Method Article
Establishment of Rat Models Mimicking Gender-affirming Hormone Therapies
* These authors contributed equally
In This Article
Summary
The protocol describes the development of two rodent models mimicking gender-affirming hormone therapies through subcutaneous administration of testosterone or estradiol plus cyproterone acetate (used in human therapies for transgender people): setting of the doses, identification of relevant biomarkers, and evaluation of the effects.
Abstract
Transgender (TG) people are individuals whose gender identity and sex assigned at birth do not match. They often undergo gender-affirming hormone therapy (GAHT), a medical intervention that allows the acquisition of secondary sex characteristics more aligned with their individual gender identity, providing consistent results in the improvement of numerous socio-psychological variables. However, GAHT targets different body systems, and some side effects are recorded, although not yet fully identified and characterized. Therefore, TG people undergoing GAHT may be considered as a susceptible sub-group of population and specific attention should be paid in the frame of risk assessment, e.g., through the use of targeted animal models. The present work describes the procedures set to implement two rat models mimicking GAHT: the demasculinizing-feminizing model (dMF) mimicking the GAHT for TG women and the defeminizing-masculinizing model (dFM) mimicking the GAHT for TG men. The models have been implemented through the administration of the same hormones used for human GAHT, namely, β-estradiol plus cyproterone acetate for dMF and testosterone for dFM, by the same routes of exposure for a 2 week period. Rats are checked daily during the treatment to evaluate health status and potentially aggressive behaviors. At sacrifice, blood and target tissues have been sampled and stored for biochemical, molecular, and histopathological analysis. Sex-specific parameters, namely, sperm count and clitoral dimensions, have also been evaluated. In addition, CYP450 isoforms, exclusively and/or preferentially expressed in male and female rat liver, are identified and characterized as novel biomarkers to verify the success of GAHT and to set the model. Thyroid involvement has also been explored as a key target in the endocrine system.
Introduction
The individual's psychological perception of being male, female, neither, both, or somewhere in between1 is called gender identity. It can match the biological sex (cisgender) or can be different (transgender - TG). A TG man is an individual born as a female but who identifies himself as a man. A TG woman is born as a male but identifies herself as a woman2. It is estimated that, at present, there are 25 million TG people worldwide3, most of them suffering from gender dysphoria, a psychological condition characterized by incongruence between their gender and the one they were assigned at birth4, which can result in social discrimination and difficulties at work and in family, often leading to depression, anxiety, and stress5. For such reasons, TG people often go through gender-affirming hormone therapy (GAHT) and/or gender-affirming surgery. GAHT for TG men is characterized by the administration of testosterone (T) (Table 1), and for TG women, estrogens (E2) plus antiandrogens (Table 2)6.
GAHT usually lasts for the entire life of the individual, acting continuously on the endocrine system7,8. So, it is important to analyze the GAHT's impact on the health of TG people and its potential long-lasting effects. Moreover, TG people are exposed, as the general population, to chemical contaminants -- in particular, the endocrine disrupters (ED) -- that target the endocrine system as the GAHT, resulting in overstimulation9.
ED are a group of chemicals affecting the organisms and/or their progeny by altering different hormonal and metabolic processes, such as the secretion, activation, synthesis, release, and binding of natural hormones. Since ED are widespread in the environment, food and products of everyday use (e.g., plastic bottles and containers, liners of metal food cans, detergents, flame retardants, food, toys, cosmetics and pesticides, etc.) general population is continuously exposed during the whole life10. In addition, even low-dose exposure to EDs can lead to tissue and organ damage, and a common phenomenon associated with ED exposure is the occurrence of sex-specific effects both in laboratory animals and in humans11. As an example, exposure to the food contact material, bisphenol A (BPA), is linked to health risks such as endometriosis and polycystic ovary syndrome in women, as well as reduced fertility, due to its estrogenic effects12. In men, BPA can lower levels and diminish sperm quality13. Additionally, pesticides are associated with a higher risk of breast cancer in women and significant fertility issues in men13. So far, no specific tools are available to study the toxicological effects of environmental contaminants, including ED, in TG people9.
The present study aims to describe methods and parameters selected for the development of two TG animal models: a demasculinizing-feminizing model (dMF) and a defeminizing-masculinizing model (dFM). In particular, the selection of suitable dose levels, time, and way of administration of the hormones on the basis of the current human therapies are assessed14. Moreover, tissue and functional biomarkers that define the models uniquely are identified and characterized. In addition, the efficacy and tolerability of therapy in animals as well as the selection of the most appropriate markers for use in long-term studies, are described in detail together with the techniques used for such purposes.
In order to characterize the models, the following endpoints are analyzed: testosterone (T) serum level (the best biomarker to evaluate the success of GAHT in both models9,15); estradiol (E2) serum level (both models); thyroid stimulating hormone (TSH) and thyroxine (T4) (dMF model); sperm count (dMF model); clitoral dimension (dFM model); histopathological analysis of reproductive organs, liver and thyroid (for both models). In addition, gene expression of the following sex-specific liver cytochrome P450 isoforms (CYP450s, for both models) are also analyzed16,17: CYP2C11 (specifically expressed in the male liver), CYP3A18 (expressed 25 times more in male liver than in female), CYP2C12 (specifically expressed in the female liver), and CYP2C6 (predominantly expressed in female liver, but present at lower levels, also in the male).
Protocol
The studies are performed following Directive 2010/63/EU, the Italian Legislative Decree n. 26 of 4 March 2014, and the OECD Principles of Good Laboratory Practice (GLP). The study protocol was approved by the Italian Ministry of Health (authorization no. 806/2021-PR). Here, 16 young sexually mature Sprague-Dawley rats of both sexes (304 ± 13 g male rats and 190 ± 7 g female rats, 8-9 weeks old) are purchased and housed in two/cage under standard laboratory conditions (22 ± 0.5 °C, 50%-60% relative humidity, 12 h of dark-light alternation with 12-14 air changes per hour) with water and food available ad libitum. In all the cages, for each animal's environmental enrichment, insert wood gnawing blocks and replace them weekly.
NOTE: The animals aged 8-9 weeks have been chosen since GAHT can start during adolescence (Tanner stages 2 to 3), corresponding to 8-9 weeks in rodents, and it lasts for a long time, potentially for the whole life of TG people9.
1. Group sizing and animal care
- Use young adult male and female Sprague-Dawley rats (8-9 weeks old) as a model since rat is the preferred species indicated by OECD (e.g., Guideline 421 Reproductive/Developmental Toxicity Screening Test) and the only rodent species proposed and considered in OECD Guideline 407 (28-day oral toxicity study at a repeated dose in rodents, updated with the parameters for the detection of EIs).
