannan and galactoglucomannan by catalyzing the hydrolysis of α-1,6-linked terminal galactose residues40. Galactose is essential for cell metabolism as it facilitates energy production and storage in various body tissues and serves as a precursor for glycosylation41. Supplementation with galacto-oligosaccharides can enhance iron absorption in the intestine of rats and increase blood hemoglobin levels42. In this study, we investigated the underlying mechanisms of SLBXS in treating anemia using a combination of metabolomics and network pharmacology. We found that the HIF-1 signaling pathway and galactose metabolism pathway are significantly important in the treatment of anemia with SLBXS. This study provides new insights into the mechanisms of SLBXS in treating anemia. Future research should focus more on identifying and understanding the active ingredients involved.
Offenlegungen
The authors have nothing to disclose.
Danksagungen
This work was supported by the Special Training Plan for Minority Science and Technology Talents, Natural Science Foundation of Xinjiang Uyghur Autonomous Region (2020D03021), the Funds for Key Program for Traditional Chinese Medicine of Hubei University of Chinese Medicine (2022ZZXZ004), and Tianshan Innovation Team Program (2020D14030).
Materialien
| Name | Company | Catalog Number | Comments |
| Acetylphenylhydrazine | Shanghai Aladdin Biochemical Technology Co., Ltd. | C13979660 | |
| Automatic chemiluminescence imaging analysis system | Shanghai Tanon Life Science Co., Ltd. | Tanon-5200 | |
| Bicinchoninic acid assay kit | ThermoFisher Scientific | QPBCA-1KT | |
| Capillary column | Agilent J&W Scientific, Agilent Technologies, Inc. | DB-5MS | |
| Cell lysis buffer for Western and IP | Beyotime Biotechnology | P0013 | |
| Chlorotrimethylsilane | Shanghai Aladdin Biochemical Technology Co., Ltd. | C104814 | |
| Electronic balance | Mettler-Toledo International Inc. | ME203E | |
| Electronic scale | Mettler-Toledo International Inc. | LE104E | |
| Fu-Fang-E-Jiao Syrup | Dong E E Jiao Co., Ltd. | 214020031 | |
| Fully automatic hemocyte analyzer | Shenzhen Mindray Animal Care Technology Co., Ltd. | IDEXX ProCyte Dx | |
| GC-MS system | Agilent Technologies, Inc. | 7890B-5977B | |
| GLA primary antibody | Bioworld Technology | BS77041 | |
| Glass homogenizer | Shanghai Lei Gu Instruments Co., Ltd. | B-013001 | |
| Glass rod | Shanghai Lei Gu Instruments Co., Ltd. | B-003904 | |
| GraphPad Prism software | GraphPad, La Jolla | Version 9.0 | |
| Heparinized sample tubes | Changde BKMAM Biotechnology Co., Ltd. | B-ACT1P5 | |
| HIF-1α primary antibody | Bioworld Technology | BS3514 | |
| HMDB database | http://www.hmdb.ca/ | — | |
| Isoflurane | Hebei Jindafu Pharmaceutical Co., Ltd. | 20231202 | |
| Male C57BL/6 mice | Liaoning Changsheng Biotechnology Co., Ltd. | No. SCXK [Liao] 2015-0001 | |
| MassHunter | Agilent Technologies, Inc. | B.08.00 | |
| MetaboAnalyst 5.0 | https://www.metaboanalyst.ca/ | — | |
| Methoxyamine hydrochloride | Shanghai Aladdin Biochemical Technology Co., Ltd. | E1818113 | |
| n-hexane | Shanghai Aladdin Biochemical Technology Co., Ltd. | C14878803 | |
| NIST database | http://webbook.nist.gov/chemistry/ | — | |
| NOS3 primary antibody | Bioworld Technology | BS3625 | |
| Pyridine | Shanghai Aladdin Biochemical Technology Co., Ltd. | C13026996 | |
| Saline | BIOSHARP LIFE SCIENCES | 2308262009 | |
| Shi-Liu-Bu-Xue Syrup | Xinjiang Uygur Pharmaceutical Co., Ltd. | 211277 | |
| Surgical manipulation plate | DIXSG | ZK-JPB-A | |
| VEGFA primary antibody | Bioworld Technology | AP0742 | |
| β-actin | ABclonal (Shanghai) Trading Co., Ltd. | AC026 |
Referenzen
- Munoz, M., Gomez-Ramirez, S., Bhandari, S. The safety of available treatment options for iron-deficiency anemia. Expert Opin Drug Saf. 17 (2), 149-159 (2018).
