Method Article
In Vivo Confocal Fluorescence Imaging of Neural Activity Induced by Sensory Stimulation in Partially Restrained Larval Zebrafish
摘要
Here, we present a detailed protocol to examine neural activity in brain regions of transgenic zebrafish that express GCaMP calcium indicators using confocal microscopy.
摘要
Zebrafish larvae are a promising vertebrate model system for studying the neural mechanisms of behavior. Their translucence and relatively simple neural circuitry facilitate the use of optogenetic techniques in cellular analyses of behavior. Fluorescent indicators of in vivo neural activity, such as GCaMP6s, have been widely used to study the neural activity associated with simple behaviors in larval zebrafish. Here, we present a protocol for detecting sensory-induced activity in semi-restrained zebrafish larvae using the transgenic line Tg(elav3:GCaMP6s). In particular, we use the chemical agent allyl isothiocyanate to induce a robust, reproducible fluorescent response in a brain region at the border of the hindbrain and spinal cord. We discuss the potential uses of GCaMP6s for optical monitoring of neural activity during a range of behavioral paradigms and the limitations of this technique. Our protocol outlines an accessible approach for monitoring dynamic, behavior-related in vivo neural activity in the larval zebrafish brain.
引言
Zebrafish represent a vertebrate animal model with tractability for detailed cellular-molecular neurobiological investigations. Larval zebrafish possess ~100,000 neurons at 5 days post fertilization (dpf), significantly less than mammalian brains. Furthermore, zebrafish are relatively translucent, a property that facilitates optical studies of neural structure and function1,2,3,4,5. Several optogenetic tools have been developed for use in zebrafish, including high-fidelity calcium indicators6, voltage sensors7,8, and activity-dependent markers of neural activity9,10,11,12,13. These tools are complementary to other advantages possessed by this model, such as amenability to genetic modifications14,15,16,17 and the readiness with which zebrafish larvae absorb chemicals present in bathing solutions18,19,20,21.
A variety of methods are useful for zebrafish optical physiology, particularly two-photon, light sheet, and confocal microscopy. Each of these technologies must balance two related problems of resolution: optical access, including light scattering by surrounding tissue, and sampling speed, especially for capturing action potential kinetics at the sub-millisecond scale22. There have been dramatic improvements in in vivo calcium imaging using two-photon microscopy, but this method is often restricted to a field of view of <1 mm2, and typically, only a single plane of depth can be acquired, thus limiting capture of activity across large regions of neural circuitry22. For light sheet microscopy, the potential to record the activity of almost all neurons in the brain resolves the field-of-view limitation of two-photon microscopy, but current camera speeds physically limit capture to roughly three brain volumes per second at 40 planes per brain volume in the larval zebrafish1,23. Confocal microscopy is inferior in both depth resolution and capture speed to two-photon and light sheet microscopy. Confocal microscopy has the advantages of widespread accessibility to laboratories worldwide and the capacity to achieve whole-brain reconstructions of neural activity using reporters of neural activity, such as cFos and p-ERK9. Furthermore, if small brain regions are targeted, the confocal microscope can provide adequate temporal resolution of neural activity.
The present paper describes a method that uses confocal microscopy to record neural activity in transgenic zebrafish expressing GCaMP6s pan-neuronally. Several similar protocols using zebrafish larvae have been developed to understand the function of neural pathways24,25,26,27,28,29. Key features of several of these protocols, such as time-lapse imaging, fluorescent indicators of calcium dynamics, and live imaging, have been combined to measure neural activity in a small population of neurons in the zebrafish central nervous system in response to allyl isothiocyanate (AITC), an aversive chemical irritant11,26,27,29,30,31. AITC elicits a brain-wide response focused in the hindbrain area11. One cluster of neurons just caudal to the hindbrain has a role in locomotion and a prolonged response to AITC. This response outlasts the removal of the aversive stimulus30. By restricting the field of view, we have succeeded in detecting neural activity in this neural cluster as reflected by the fluorescence change in neurons expressing GCaMP6s. We provide techniques, guidelines, and best practices to achieve sufficient spatiotemporal resolution using confocal microscopy. In addition, we discuss the limitations of our optical recording method. Despite these limitations, the method should permit the investigation of a variety of neurobiological phenomena, including memory and sensorimotor processing.