- Use the G*Power software for the group sizing. For both the dFM and the dMF models, select four test groups: one control group (C) and three groups of animals treated with different doses of GAHT. Do the sizing with reference to the comparison between two groups (treatment dose versus C) with the Mann-Whitney test, a two-tailed significance level alpha = 0.0167 (corresponding to alpha = 0.05 with Bonferroni correction applied to the 3 comparisons between each treatment dose and the C), and 1-beta power = 0.80.
- The calculated number of rats/groups is as follows:
for dFM model, animals/group n=4, according to Kinnear et al.18 indicating after 2 weeks of treatment, the following levels: in the control group: 0.2 ± 0.3 ng/mL, in the hormone therapy group: 16 ± 5 ng/mL (mean ± standard deviation), corresponding to an effect dimension measured with Cohen's d = 4.46 (very large effect).
for dMF model: animals/group n=3, according to Gómez et al.19 indicating after 2 weeks of treatment, the following levels: control group: 1.901 ± 0.413 ng/mL and hormone therapy group: 0.043 ± 0.023 ng/mL (mean ± standard error) corresponding to an effect dimension measured with Cohen's d = 6.35 (very large effect). - To align the two models, choose n=4 animals per sex/group, for a total of N = 32 animals.
- The calculated number of rats/groups is as follows:
- After 1 week of acclimatization, divide rats by sex into two experimental groups (dMF and dFM, 4 rats/group), each composed of one C (control male, CM and control female, CF) and three treatment groups of the selected GAHT.
- Monitor all rats 2x a day (at 8:30 a.m. and 4:00 p.m.) to check general health conditions and potential aggressiveness due to GAHT administration. Record body weight (bw) and feed consumption 2x a week, using an analytical balance with the dynamic weighting function optimal for laboratory animals' weight.
- In the dFM experimental model, measure the clitoral diameter of all rats immediately before the start of treatment (point 0) and at the end of treatment (point 13), using a digital gauge for precision readings.
- Weigh all animals before the sacrifice and, in the dMF model, immediately after sacrifice remove the epididymis in order to perform the sperm count.
2. Dose selection and preparation
- Use the following three doses of hormones to be administered to dFM rats: 5, 10.5 and 22.5 mg/kg per week, for the dMF model, use the following three selected dose levels (DL): DL1 E2 0.045 mg/kg + CPA 0.2 mg/kg, DL2 E2 0.09 mg/kg + CPA 0.2 mg/kg and DL3 E2 0.18 mg/kg + CPA 0.2 mg/kg.
NOTE: All the doses are selected taking into account the main human clinical guidelines used for TG people15 and the limited data available in the literature. - Prepare all the stock solutions weekly using a chemical safety hood. Properly mix all the substances into the sesame oil as a vehicle, ensuring their complete solubilization.
NOTE: For both models, use a dose conversion calculator (https://dosecal.cftri.res.in/) to enter the dose to convert, the species for which the dose has been set, the animal species, and the weight for which to convert the dose. For the dFM model (Table 3), human hormone therapy, a maximum dose of 100 mg of T per week20. The dose calculated in rats is 10.5 mg/kg of T per week. The second dose is 22.5 mg/kg per week in rats18. The third dose is 5 mg/kg per week, identified by maintaining the factor of 2.1 between the two calculated doses. For the dMF model see Table 4, the dosages are based on recommended regimens for humans21 and data available through in vivo studies14,19,22.
3. Animal treatment
- Perform subcutaneous administration of T (for the dFM model) and E2 plus CPA (for the dMF model) by pinching and lifting the skin at the injection site, forming a kind of curtain. Then, insert the needle of a 1 mL syringe parallel to the animal's back. Once inside, slowly inject the required volume (100 µL for T; 200 µL for E2+CPA)23.
- Treat the animals as follows: for the dFM model, administer T enanthate dissolved in sesame oil (vehicle) 2x/week for 2 weeks (100 µL for each subcutaneous injection of T). For the dMF model, administer E2 plus CPA acetate dissolved in sesame oil (vehicle) 5x/week for 2 weeks (200 µL for each subcutaneous injection). Treat the rats of C group in the same way with the vehicle only (sesame oil).
4. Blood sampling, sacrifice, and tissue sampling
- After 2 weeks of treatment, just before the sacrifice, use a gaseous anesthesia system and anesthetize all animals. Induce anesthesia by dose varying from 2% to 3.5% isoflurane in 100% oxygen at a rate of 1.5 L/min till loss of the reflexes in a clear, polystyrene induction chamber. Place the rat in the prone position on the heating pad and regulate the dose of isoflurane at a maintenance dose varying from 1.5% to 3.5% administered by a standard rat nose mask throughout the intracardiac blood sampling procedure.
- Prepare a 5 mL syringe with a 21G needle, eliminating the vacuum. Probing the heart with the fingers, carefully insert the needle into the lateral thoracic wall perpendicular to the body at approximately the point of flexed elbows, between ribs 5 and 6. Once inside the heart ventricle, the blood enters the syringe by capillarity. Retract the syringe piston slowly and continuously until blood draws.
- After the blood sampling, place the rats into the CO2 chamber in order to perform euthanasia using CO2 asphyxiation with a fill rate of 30%-70% displacement of the chamber volume per minute. After 2-3 min, verify the animals' death by monitoring breathing and heartbeat24.
- After the sacrifice, place every animal on the dissection table in a supine position, with the front legs facing upwards and the hind legs facing down, and fix them with pins. Sprinkle the animal's abdomen with a disinfectant solution.
- Excise the following organs: testes, epididymis, uterus, ovaries, liver, and thyroid. Weigh and store them in 50 mL tubes with 35 mL of 10% buffered formalin (all organs) or Bouin's solution (testis only) or collect them in cryogenic tubes (an organ/tube) and immediately freeze them in liquid nitrogen (for storing at -80 °C).
- Firstly, cut the skin with scissors on the midline, from the pubis to the upper abdomen. Make two lateral cuts on the rib cage and reflect the skin on both sides of the incision to expose the thoracic viscera.
- Remove the reproductive organs first; in male rats, remove the testes, oval-shaped paired organs located in the abdominal cavity, in the intra-abdominal position (scrotal sac). Cut and press the scrotal sac to ensure that the testes are sticking out, then grasp them gently. Hold the visceral fat with pliers, then cut the testes away from the viscera. After the excision, weigh and store the testes in Bouin's solution for histopathological analysis.
- In female rats, first grab the uterus delicately, then cut the ovaries (which are located at the end of both the uterine horns) from the visceral adipose tissue. After the excision, weigh the ovaries.