- Soliman, A. T., De Sanctis, V., Yassin, M., Wagdy, M., Soliman, N. Chronic anemia and thyroid function. Acta Biomed. 88 (1), 119-127 (2017).
- Pivina, L., Semenova, Y., Dosa, M. D., Dauletyarova, M., Bjorklund, G. Iron deficiency, cognitive functions, and neurobehavioral disorders in children. J Mol Neurosci. 68 (1), 1-10 (2019).
- Lumbiganon, P., et al. Indirect causes of severe adverse maternal outcomes: a secondary analysis of the WHO Multicountry Survey on Maternal and Newborn Health. BJOG. 121 (Suppl 1), 32-39 (2014).
- Shaddy, R. E., et al. Systematic literature review on the incidence and prevalence of heart failure in children and adolescents. Pediatr Cardiol. 39 (3), 415-436 (2018).
- China Medical Association of Minorities. . Guidelines for clinical use of minority drugs. , (2018).
- Yu, W. Q. Clinical efficiency of Pomegranate blood-enriching syrup in the treatment of iron deficiency anemia. J Med Pharm Chin Minor. 26 (11), 11-12 (2020).
- Zhang, D. N., et al. Mechanism of Shiliu Buxue Syrup for anemia using integrated metabolomics and network pharmacology. Anal Biochem. 653, 114774 (2022).
- Nielsen, J. Systems biology of metabolism. Annu Rev Biochem. 86, 245-275 (2017).
- Wang, M., et al. Metabolomics highlights pharmacological bioactivity and biochemical mechanism of traditional Chinese medicine. Chem Biol Interact. 273, 133-141 (2017).
- Ben, C. N. Establishment and experimental research of "Blood Deficiency" animal model. Lab Animal Sci. (03), 5-10 (1994).
- Cao, S., et al. Integrative transcriptomics and metabolomics analyses provide hepatotoxicity mechanisms of asarum. Exp Ther Med. 20 (2), 1359-1370 (2020).
- Yang, H. Y., Liu, M. L., Luo, P., Yao, X. S., Zhou, H. Network pharmacology and metabolomics in the study of traditional Chinese medicine for cardiovascular diseases. Phytomedicine. 104, 154268 (2022).
- Croci, S., Pedrazzi, G., Passeri, G., Piccolo, P., Ortalli, I. Acetylphenylhydrazine induced haemoglobin oxidation in erythrocytes studied by Mossbauer spectroscopy. Biochim Biophys Acta. 1568 (1), 99-104 (2001).
- Liebowitz, J., Cohen, G. Increased hydrogen peroxide levels in glucose 6-phosphate dehydrogenase deficient erythrocytes expose to acetylphenylhydrazine. Biochem Pharmacol. 17 (6), 983-988 (1968).
- Tarasenko, T. N., McGuire, P. J. The liver is a metabolic and immunologic organ: A reconsideration of metabolic decompensation due to infection in inborn errors of metabolism (IEM). Mol Genet Metab. 121 (4), 283-288 (2017).
- Topalovic, A., Knezevic, M., Gacnik, S., Mikulic-Petkovsek, M. Detailed chemical composition of juice from autochthonous pomegranate genotypes (Punica granatum L.) grown in different locations in Montenegro. Food Chem. 330, 127261 (2020).
- Legua, P., et al. Total phenols and antioxidant capacity in 10 Moroccan pomegranate varieties. J Food Sci. 77 (1), C115-C120 (2012).
- Rodrigo, R., Miranda, A., Vergara, L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin Chim Acta. 412 (5-6), 410-424 (2011).
- Hussain, T., et al. Oxidative stress and inflammation: What polyphenols can do for us. Oxid Med Cell Longev. 2016, 7432797 (2016).
- Sudheesh, S., Vijayalakshmi, N. R. Flavonoids from Punica granatum--potential antiperoxidative agents. Fitoterapia. 76 (2), 181-186 (2005).
- Riaz, A., Khan, R. A. Anticoagulant, antiplatelet and antianemic effects of Punica granatum (pomegranate) juice in rabbits. Blood Coagul Fibrinolysis. 27 (3), 287-293 (2016).