研究方案
All procedures using animals were approved by the Institutional Animal Care Use Committee at California State University, Fullerton (Protocol # 2023-1310).
1. Staging larval zebrafish in low-melting point agarose
- Breed adult transgenic animals of pan-neuronally expressing Tg(elav3:GCaMP6s)6 with two males to two females in accordance with institutional animal care guidelines. Adults are placed in special breeding chambers so that released eggs are captured in a lower chamber with a false bottom that is not accessible to adults. At first light or when a separation divider is removed, females release eggs, and males fertilize eggs captured in the false bottom.
- Remove adults and collect fertilized eggs using an egg strainer and place in embryo (E3) medium (5 mM NaCl, 0.33 mM MgSO4, 0.33 mM CaCl2, 0.17 mM KCl, 1 mM HEPES, 0.00001% methylene blue, pH 7.0)30 with 14 h light/10 h dark at 28.5 °C inside an incubator
NOTE: The E3 recipe used has a low concentration of methylene blue that does not impact fluorescence or lead to any significant auto-fluorescence signal. We do not require the use of 1-phenyl 2-thiourea (PTU) at the developmental ages specified in the protocol due to the natural translucence afforded by the GCaMP6s transgenic line. - Mount animals 2-7 days post-fertilization (dpf) using 3% low melting point agarose dissolved in E3 medium. Heat agarose to a temperature at which the zebrafish can be manipulated without damage to the organism. Before the low melting point agarose cools and solidifies (<1 min), position larvae dorsal side up in a Petri dish with a glass bottom so that the brain region to be inspected is as close to the objective as possible, as shown in Figure 1. Depending on the experiment, typically, 8 to 10 zebrafish per experimental group is sufficient.
NOTE: Animals were not anesthetized in this protocol. - After the agarose has set with the larva in place (approximately 2 to 3 min), add 5 mL of E3 solution. Then, cut the agarose with a scalpel as required by the experimental protocol.
NOTE: Different parts of the agar should be cut away, depending on what portions of the zebrafish need to be freed from restraint and/or receive a different type of stimulus. - Move the Petri dish containing the zebrafish to the confocal microscope stage and allow the fish to acclimate for 20 min.
2. Setting up and imaging under confocal microscopy with stimulus application
- After the acclimation period, center the fish under the 40x (NA 1.0) water immersion objective at room temperature using a brightfield with a light source (~475 nm).
- Confocal microscope acquisition settings will depend upon the qualities and expression level of the fluorescent molecule, the depth and optical properties of the tissue, the size of the field of view, and the recording speed. Determine appropriate settings using the range indicator to ensure optimal capture of emitted light according to experimental needs.
- Ensure proper adjustment of the laser power and master gain to capture fluorescent light in the optimal range, thereby preventing unnecessary loss of the GCaMP6s-dependent fluorescence signal. Use the list of key confocal settings and factors given below to determine the appropriate settings.
- Pinhole size: Determine this by the objective used and set to accept a single focal plane while restricting other focal planes. For this protocol, a pinhole of 32 µm is used.
- Laser power and wavelength: Adjust laser power based on the expression level of the fluorescent molecule and the depth and optical properties of the tissue. Use the range indicator to ensure the laser does not exceed the range of the photodetector. Set the excitation wavelength to the wavelength of light that optimally excites the fluorescent molecule. For this experiment, because the fish were heterozygous for the GCaMP6s transgene and expression levels were low, use 5% laser power at 488 nm wavelength, 650 master gain. With strong transgene expression, laser power ranges from 1.5% to 3%, which is usually sufficient.
NOTE: If the laser power is set too low, information (light detected by the photomultiplier tube) will be lost. If the laser power is set too high, the emitted light might exceed the range of the detector or damage the sample (photobleach). - Master gain: The master gain determines the sensitivity of the photodetector. Use the range indicator to ensure the master gain is not set too high, thereby exceeding the range of the photodetector. For this experiment, the master gain is set to 650.
- Field of view: Ensure the field of view is sufficiently large to capture the tissue of interest. This is a major limitation for confocal microscopes generally because the size of the field of view will limit the speed of capture, as shown in Figure 2. For the experiments involving AITC, set the field of view to 79.86 µm2.
- Speed of capture: As the capture speed is increased, the amount of light captured by the photodetector will be reduced. As less light is captured, the image quality will be degraded. Ensure capture speed balances the quality of the image and the speed required to capture the physiological event of interest. Here, the capture speed is set to 1.20 f/s.