- In both female and male rats, move on to the right side of the abdominal cavity, where the liver is located. The liver is a multilobulated organ and it is possible to identify many parts: left medial (smaller), left lateral (major), right medial (smaller), right lateral (major), caudate, and square lobe25. Grab the liver with forceps, taking care not to break it. Grasp the xiphoid process with pliers and completely cut the diaphragm with scissors.
- Use forceps to lift the xiphoid process and extract the liver from the abdominal cavity. Separate the liver from the diaphragm with scissors. After that, weigh the liver and store.
- For thyroid extraction, with the help of scissors, cut perpendicularly along the neck of the animal, remove the skin and musculature, and gently trim the ends of the trachea, in which the thyroid gland is located. Store the thyroid for further analysis26.
- Perform sperm count as described below.
- After testis removal, grab the epididymis, which is attached to the posterior margin of the respective testis and contained in the scrotum.
- In this experiment, semen samples are taken from the tail of the right epididymis. At the time of collection, clean the tail of fat and connective tissue using scissors, separate it from the remaining part, and place it in a Petri dish (35 mm x 10 mm) containing 1 mL of DMEM. Cut with scissors and flow with a Pasteur pipette to facilitate the release of the content.
- Transfer all the liquid obtained into a 15 mL tube and bring the volume to 10 mL with DMEM (dilution factor 1:10=0.1). Pipette the content to homogenize and create a suspension. After a slight shaking, take 10 µL of the suspension to load the Neubauer chamber. Load the camera and after 3 min of camera loading, start the counting.
5. Enzyme-linked immunosorbent assay (ELISA) assay
- Collect blood samples (about 2-5 mL per animal) in 2 mL tubes and allow them to coagulate at room temperature for 1 h. Spin all blood samples in a centrifuge 2x for 15 min at 4 °C and 3000 x g.
- Centrifuged blood has a multi-layered appearance with three layers of different colors. The top layer consists of plasma (usually yellow or transparent), the liquid portion of blood; the layer below the plasma may have a whitish or gray coloration and is the Buffy coat. The lowest layer contains red blood cells, and the color may appear dark red or bright red. To determine sex hormone levels, collect the serum of all samples with a pipette (without touching the buffy coat or the erythrocyte layers), divide them into aliquots (500 µL) and store them at -80 °C.
- In both models, perform ELISA test to detect and/or measure the presence of proteins, antibodies or antigens in the sample for the determination of sex hormones. Use the direct method and follow the instructions of each ELISA kit. In this study, serum levels of the following hormones were determined in rats: E2, Rat Estradiol ELISA; T, Mouse/Rat Testosterone Elisa; TSH, Rat ELISA Kit and T4, Rat ELISA Kit.
6. Histopathological analysis
- Prepare histological slides of tissues according to the following procedures.
- Fix the samples of the uterus, ovary, thyroid and liver at the time of extraction in 10% buffered formalin for at least 48 h.
CAUTION: Work under a chemical hood. Do not inhale the substance/mixture. Avoid generating vapor/aerosols. - Wash samples under running water for 1 h and then store in 80% ethyl alcohol (C2H5OH) until processing, to preserve the protoplasmic structure from alterations resulting from cellular death.
- Fix the testis in Bouin's solution consisting of 15 mL of saturated solution of picric acid, 5 mL of concentrated formalin (33%-40%), and 1 mL of glacial acetic acid for 48 h. Wash samples with 80% ethyl alcohol.
CAUTION: Work under a chemical hood. - Perform inclusion in solid paraffin using an automatic tissue processor. At the end of the inclusion process, place the samples in small metal containers in which melted paraffin has been poured and cool on a frozen plate, forming the blocks for the cut. Orient them depending on the desired cutting section.
- After that, cut the samples using a rotating microtome. Collect 5-6 µm sections using small brushes, spread them into water at 37 °C for a few seconds, and place them on microscope glass slides. Leave the slides to drain perpendicularly for a few minutes and dry them in the oven at 36 °C for at least 1 h.
- Perform hematoxylin and eosin staining as described below.
- Deparaffinize the slides by dipping them in clearing agent of terpene origin (xylene substitute) for 5 min.
- Rehydrate the slides by dipping them through the following series of graded ethanol. For each solution, 2 min per dip: 100% ethanol, 95% ethanol, 80% ethanol, 50% ethanol, and distilled water.
NOTE: The can be a stop point for the protocol, and the slides can be left at RT in water for several hours. Optionally, the slides can also be stored at 4 °C in water. - Place the slides in Mayer's hematoxylin for 5 min. Immediately transfer them back to the container with tap water for 5 min. Run tap water into the back corner of the container farthest away from the sections. Periodically empty the container until the purple coloring in the water is no longer present.
NOTE: To prevent sections from peeling off from the slides, do not run water directly onto the slides. - Place the slides into the eosin Y 1% aqueous solution for 2 min. Transfer the slides to distilled water for 15 s. Dehydrate the slides by dipping them through the following series of graded ethanol. For each solution, 15 s per dip: 50% ethanol, 80% ethanol, 95% ethanol.
- Place the slides in 100% ethanol for 1 min and change it to fresh 100% ethanol for another 1 min. Place the slides in a clearing agent of terpene origin (xylene substitute) for 2 min and change it to a fresh clearing agent of terpene origin (xylene substitute) for another 2 min. Remove one slide at a time and place a coverslip.
- Cover the sections with mounting medium and place a coverslip on the bottom of the slide.
NOTE: Ensure the mounting medium pulls the slide into position. If the coverslip is not flat, gently tap it into place. Blot any excess mounting medium using a paper towel. - Dip a cleaning wipe into the clearing agent of terpene origin (xylene substitute) and wipe the back of the slide to remove any dripped medium. Place the slide flat on a mobile solid surface, like a piece of cardboard. Add coverslip to all the remaining slides as described in step 6.7.6. Allow the slides to dry and the mounting medium to harden at RT in the hood.
CAUTION: Work under a chemical hood.
- After the staining, cover the samples with a cover slip and evaluate them using an optical microscope. Histopathological changes have been described based on distribution, severity, and morphological characteristics.
- Perform injury scoring semi-quantitatively, using a 5-point assessment scale (0 to 4), considering the severity of the changes based on criteria explained by Shackelford et al.27 and summarized as follows:
Grade 0: no change
Grade 1: minimum change. The effect is barely visible on the tissue; small and infrequent. Affects a portion of tissue less than or equal to 10%.
Grade 2: light change. Represents a small histological change. This grade is used when the tissue, or its structure, has shown a change in volume between 11% and 20%.