- Balasubramani, S. P., Varghese, R. K., Vishnuprasad, C. N., Venkatasubramanian, P. Pomegranate juice enhances iron dialysability and assimilation in in-vitro cell-free and cell-based models. Plant Foods Hum Nutr. 75 (2), 272-278 (2020).
- Jilani, T., Iqbal, M. P. Does vitamin E have a role in treatment and prevention of anemia. Pak J Pharm Sci. 24 (2), 237-242 (2011).
- Farrell, P. M., Bieri, J. G., Fratantoni, J. F., Wood, R. E., di Sant'Agnese, P. A. The occurrence and effects of human vitamin E deficiency. A study in patients with cystic fibrosis. J Clin Invest. 60 (1), 233-241 (1977).
- Ukab, W. A., Sato, J., Wang, Y. M., van Eys, J. Xylitol mediated amelioration of acetylphenylhydrazine-induced hemolysis in rabbits. Metabolism. 30 (11), 1053-1059 (1981).
- Ahuja, V., et al. Biological and pharmacological potential of xylitol: A molecular insight of unique metabolism. Foods. 9 (11), 1592 (2020).
- Nikas, I. P., Paschou, S. A., Ryu, H. S. The role of nicotinamide in cancer chemoprevention and therapy. Biomolecules. 10 (3), 477 (2020).
- Katayoshi, T., Yamaura, N., Nakajo, T., Kitajima, N., Tsuji-Naito, K. Porcine placental extract increases the cellular NAD levels in human epidermal keratinocytes. Sci Rep. 12 (1), 19040 (2022).
- Bartoszewski, R., et al. Primary endothelial cell-specific regulation of hypoxia-inducible factor (HIF)-1 and HIF-2 and their target gene expression profiles during hypoxia. Faseb J. 33 (7), 7929-7941 (2019).
- Lee, S. H., Golinska, M., Griffiths, J. R. HIF-1-independent mechanisms regulating metabolic adaptation in hypoxic cancer cells. Cells. 10 (9), 2371 (2021).
- Sato, T., Takeda, N. The roles of HIF-1α signaling in cardiovascular diseases. J Cardiol. 81 (2), 202-208 (2023).
- Ratcliffe, P. J. Oxygen sensing and hypoxia signalling pathways in animals: The implications of physiology for cancer. J Physiol. 591 (8), 2027-2042 (2013).
- Li, L. J., Huang, Q., Zhang, N., Wang, G. B., Liu, Y. H. miR-376b-5p regulates angiogenesis in cerebral ischemia. Mol Med Rep. 10 (1), 527-535 (2014).
- Tejero, J., Shiva, S., Gladwin, M. T. Sources of vascular nitric oxide and reactive oxygen species and their regulation. Physiol Rev. 99 (1), 311-379 (2019).
- Fujii, J., et al. Erythrocytes as a preferential target of oxidative stress in blood. Free Radic Res. 55 (5), 562-580 (2021).
- Huang, J. W., et al. High expression of HIF-1α alleviates benzene-induced hematopoietic toxicity and immunosuppression in mice. Environ Pollut. 311, 119928 (2022).
- Zhang, Z., Yao, L., Yang, J., Wang, Z., Du, G. PI3K/Akt and HIF-1 signaling pathway in hypoxia-ischemia (Review). Mol Med Rep. 18 (4), 3547-3554 (2018).
- Del Balzo, U., et al. Nonclinical characterization of the hypoxia-inducible factor prolyl hydroxylase inhibitor roxadustat, a novel treatment of anemia of chronic kidney disease. J Pharmacol Exp Ther. 374 (2), 342-353 (2020).
- Bhatia, S., Singh, A., Batra, N., Singh, J. Microbial production and biotechnological applications of α-galactosidase. Int J Biol Macromol. 150, 1294-1313 (2020).
- Conte, F., van Buuringen, N., Voermans, N. C., Lefeber, D. J. Galactose in human metabolism, glycosylation and congenital metabolic diseases: Time for a closer look. Biochim Biophys Acta Gen Subj. 1865 (8), 129898 (2021).
- Wang, Y. Y. . Investigate the effect of galacto-oligosaccharide supplementation on intestinal absorption of iron in rats. [Master's Thesis]. , (2019).
Nachdrucke und Genehmigungen
Genehmigung beantragen, um den Text oder die Abbildungen dieses JoVE-Artikels zu verwenden
Genehmigung beantragenThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Alle Rechte vorbehalten