- Center the field of view on a neuronal cluster caudal to the commissura infima Halleri in the rostral portion of the spinal cord30. This cluster is quite small at ~2.5 dpf but is much larger later in development (7 dpf).
NOTE: If agarose mounting in step 1 is suboptimal, that is, the animal is positioned at an angle or too deeply in the agarose, the image quality will be adversely affected. An image of the zebrafish spinal cord in a well-placed animal and a contrasting image of the spinal cord in a suboptimally placed zebrafish are shown in Figure 3. - Conduct a time series scan using a field of view of 79.86 µm2 at 1.20 frames per s (fps) with a spatial resolution of 0.119 µm2 per pixel. Adjust the size of the field of view and speed of image acquisition according to the desired application.
- At 2 min after the onset of imaging, add 41.67 µL of either a stock solution of AITC (10 µM final concentration) or E3 solution to the dish with a pipette. Continue recording for ~30 s to observe any changes in the GCaMP6s activity in the region of interest with time-lapse imaging. After the recording is completed, free the animal from the agarose and euthanize it as per institutional animal care guidelines.
NOTE: While recording GCaMP6s activity, the confocal light itself can be a stimulus. This must be considered in the analysis of the imaging results. The recording time is a function of the number of frames and the capture rate of each frame, which can be adjusted as necessary for the desired time.
3. Analysis of GCaMP signal using FIJI
- After recording, if the output is not in the .tif file format for downstream FIJI analysis, export the image files as .tif files from the microscope software suite.
- Download FIJI for analysis of neural traces. Open FIJI, import the .czi files into the program, and when prompted, use the default settings in the BioFormats plugin.
- Highlight an area/neuron of interest using either the Circle or Freehand tools by clicking on Their Respective Icons. (Hovering over the icons will flash the name of the tool in the program.)
NOTE: Because the area of interest varies in size, fixed areas/circles are not possible. Software that allows for registration of fluorescent changes over time as voxels, such as the Thunder package32, could allow for the detection of individual neurons; however, for the ~20 neurons in the current experiment, detection of the membrane borders by a blind observer would be likely to be superior to an automated detection system. - After an ROI has been selected, in the FIJI toolbar, click Analyze > Tools > ROI Manager. After that, click Add [t], and the selected ROI will be added to the ROI Manager window.
- Within the ROI Manager, click More > Multi Measure. Leave the settings at default and click OK. FIJI will produce raw data that can be manipulated as a CSV file using Python or as raw data in a spreadsheet.
- To normalize the raw data and represent it as a neural trace for a given region of interest, average the last ~30 s values of the 2 min window before the stimulus, and normalize all post-stimulus values to that average baseline value:
normalized fluorescence intensity = (instantaneous fluorescence of a given region of interest post-stimulus)/(average fluorescence of ~30 s of a 2 min interval pre-stimulus) - Plot the data as neural traces, as shown in Figure 4.
NOTE: In some types of microscopies, such as light sheet microscopy, where a volume of the brain is recorded, movement artifacts are addressed via post-processing spatial registration to a reference brain volume1,11. However, because we are only recording from a single plane, no adjustments can be made for movement artifacts. Therefore, for the results shown here, no frames were removed. However, should movement artifacts significantly alter the results, automated systems could identify and correct frames with movement artifacts1, 11, or a blinded observer could remove such frames.
结果
Administration of allyl isothiocyanate causes a calcium-associated neural signal in larval zebrafish
The administration of AITC (step 2.6) causes a widespread increase in GCaMP6s-associated neural activity across the brain of the larval zebrafish11,30. We observed an increased fluorescent signal in a small region of the brain after applying AITC, as shown in Figure 4.