Grade 3: moderate change. The change is evident in the tissue. The degree indicates the presence of variations between 21%-40% of the tissue or its structure
Grade 4: marked change. Overwhelming histological change of tissue or its structure. Variations affect 41%-100% of the tissue. - Perform quantitative histomorphometrical analysis on uterus, ovary, testis and thyroid. Examine tissue sections using an image analysis system applied to an optical microscope and follow the steps described below.
- Uterus: Using a 2x objective measure the total area of each transverse section of the right uterine horn, the external and internal myometrium, and the uterine lumen. Calculate the area of the endometrium and myometrium and the ratio between the area of the endometrium and the myometrium28.
- Ovary: Use one of the whole-section in the central position of the ovary to reveal follicles in various stages of development; count the primary, secondary, corpus luteum, Graaf, and atresic follicles using a 4x objective. Perform classification of follicles according to Fortune29. In addition, measure the area of the ovary for each sample and its follicular density (number of follicles/ovary area x 100)30.
- Testis: Using the 10x objective, measure 20 tubules/sample; in addition, measure total lumen area, total area, longitudinal diameter, and transverse diameter28.
- Thyroid: Measure the following parameters: follicular density (ratio between the number of follicles and a given area, 10x objective); indirect follicular cell height (mean ratio of follicle area and colloid area in five randomly selected follicles/sample 40x objective); the mean ratio of follicular epithelium areas and the nuclei number (in the same follicle to ascertain follicular maturation); follicular cell height (mean of five cell height in five randomly selected follicles/sample, 64x objective)31.
7. Gene expression
- For both models, perform the gene expression analysis using liver samples stored at -80 °C. Follow the protocol of total RNA purification using a kit, starting from a lysate of animal tissue.
- To obtain the liver lysate, grind the tissue sample (10 mg) with a minipinner, add 600 µL of RL BUFFER (provided with the kit) to the tissue sample, and continue to grind until the sample has been homogenized.
- Immediately after the extraction, quantify the RNA obtained and evaluate its quality (for denaturation or the presence of any protein residues) using a fluorospectrometer. Perform spectrophotometric absorbance readings at wavelengths of 260 nm and 280 nm, where nucleic acids and proteins respectively absorb.
- After the evaluation of the total RNA concentration, using a cDNA Synthesis Kit following the manufacturer's instructions, reverse transcribe the appropriate volume containing 1 µg of total RNA from each sample to cDNA.
- Analyze the obtained cDNA by RT-PCR.
- To analyze gene expressions of genes of interest, specific forward and reverse primers have been designed for each target gene, as well as for the reference gene constitutively expressed glyceraldehyde phosphate dehydrogenase (GAPDH). Use the web application Primer-BLAST (www.ncbi.nlm.nih.gov/tools/primer-blast) to choose the best primers that would guarantee the specificity of the pairing only to the gene of interest and obtain the oligonucleotides synthesized by a commercial supplier. The sequences of the used primer pairs are reported in Table 5.
- Resuspend the lyophilized primers with RNAse-free distilled water at a final concentration of 100 mM. Use a dedicated kit to perform qPCR analysis. Carry out the reactions in a final volume of 20 µL, diluting the annealed cDNA 1:40. Load each sample in duplicate in 96-well PCR plates.
- Perform qPCR runs on thermal cycler following this program: 1 cycle at 95 °C for 10 min; 40 cycles at 95°C for 15 s, 58 °C for 30 s, and 72 °C for 1min. At the end, perform a dissociation cycle from 55° C to 95° C (30 s/°C) to verify the specificity of the amplified product. Using the machine's dedicated software, obtain threshold cycle values (Cycle Threshold, Ct) for each sample. Express the results as ΔΔ Ct (delta delta Ct) according to the Pfaffl formula32:
ΔCt Target gene = Ct Control - Ct Treated
ΔCt Reference gene = Ct Control - Ct Treated
ΔCt = ΔCt Target gene - ΔCt Reference gene
8. Data analysis
- Perform data management using a spreadsheet. Perform analysis using statistical analysis software. Design graphics using specific software.
- Represent bw gain, feed consumption, absolute and relative organ weight, hormone serum levels, tissue morphometrical data, and gene expression data as mean ± standard deviation. Perform a non-parametric Kruskal-Wallis analysis, followed by post-hoc pairwise comparisons (Mann-Whitney test).
- Analyze histological data using a 2-way Fisher Exact Test to identify significant differences from the Control group, including samples that are assigned to a category without any reference to severity gradations (total finding incidence). Use the Cochran-Armitage Trend Test to identify the dose-response trend. Consider differences among groups as significant if the p-value is < 0.05.
Results
As demonstrated by Tassinari et al.14 and by Tammaro et al.33, the following results showed the success of GAHT on rats and the appropriateness of the models.
No mortality or abnormal behavior, such as aggressiveness, are recorded, and no clinical signs of toxicity or suffering (e.g., decreased activity, piloerection, and an ungroomed appearance) are observed14.
Plasma T concentrations in adult male rats and female rats during estrus and proestrus are 5.71 ± 0.84 ng/ml and 1.24 ± 0.29 and 0.80 ± .36 ng/ml, respectively34. The mean circulating level of T in the gonadally intact rats is 3.5 ± 0.5 ng/mL35. The T and E2 levels measured in the control rats in both models fell into the physiological range indicated in the literature33. The physiological range of E2 for female rats is between 5-140 pg/mL36. The physiological range of E2 for male rats is between 2-175 pg/mL37.
Model dFM
Considering the variation in clitoral diameter, calculated as the difference between the diameter measured on the last day (point 13) and the first day of treatment (point 0), the diameter is increased in all treatment groups (DL1= 0.45; DL2=095; DL3=2.05 mg di T per administration) compared to the C group (Table 6).
The bw gain is significant in the DL2 and DL3 groups in comparison to the C group; feed consumption significantly increased in the DL3 group compared to the C (Figure 1).
T serum levels increased in all treatment groups, reaching the range of the corresponding cisgender (C MALE=CONTROL MALE); E2 levels decreased in all treatment groups, reaching the range of the corresponding cisgender (Figure 2).
Absolute and relative ovary weight decreased in the DL2 and DL3 groups, in comparison to the C group (Table 7; Figure 3), with a decreased uterus absolute weight in the DL2 group (Table 7).
Histopathological analysis of ovaries in the DL3 group showed a dose-dependent significant increase of hyperaemic vessels upon comparison to the C group. The DL3 group showed a significant increase in the number of primary and secondary follicles, and a significant decrease in Graaf follicles when compared to the C group (Table 8).
The uterus showed increased hyperemic vessel, significantly in the DL3 group. Lumen areas, myometrium areas, endometrium areas, and luminal epithelium height are reduced in all treatment groups (Table 8).
Gene expression analysis showed significant up-regulation of the male sex-specific genes (Cyp2C11; Cyp3A18)17 in DL2 and DL3 groups and a significant down-regulation of the female sex-specific gene (Cyp2C12)14 in DL2 and DL3 groups in comparison with C group (Figure 4).
Model dMF
No death or adverse clinical effects have been recorded for the dMF model. Sperm count is dose-dependently and statistically decreased in all treatment groups (DL1: 0.045 + 0.2, DL2: 0.09 + 0.2, and DL3: 0.18 + 0.2 mg E2+CPA) in comparison to the C group (Figure 5).
The bw gain and feed consumption significantly decreased in all treatment groups (DL1: 0.045 + 0.2, DL2: 0.09 + 0.2, and DL3: 0.18 + 0.2 mg E2+CPA) compared to the C group (Figure 6).
T serum levels are significantly decreased, and E2 serum levels are statistically increased in all treatment groups (DL1: 0.045 + 0.2, DL2: 0.09 + 0.2, and DL3: 0.18 + 0.2 mg E2+CPA) in comparison to C group (Figure 7). As expected, no difference is shown between the T serum levels of the treatment groups and the C female group.
TSH serum levels are dose-dependently increased in all treatment groups, significantly in the DL2 group and with borderline significance in DL3 (p=0.06) compared to the C group (Figure 8).
Testis absolute weight is significantly decreased in all treatment groups (DL1: 0.045 + 0.2, DL2: 0.09 + 0.2, and DL3: 0.18 + 0.2 mg E2+CPA) in comparison to C; no differences in relative weight are seen among treatment in comparison to C group (Table 9).
Germ cell depletion and increased tubule degeneration in testes are observed in a dose-dependent manner with histopathological analysis. (DL1, 1/4 sample: grade 1; DL2, 2/4 samples: grade 1; DL3, 2/4 samples: grade 1 plus 2/4 samples: grade 2) and statistically significant decreased tubule lumen area in the DL3 group compared to the C group (Table 10; Figure 9).
Thyroid histopathological analysis revealed follicular cell hypertrophy with central follicles tightly packed and smaller than normal in all treatment groups (DL1: 0.045 + 0.2, DL2: 0.09 + 0.2 and DL3: 0.18 + 0.2 mg E2+CPA) in comparison to C group (Figure 10). Follicular density in all treatment groups is significantly increased with an increase in the number of follicles. However, the follicle dimension is decreased with the reduction in area of both follicle and colloid in all treatment groups compared to the C. The height of epithelium cell t and area of follicles are unaffected (Table 10).
Gene expression analysis indicates that Cyp2c11 (male-specific isoform) is down-regulated in the DL3 group and Cyp3a18 (male-predominant isoform) is significantly down-regulated in all treatment groups (Figure 11A,B). Cyp2c12 (female-specific isoform) is up-regulated in all treatment groups, statistically significant in DL1 and DL2 (Figure 11C), the Cyp2c6 (female predominant isoform) is significantly up-regulated in the DL2 group when compared to C (Figure 11D).

Figure 1: General toxicity data in the defeminizing-masculinizing model. (A) Body weight gain of Sprague-Dawley female rats subcutaneously treated with different doses of testosterone enanthate 2x a week for 2 weeks: C: 0-sesame oil; DL1: 0.45; DL2: 0.95; DL3: 2.05 mg. (B) Food consumption of Sprague-Dawley female rats subcutaneously treated with different doses of testosterone enanthate 2x a week, for 2 weeks: CF: 0-sesame oil; DL1: 0.45; DL2: 0.95; DL3: 2.05 mg. Data are presented as mean ± standard deviation. Statistical significance: * p < 0.05 Mann-Whitney test. This figure has been modified from14. Please click here to view a larger version of this figure.

Figure 2: Hormone serum levels in the defeminizing-masculinizing model (dFM). Biochemical evaluation of hormones by ELISA assay of Sprague-Dawley female rats subcutaneously treated with different doses of testosterone enanthate 2x a week, for 2 weeks: C: 0-sesame oil; DL1: 0.45; DL2: 0.95; DL3: 2.05 mg and C MALE (control male) 0 mg. (A) testosterone, (B) estradiol. Data are presented as mean ± standard deviation. Statistical significance: * p < 0.05 Mann-Whitney test. This figure has been modified from14. Please click here to view a larger version of this figure.

Figure 3: Ovarian histopathological features. Ovary of Sprague-Dawley female rats subcutaneously treated 2x a week for 2 weeks with (A) 0-sesame oil Panel and (B) 2.05 mg of testosterone enanthate. Scale Bar 10 µm (original magnification: 20x; hematoxylin and eosin stain). This figure has been modified from14. Please click here to view a larger version of this figure.

Figure 4: Gene expression in the feminizing-masculinizing model. Gene expression analysis of (A) male-specific genes Cyp2c11 and Cyp3a18 and (B) female-specific genes Cyp2c6 and Cyp2c12 by real-time PCR in female rat livers subcutaneously treated with different doses of testosterone enanthate 2x a week, for 2 weeks: C 0-sesame oil, DL1 0.45, DL2 0.95, and DL3 2.05 mg. Data are presented as mean ΔΔCt values ± standard deviation, with control samples as calibrators and GAPDH as the reference gene. Statistical significance: * p < 0.05 Mann-Whitney test. This figure has been modified from14. Please click here to view a larger version of this figure.

Figure 5: Sperm count. (A) Sperm count of Sprague-Dawley male rats subcutaneously treated with estradiol valerate plus cyproterone acetate, 5x a week for 2 weeks: Control (C): 0-sesame oil, DL1: 0.045 + 0.2, DL2: 0.09 + 0.2 and DL3: 0.18 + 0.2 mg. Data are presented as mean ± standard deviation. Statistical significance: * p < 0.05 Mann-Whitney test. (B) Light microscopic photos of sperm count on Neubauer chamber (original magnification 10x; area of 1/16 mm2). This figure has been modified from33. Please click here to view a larger version of this figure.

Figure 6: General toxicity data in the demasculinizing-feminizing model. (A) Body weight gain (BW) of Sprague-Dawley male rats subcutaneously treated with estradiol valerate plus cyproterone acetate, 5x a week for 2 weeks: Control (C): 0-sesame oil, DL1: 0.045 + 0.2, DL2: 0.09 + 0.2 and DL3: 0.18 + 0.2 mg. (B) Feed consumption. Data are presented as mean ± standard deviation. Statistical significance: * p < 0.05 Mann-Whitney test. This figure has been modified from33. Please click here to view a larger version of this figure.