Balancing resolution of calcium-associated neural signals, capture speed, and area of capture
Increasing capture speed reduces the resolution, which must be considered in experimental design. We present a variety of speeds and their corresponding resolutions using zebrafish embryos at ~2.5 dpf in Figure 5. When recording neural activity, the capture speed needs to be sufficiently fast to record the activity. Although the voltage changes due to an action potential are relatively fast (1-2 ms), calcium interactions with GCaMP will prolong the duration of the action potential signal. GCaMP6s, the molecule used here, has particularly slow kinetics. Single action potentials using GCaMP molecules can be detected and correlated in cell culture6, but it is unclear if this precision of signal detection could occur in vivo with larval zebrafish. However, the higher the capture speed, the more likely it is that neural activity-related temporal information will be captured, assuming signal detection is not significantly reduced by the higher capture speeds. Furthermore, the size of the field of observation will also influence capture speeds with a standard confocal microscope. By reducing the field of view, one increases capture speed but loses information about neural activity in other brain areas. To capture GCaMP6 signals, applications using confocal microscopy must be limited to a small brain area (50-150 µm2). The capture speed should be faster than the signal being observed to reduce aliasing. However, the capture speeds of confocal microscopy will inevitably result in the loss of some information in the form of emitted light. These relative costs and benefits must always be considered to address the desired experimental question effectively.

Figure 1: Depiction of the position of a larval zebrafish partially restrained for imaging. The larva is embedded in a low melting point agarose and positioned dorsal side up. Rostral and caudal portions of the agarose were removed to allow the zebrafish skin to be exposed to AITC. Please click here to view a larger version of this figure.
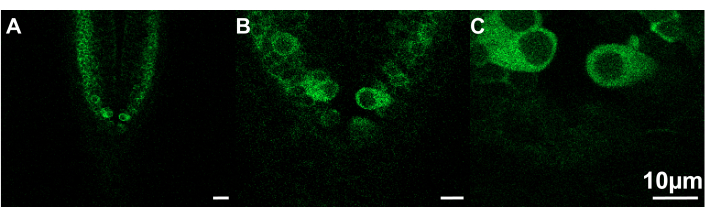
Figure 2: Examples of images of different sizes that can be viewed at different capture speeds. (A) Frame size = 319.45 x 319.45 µm; capture speed =.30 fps. (B) Frame size = 106.48 x 106.48 µm; capture speed = 7.67 fps. (C) Frame size = 53.24 x 53.24 µm; capture speed = 9.80 fps. Zebrafish, ~2.5 dpf; scale bar 10 µm. Please click here to view a larger version of this figure.

Figure 3: Confocal images of the caudal portion of the hindbrain of two zebrafish larvae. (A) The larva (~2.5 dpf) is positioned at an unideal angle and too deeply in the agarose, preventing crisp imaging of the neurons. (B) Here, the larva (~2.5 dpf) is properly positioned near the surface of the agarose. Scale bar 20 µm. Please click here to view a larger version of this figure.

Figure 4: AITC increases the fluorescence intensity of neurons located at the border of the hindbrain and spinal cord of the zebrafish brain. (A) Representative confocal images of zebrafish (7 dpf) neurons when exposed to AITC or E3. Images of neurons before (A1) and after (A2) application of AITC compared to (B) images of neurons before (B1) and after (B2) application of the control solution (E3). Scale bar 10 µM. Traces of the normalized fluorescent state of several neurons located in the hindbrain of embryonic zebrafish exposed to AITC (A3) or E3 (B3). Note that the neurons exhibited increased fluorescence after applying AITC to the bath, whereas increased fluorescence was not observed in the neurons after applying E3. Please click here to view a larger version of this figure.

Figure 5: Capture speed of GCaMP6s determines the amount of light captured and the resulting resolution of the image. (A) Images of neurons captured every 10.13 s (0.10 fps) give a relatively high resolution of neurons in the hindbrain and spinal cord. With an increase in capture speed, temporal information is gained, but spatial resolution is lost: (B) capture speed = 0.79 fps, (C) capture speed = 3.16 fps, and (D) capture speed = 7.67 fps. Zebrafish, 2 dpf; scale bar 20 µm. Please click here to view a larger version of this figure.
讨论
We have shown that neural activity can be recorded in the brains of zebrafish larvae using GCaMP6s together with confocal microscopy; the lower capture speeds required due to the slower kinetics of GCaMPs can be compensated for by reducing the brain area observed6. Reporters with faster temporal dynamics (i.e., GCaMP6f) are available, but the superior temporal resolution usually comes at the cost of reduced fluorescence signal6. The confocal microscope is limited to relatively slower recording speeds22, so reporter molecules should be selected with consideration of the temporal limitations of confocal microscopy.