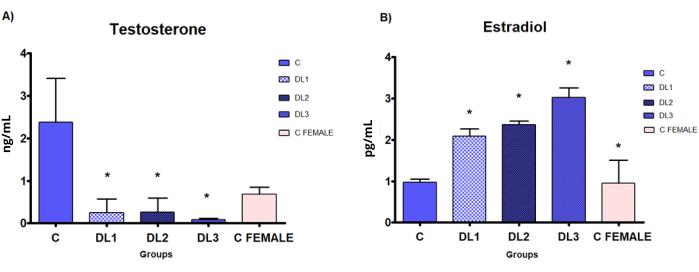
Figure 7: Hormone serum levels in the demasculinizing-feminizing model. Biochemical evaluation of hormones by ELISA assay of Sprague-Dawley male rats subcutaneously treated with estradiol valerate plus cyproterone acetate, 5x a week for 2 weeks: Control (C): 0-sesame oil, DL1: 0.045 + 0.2, DL2: 0.09 + 0.2 and DL3: 0.18 + 0.2 mg. (A) testosterone, (B) estradiol. Data are presented as mean ± standard deviation. Statistical significance: * p < 0.05 Mann-Whitney test. This figure has been modified from33. Please click here to view a larger version of this figure.
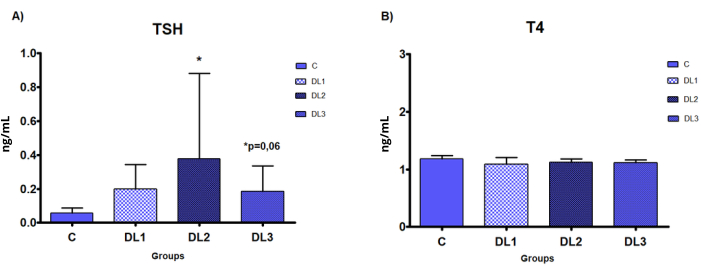
Figure 8: Thyroid biomarker serum levels in the demasculinizing-feminizing model. Biochemical evaluation of hormones by ELISA assay of Sprague-Dawley male rats subcutaneously treated with estradiol valerate plus cyproterone acetate, 5x a week for 2 weeks: Control (C): 0-sesame oil, DL1: 0.045 + 0.2, DL2: 0.09 + 0.2 and DL3: 0.18 + 0.2 mg. (A) TSH, (B) T4. Data are presented as mean ± standard deviation. Statistical significance: * p < 0.05 Mann-Whitney test. This figure has been modified from33. Please click here to view a larger version of this figure.

Figure 9: Testis histopathological features. Testis tubule degeneration with depletion of germ cells in Sprague-Dawley male rats subcutaneously treated with estradiol valerate plus cyproterone acetate, 5x a week for 2 weeks: Control (C): 0-sesame oil, DL1: 0.045 + 0.2, DL2:0.09 + 0.2 and DL3: 0.18 + 0.2 mg. Scale Bar 10 µm (original magnification 10x ; hematoxylin and eosin stain). This figure has been modified from33. Please click here to view a larger version of this figure.

Figure 10: Thyroid histopathological features. Thyroid hypertrophy in Sprague-Dawley male rats subcutaneously treated with estradiol valerate plus cyproterone acetate 5x a week for 2 weeks: Control (C): 0-sesame oil, DL1: 0.045 + 0.2, DL2: 0.09 + 0.2 and DL3: 0.18 + 0.2 mg. Scale bar 10 µm (original magnification 10x; hematoxylin and eosin stain). This figure has been modified from33. Please click here to view a larger version of this figure.

Figure 11: Gene expression in the demasculinizing-feminizing model. Gene expression analysis of (A) male-specific genes Cyp2c11; (B) Cyp3a18 and (C) female-specific genes Cyp2c12 and (D) Cyp2c6 by real-time PCR in male rats livers subcutaneously treated with different doses of estradiol plus cyproterone acetate, 5x a week for 2 weeks: Control (C): 0-sesame oil, DL1: 0.045 + 0.2, DL2: 0.09 + 0.2 and DL3: 0.18 + 0.2 mg. Data are presented as mean ΔΔCt values ± standard deviation, with control samples as calibrators and GAPDH as the reference gene. Statistical significance: * p < 0.05 Mann-Whitney test. This figure has been modified from14. Please click here to view a larger version of this figure.
Table 1: Hormone therapy for transgender man: route of administration, formulation, and dosage Please click here to download this Table.
Table 2: Hormone therapy for transgender women: route of administration, formulation, and dosage Please click here to download this Table.
Table 3: Hormone dose levels of defeminizing-masculinizing model. Dose levels selected for the dFM model in Sprague-Dawley female rats subcutaneously treated with different doses of testosterone enanthate 2x a week for 2 weeks: Control (C) 0-sesame oil; DL1 0.45, DL2 0.95 and DL3 2.05 mg. Please click here to download this Table.
Table 4: Hormone dose levels of demasculinizing-feminizing model. Dose levels selected for dMF model in Sprague-Dawley male rats subcutaneously treated with estradiol valerate plus cyproterone acetate, 5x a week for 2 weeks: Control (C): 0-sesame oil, DL1: 0.045 + 0.2, DL2: 0.09 + 0.2 and DL3: 0.18 + 0.2 mg. Please click here to download this Table.
Table 5: Primers forward and reverse design. Please click here to download this Table.
Table 6: Clitoral dimensions. Variation in clitoral diameter observed in millimeters (mm) of Sprague-Dawley female rats when different doses of testosterone enanthate 2x was subcutaneously delivered for a week for 2 weeks: C 0-sesame oil; DL1 0.45, DL2 0.95, and DL3 2.05 mg. Statistical significance: § p < 0.05 Fisher exact test; # p < 0.05 linear trend; * p < 0.05 Mann-Whitney test. n: number. SD: standard deviation. Please click here to download this Table.
Table 7:Absolute and relative weight of ovary and uterus. Ovary and uterus absolute and relative weight of Sprague-Dawley female rats subcutaneously treated with different doses of testosterone enanthate 2x a week for 2 weeks: C 0-sesame oil, DL1 0.45, DL2 0.95 and DL3 2.05 mg. Statistical significance: § p < 0.05 Fisher exact test; ## p < 0.01 linear trend; * p < 0.05 Mann-Whitney test. n: number. SD: standard deviation. This table has been modified from14. Please click here to download this Table.
Table 8:Histopathological data of the defeminizing-masculinizing model. Histopathological endpoints in target organs of Sprague-Dawley female rats subcutaneously treated with different doses of testosterone enanthate 2x a week for 2 weeks: C 0-sesame oil, DL1 0.45, DL2 0.95 and DL3 2.05 mg. Statistical significance: § p < 0.05 Fisher exact test; ## p < 0.01 linear trend; * p < 0.05 Mann-Whitney test. n: number. SD: standard deviation. This table has been modified from14. Please click here to download this Table.
Table 9:Absolute and relative weight of testes. The absolute and relative weight of testes of Sprague-Dawley male rats subcutaneously treated with estradiol valerate plus cyproterone acetate, 5x a week for 2 weeks: Control (C): 0-sesame oil, DL1: 0.045 + 0.2, DL2:0.09 + 0.2 and DL3: 0.18 + 0.2 mg. Statistical significance: * p < 0.05 Mann-Whitney test. N: sample number; SD: standard deviation. This table has been modified from33. Please click here to download this Table.
Table 10: Histopathological data of the demasculinizing-feminizing model. Histopathological data of testes and thyroid of Sprague-Dawley male rats subcutaneously treated with estradiol valerate plus cyproterone acetate, 5x a week for 2 weeks: Control (C): 0-sesame oil, DL1: 0.045 + 0.2, DL2:0.09 + 0.2 and DL3: 0.18 + 0.2 mg. Statistical significance: § p < 0.05 Fisher exact test; ° p = 0.08, * p < 0.05 Mann-Whitney test; ## p < 0.01 Cochran-Armitage Trend Test. N: sample number; SD: standard deviation. This table has been modified from33. Please click here to download this Table.
Discussion
The implementation of rodent models mimicking GAHT is crucial to studying the potential specific susceptibility and vulnerability of TG people and the long-term outcomes of the therapies, usually lasting all their lives.
Given the scarce number of similar studies in the literature, the critical point of this experiment is the selection of the doses to set the models; such doses should be sufficiently low to be compatible with long-term administration in animals without causing adverse effects, toxicity, and/or death. Another critical point is the best route of administration to adopt, taking into account the preferred route in humans. Indeed, although in the dFM model, the T is administered according to the clinical practice in TG men15In the dMF model, the route of administration did not overlap with the clinical practice in TG women15. Further experiments are needed to establish a more reliable method. In fact, the two rat models are designed to mimic as much as possible the human treatments. They provide pivotal data on the impact of both GAHTs on several body functions, including reproduction, thyroid homeostasis and liver metabolism, filling part of the existing gap.
Several additional measures can be applied to improve the two models and make them even more robust and transferable to humans. For example, using subcutaneous administration through the implantation of polymer reservoirs that release E2 or T in a controlled daily manner, which closely mimics the hormone-release patches widely used in clinical settings today15. Additionally, extending the duration of the treatment can better simulate the chronic exposure of patients who choose to undergo GAHTs.
Special mention should be made concerning the expression of sex-specific hepatic CYPs, which are used to evaluate the success of the therapies. In fact, this parameter has been set and developed for the first time in the present work and represents a valuable tool to confirm the implementation of the models.
Finally, further studies are needed to evaluate the reversibility of reproductive perturbations due to GAHT to improve the fertility preservation of TG people who decide to have children38.
Disclosures
The authors declare that the research was conducted without any commercial or financial relationships that could potentially create a conflict of interest.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| Analytical balance ABJ 320-4NM | Kern | Z741091 | |
| Bouin | Biooptica | 05-M01008 | |
| Centrifuge 5415 R | Eppendorf | For eppendorf | |
| Cyproterone Acetate | Sigma-Aldrich | C3412 | |
| D-MEM medium | Gibco | ||
| ExcelTaq 2X Fast Q-PCR Master Mix (SYBR, ROX), 200 RXN | Smobio | TQ1210 | |
| Formalin solution, neutral buffered, 10% | Sigma-Aldrich | HT501128 | |
| GraphPad Prism software version 5.0 for Windows | GraphPad Software | ||
| Hematoxylin | Biooptica | 05-06002/L | |
| Heosin | Biooptica | 05-10007/L | |
| Imaging Software | Nikon | NIS-BR | |
| JMP 10 statistical software | SAS Institute | ||
| Microm | Thermo Scientific | HM 325 | |
| Microscopy | Nikon Microphot FX | ||
| Mouse/Rat Testosterone ELISA | Biovendor | RTC001R | 96T |
| NanoDrop 1000 Spectrophotometer | Thermo Scientific | ND-1000 | |
| Paraffina | Biooptica | 087910 | |
| Portable Balances SCOUT STX2202 | OHAUS | 30253064 | |
| Primers | Life Technologies | Designed by PrimerBlast | |
| Rat Estradiol ELISA | Biovendor | RTC009R | 96T |
| Rat TSH(Thyroid Stimulating Hormone) ELISA Kit | ELK Biotechnology | ELK2283 | 96T |
| Rat TSH(Thyroid Stimulating Hormone) ELISA Kit | ELK Biotechnology | ELK2283 | 96T |
| SensiFASTcDNA Synthesis Kit | Bioline | BIO-65053 | 50 reaction |
| Sesam Oil | ACROS | AC241000010 | |
| Sprague Dawley rats male and female | Envigo | 8/9 weeks old | |
| Standard diets | Mucedola | 4RF18 | |
| T4(Thyroxine) ELISA Kit | ELK Biotechnology | ELK8716 | 96T |
| Testosterone enanthate | Sigma-Aldrich | T3006 | |
| Thermal Cycler LineGene 9600 Plus Bioer | Aurogene | ||
| Tissue processor | Shandon Excelsior ES, Thermo Scientific) | ||
| Total RNA purification KIT | Norgen | 17200 | 50 column |
| Victor 3 Multilabel reader | Perkin Elmer | ||
| β-Estradiol 17-valerate | Sigma-Aldrich | E1631 |
References
- Rokach, A., Patel, K. . Human sexuality: Function, dysfunction, paraphilias, and relationships. , (2021).
- American Psychological Association. Guidelines for psychological practice with transgender and gender nonconforming people. Am Psychol. 70 (9), 832-864 (2015).
- Ammari, T., Sluiter, E. C., Gast, K., Kuzon, W. M. Female-to-male gender-affirming chest reconstruction surgery. Aesthet Surg J. 39 (2), 150-163 (2019).
- Garg, G., Elshimy, G., Marwaha, R. . Gender Dysphoria. , (2023).