The protocol is limited by two key features: 1) the requirement for the animal to be placed in agarose during recording and 2) the relatively slow capture speed of confocal microscopy. Balancing the field of view and capture speeds is key to capturing neural activity with sufficient resolution for a given application. If AITC-induced activity is truncated or diminished, this could indicate that the larval zebrafish may not be able to detect the stimulus or that the AITC may be insufficiently potent. In the latter case, refreshing the stock and keeping it frozen at -20 °C will extend its efficacy. If the fish cannot detect the stimulus, the animal may have been implanted too deeply in the agar. A critical step is the positioning of a fish during Step 1 of the protocol, as this is crucial for optimal optical access during the application of the drug.
Due to the relatively slow scan speeds of the confocal microscopy, whole-brain recordings are not technically possible. Significantly, the protocol outlined here can be used to measure small brain areas after identification with complementary techniques. In particular, by using techniques that leverage molecules that mark neural activity with activity-dependent reporters9,11, the experimenter can target key brain areas of interest for the application of the methods outlined in this article. Brain areas of interest can then be referenced to the annotated zebrafish brain atlas, z brain atlas11.
With minor adjustments, the techniques and guidelines discussed here can be employed to investigate a range of brain functions, including sensorimotor processing, learning and memory, and sensation and perception.
披露声明
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
致谢
This work was supported by a grant to ACR from the National Institutes of Health (SC2GM1304854) and a grant to DLG from the National Science Foundation (2050850).
材料
| Name | Company | Catalog Number | Comments |
| Low Melting Point Agarose | Invitrogen | 16520-100 | Diluted to 3% |
| Allyl Isothiocyanate (AITC) | Sigma Aldrich | 377430 | Chemical stimulant |
| E3 | N/A | N/A | Water-medium for zebrafish larvae |
| Glass Bottom Dishes | Thermo Fisher Scientific | 12-567-400 | Used to hold zebrafish during imaging experiments |
| Micropipette (10-100 uL) | Cole-Parmer | 21600-14 | Apparatus used for creating AITC dilutions |
| Microscope Slides | Fisherbrand | 12-550-A3 | Used to screen for phenotype |
| Mirror Finish Forceps | DUMONT | 11251-23 | Used to orient zebrafish in agarose |
| myTEMP Mini Digital Incubators | Benchmark | H2200-HC | Holding area for zebrafish; set to 28.5°C |
| Nitrile Gloves | MedPRIDE | MPR-50504 | Basic PPE |
| Petri Dishes | VWR | 89107-632 | Container for zebrafish |
| Posi-Click Tubes | DENVILLE | C-2171 | Used for AITC dilution |
| Samco Polyurethane Transfer Pipettes | Thermo Fisher Scientific | 225 | Apparatus used to select animal/administer diluted bolus of AITC |
| Stemi SV11 Apo Microscope | Zeiss | 1.25496E+11 | Used to stage zebrafish |
| Transgenic Larval Zebrafish (2 to 7 DPF) | N/A | N/A | Animal test subjects; Tg(elav3:GCaMP6s) strain |
| Zeiss Confocal Microscope (Model LSM9) | Zeiss | 3523004097 | Imaging of fish |
参考文献
- Ahrens, M. B., Orger, M. B., Robson, D. N., Li, J. M., Keller, P. J. Whole-brain functional imaging at cellular resolution using light-sheet microscopy. Nat Method. 10 (5), 413-420 (2013).
- Meyer, M. P., Smith, S. J. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J Neurosci. 26 (13), 3604-3614 (2006).
- Sagasti, A., Guido, M. R., Raible, D. W., Schier, A. F. Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Curr Biol. 15 (9), 804-814 (2005).
- Son, J. H., et al. Transgenic FingRs for live mapping of synaptic dynamics in genetically-defined neurons. Sci Rep. 6, 18734 (2016).
- Zada, D., Tovin, A., Lerer-Goldshtein, T., Vatine, G. D., Appelbaum, L. Altered behavioral performance and live imaging of circuit-specific neural deficiencies in a zebrafish model for psychomotor retardation. PLoS Genet. 10 (9), e1004615 (2014).
- Chen, T. W., et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature. 499 (7458), 295-300 (2013).
- Abdelfattah, A. S., et al. and photostable chemigenetic indicators for extended in vivo voltage imaging. Science. 365 (6454), 699-704 (2019).
- Abdelfattah, A. S., et al. Sensitivity optimization of a rhodopsin-based fluorescent voltage indicator. Neuron. 111 (10), 1547-1563.e9 (2023).
- Gao, Y. J., Ji, R. R. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury. Open Pain J. 2, 11-17 (2009).
- Kawashima, T., Okuno, H., Bito, H. A new era for functional labeling of neurons: activity-dependent promoters have come of age. Front Neural Circuits. 8, 37 (2014).
- Randlett, O., et al. Whole-brain activity mapping onto a zebrafish brain atlas. Nat Methods. 12, 1039-1046 (2015).
- Reijmers, L. G., Perkins, B. L., Matsuo, N., Mayford, M. Localization of a stable neural correlate of associative memory. Science. 317 (5842), 1230-1233 (2007).
- Mohr, M. A., Argast, P., Pantazis, P. Labeling cellular structures in vivo using confined primed conversion of photoconvertible fluorescent proteins. Nature Protoc. 11 (12), 2419-2431 (2016).
- Arrenberg, A. B., Del Bene, F., Baier, H. Optical control of zebrafish behavior with halorhodopsin. Proc Natl Acad Sci U S A. 106 (42), 17968-17973 (2009).
- Wyart, C., Del Bene, F. Let there be light: zebrafish neurobiology and the optogenetic revolution. Rev Neurosci. 22 (1), 121-130 (2011).
- Douglass, A. D., Kraves, S., Deisseroth, K., Schier, A. F., Engert, F. Escape behavior elicited by single, channelrhodopsin-2-evoked spikes in zebrafish somatosensory neurons. Curr Biol. 18 (15), 1133-1137 (2008).
- Portugues, R., Severi, K. E., Wyart, C., Ahrens, M. B. Optogenetics in a transparent animal: circuit function in the larval zebrafish. Curr Opinion Neurobiol. 23 (1), 119-126 (2013).
- Best, J. D., Alderton, W. K. Zebrafish: An in vivo. model for the study of neurological diseases. Neuropsychiat Dis Treatment. 4 (3), 567-576 (2008).
- Goldsmith, P. Zebrafish as a pharmacological tool: the how, why and when. Curr Opinion Pharmacol. 4 (5), 504-512 (2004).
- Roberts, A. C., et al. Habituation of the C-start response in larval zebrafish exhibits several distinct phases and sensitivity to NMDA receptor blockade. PloS One. 6 (12), e29132 (2011).
- Wolman, M. A., Jain, R. A., Liss, L., Granato, M. Chemical modulation of memory formation in larval zebrafish. Proc Natl Acad Sci U S A. 108 (37), 15468-15473 (2011).
- Ji, N., Freeman, J., Smith, S. L. Technologies for imaging neural activity in large volumes. Nat Neurosci. 19 (9), 1154-1164 (2016).
- Panier, T., et al. Fast functional imaging of multiple brain regions in intact zebrafish larvae using selective plane illumination microscopy. Front Neural Circuits. 7, 65 (2013).
- Distel, M., Köster, R. W. In vivo time-lapse imaging of zebrafish embryonic development. CSH Protoc. 2007, (2007).
- Liu, T. T., Hou, H., Du, J. L. A protocol for simultaneous Ca2+ and morphology imaging of brain endothelial tip cells in larval zebrafish. STAR Protoc. 2 (1), 100388 (2021).
- Wong, H. C., Drerup, C. M. Using fluorescent indicators for in vivo quantification of spontaneous or evoked motor neuron presynaptic activity in transgenic zebrafish. STAR Protoc. 3 (4), 101766 (2022).
- Graeden, E., Sive, H. Live imaging of the zebrafish embryonic brain by confocal microscopy. J Vis Exp. (26), e1217 (2009).
- Hirsinger, E., Steventon, B. A versatile mounting method for long term imaging of zebrafish development. J Vis Exp. (119), e55210 (2017).
- Renaud, O., Herbomel, P., Kissa, K. Studying cell behavior in whole zebrafish embryos by confocal live imaging: application to hematopoietic stem cells. Nat Protoc. 6 (12), 1897-1904 (2011).
- Roberts, A. C., et al. Induction of short-term sensitization by an aversive chemical stimulus in zebrafish larvae. eNeuro. 7 (6), (2020).
- Prober, D. A., et al. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J Neurosci. 28 (40), 10102-10110 (2008).
- Freeman, J., et al. Mapping brain activity at scale with cluster computing. Nat Methods. 11, 941-950 (2014).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。