- Chan, A. S. W., Wu, D., Lo, I. P. Y., Ho, J. M. C., Yan, E. Diversity and inclusion: Impacts on psychological wellbeing among lesbian, gay, bisexual, transgender, and queer communities. Front Psychol. 13, 726343 (2022).
- Unger, C. A. Hormone therapy for transgender patients. Transla Androl Urol. 5 (6), 877 (2016).
- Sofer, Y., et al. Gender‐affirming hormone therapy effect on cortisol levels in trans males and trans females. Clin Endocrinol. 100 (2), 164-169 (2024).
- Pirtea, P., Ayoubi, J. M., Desmedt, S. T. &. #. 8. 2. 1. 7. ;., Sjoen, G. Ovarian, breast, and metabolic changes induced by androgen treatment in transgender men. Fertil Steril. 116 (4), 936-942 (2021).
- Tassinari, R., Maranghi, F. Rodent model of gender-affirming hormone therapies as specific tool for identifying susceptibility and vulnerability of transgender people and future applications for risk assessment. Int J Environ Res Public Health. 18 (23), 12640 (2021).
- Schug, T. T., Janesick, A., Blumberg, B., Heindel, J. J. Endocrine disrupting chemicals and disease susceptibility. J Steroid Biochem Mol Biol. 127 (3-5), 204-215 (2011).
- van Larebeke, N., Fucic, A. . Challenges in endocrine disruptor toxicology and risk assessment. , (2020).
- You, H. H., Song, G. Review of endocrine disruptors on male and female reproductive systems. Comp Biochem Physiol C Toxicol Pharmacol. 244, 109002 (2021).
- Sifakis, S., Androutsopoulos, V. P., Tsatsakis, A. M., Spandidos, D. A. Human exposure to endocrine disrupting chemicals: effects on the male and female reproductive systems. Environ Toxicol Pharmacol. 51, 56-70 (2017).
- Tassinari, R., et al. Risk assessment of transgender people: Development of rodent models mimicking gender-affirming hormone therapies and identification of sex-dimorphic liver genes as novel biomarkers of sex transition. Cells. 12 (3), 474 (2023).
- T’Sjoen, G., Arcelus, J., Gooren, L., Klink, D. T., Tangpricha, V. Endocrinology of transgender medicine. Endo Rev. 40 (1), 97-117 (2019).
- Dhir, R. N., Shapiro, B. H. Interpulse growth hormone secretion in the episodic plasma profile causes the sex reversal of cytochrome P450s in senescent male rats. Proc Natl Acad Sci. 100 (25), 15224-15228 (2003).
- Robertson, G. R., Farrell, G. C., Liddle, C. Sexually dimorphic expression of rat CYP3A9 and CYP3A18 genes is regulated by growth hormone. Biochem Biophys Res Comm. 242 (1), 57-60 (1998).
- Kinnear, H., et al. A mouse model to investigate the impact of testosterone therapy on reproduction in transgender men. Human Reprod. 34 (10), 2009-2017 (2019).
- Gómez, &. #. 1. 9. 3. ;., et al. Effects of adult male rat feminization treatments on brain morphology and metabolomic profile. Hormones Behav. 125, 104839 (2020).
- T'Sjoen, G., et al. European society for sexual medicine position statement "Assessment and hormonal management in adolescent and adult trans people, with attention for sexual function and satisfaction". J Sex Med. 17 (4), 570-584 (2020).
- Coleman, E., et al. Standards of care for the health of transgender and gender diverse people, version 8. Int J Transgender Health. 23 (sup 1), S1-S259 (2022).
- Sudhakar, D., Huang, Z., Zietkowski, M., Powell, N., Fisher, A. R. Feminizing gender‐affirming hormone therapy for the transgender and gender diverse population: An overview of treatment modality, monitoring, and risks. Neurourol Urodynamics. 42 (5), 903-920 (2023).
- Turner, P. V., Brabb, T., Pekow, C., Vasbinder, M. A. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Laboratory Animal Sci. 50 (5), 600-613 (2011).
- Hickman, D. L. Minimal exposure times for irreversible euthanasia with carbon dioxide in mice and rats. J Am Assoc Laboratory Animal Sci. 61 (3), 283-286 (2022).
- Vdoviaková, K., et al. Importance rat liver morphology and vasculature in surgical research. Med Sci Monit. 22, 4716 (2016).
- Fiette, L., Slaoui, M. Necropsy and sampling procedures in rodents. Methods Mol Biol. 691, 39-67 (2011).
- Shackelford, C., Long, G., Wolf, J., Okerberg, C., Herbert, R. Qualitative and quantitative analysis of nonneoplastic lesions in toxicology studies. Toxicol Pathol. 30 (1), 93-96 (2002).
- Tassinari, R., et al. short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: focus on reproductive and endocrine systems and spleen. Nanotoxicology. 8 (6), 654-662 (2014).
- Fortune, J. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Animal Reprod Sci. 78 (3-4), 135-163 (2003).
- Maranghi, F., et al. Effects of the food contaminant semicarbazide following oral administration in juvenile Sprague-Dawley rats. Food Chem Toxicol. 47 (2), 472-479 (2009).
- Rasinger, J., et al. Low dose exposure to HBCD, CB-153 or TCDD induces histopathological and hormonal effects and changes in brain protein and gene expression in juvenile female BALB/c mice. Reprod Toxicol. 80, 105-116 (2018).
- Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29 (9), e45 (2001).
- Tammaro, A., et al. Risk assessment of transgender people: implementation of a demasculinizing–feminizing rodent model including the evaluation of thyroid homeostasis. Biology Direct. 19 (1), 5 (2024).
- Falvo, R. E., Kaltenbach, C. C., Pancoe, W. L. Determination of testosterone concentration in the plasma of normal and androgen-sterilized female rats, using a competitive protein binding technique. Neuroendocrinology. 10 (4), 229-234 (1972).
- Gibbs, R. B. Testosterone and estradiol produce different effects on cognitive performance in male rats. Horm Behav. 48 (3), 268-277 (2005).
- Ström, J. O., Theodorsson, A., Ingberg, E., Isaksson, I. M., Theodorsson, E. Ovariectomy and 17β-estradiol replacement in rats and mice: a visual demonstration. J Vis Exp. (64), e4013 (2012).
- Cooke, P. S., Nanjappa, M. K., Ko, C., Prins, G. S., Hess, R. A. Estrogens in male physiology. Physiol Rev. 97 (3), 995-1043 (2017).
- Raja, N. S., Rubin, E. S., Moravek, M. B. A Review of animal models investigating the reproductive effects of gender-affirming hormone therapy. J Clin Med. 13 (4), 1183 (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved