Method Article
Intravital Microscopy to Study Platelet-Leukocyte-Endothelial Interactions in the Mouse Liver
摘要
Intravital microscopy is a powerful tool that provides insight into both the temporal and spatial relationships of rapid and/or sequential processes. Herein, we describe a protocol to assess both protein-protein interactions and platelet-neutrophil-endothelial interactions in liver sinusoids in a murine model of experimental sepsis (endotoxemia).
摘要
Inflammation and thrombosis are complex processes that occur primarily in the microcirculation. Although standard histology may provide insight into the end pathway for both inflammation and thrombosis, it is not capable of showing the temporal changes that occur throughout the time course of these processes. Intravital microscopy (IVM) is the use of live-animal imaging to gain temporal insight into physiologic processes in vivo. This method is particularly powerful when assessing cellular and protein interactions within the circulation due to the rapid and sequential events that are often necessary for these interactions to occur. While IVM is an extremely powerful imaging methodology capable of viewing complex processes in vivo, there are a number of methodological factors that are important to consider when planning an IVM study. This paper outlines the process of conducting intravital imaging of the liver, identifying important considerations and potential pitfalls that may arise. Thus, this paper describes the use of IVM to study platelet-leukocyte-endothelial interactions in liver sinusoids to study the relative contributions of each in different models of acute liver injury.
引言
Inflammation and thrombosis are complex processes that occur primarily in the microcirculation. This protocol outlines the surgical preparation that allows for imaging of the liver microvasculature in vivo. Although standard histology may provide insight into the end pathways for both inflammation and thrombosis, it cannot show the temporal changes that occur throughout the process. Furthermore, this method is particularly powerful when assessing the transient cellular and protein interactions that occur within the microvascular circulation due to its ability to capture, via videomicroscopy, the often rapid and sequential interactions occurring within biological systems. This paper describes the use of intravital microscopy to study platelet-leukocyte-endothelial interactions in liver sinusoids to study the relative contribution of each in different models of acute liver injury.
While this surgical preparation and imaging modality have the potential to be adapted to a host of inflammatory and pathological models, two models of vascular inflammation are outlined here: an endotoxemia model of murine sepsis and acetaminophen (APAP)-induced liver injury. The injection of endotoxin (lipopolysaccharide; LPS) to induce an experimental model of murine sepsis began as early as the 1930s with its isolation and subsequent exploration as a key molecule in the progression of sepsis1. While this model is limited in that LPS is only a single component of Gram-negative bacteria, this is a widely accepted and utilized method that is relatively simple and quick to perform. Furthermore, it rapidly and accurately replicates the physiological manifestations of sepsis1. Given the role intestinal endotoxin absorption plays in the development of liver disease and inflammation2, this is a superb model for studying platelet-leukocyte-endothelial interactions in the mouse liver.
In addition to an LPS model of sepsis, APAP overdose provides an excellent model for the study of pathological cell-cell interactions in the liver. APAP overdose can lead to acute liver failure, marked by thrombocytopenia and platelet accumulation in the liver, subsequently blocking leukocyte-mediated liver recovery3. While the surgical preparation and imaging methodology for this model can be adapted for a number of biological questions and to study various pathological processes, both the LPS model of sepsis and APAP-induced liver injury are excellent models for the study of platelet-leukocyte-endothelial interactions in vivo.
IVM has some inherent advantages over standard histological techniques. While standard histological methods allow the study of whole or sectioned tissue samples and the appreciation of proteins and tissue architecture, these methodologies come with limitations. By design, these processes require tissue processing, which has the potential to distort or mask what is found in the living system. Tissue must be fixed during histological preparation, introducing the potential for fixation artifacts or enhanced autofluorescence. Fixation can also lead to intracellular changes in tissue, and the potential for improper fixation may lead to tissue degradation. Furthermore, histologic methods lack the potential for directly studying the temporal aspects of protein or cellular interactions and have the potential of missing ephemeral or infrequent interactions.
Conversely, fluorescence intravital microscopy allows one to avoid many of the complications and limitations inherent in standard histology. IVM avoids fixation and, thereby, the artifacts or tissue degradation that can occur during histological preparation. By design, it also allows for the imaging of tissues within the living biological system, and, as such, tissue isolation and sectioning are not required. Furthermore, it allows for the imaging and study of transient or infrequent processes, which can be difficult or impossible to capture using histology. This IVM method can also be used to capture and identify sequential processes (e.g., platelet- or leukocyte-initiated interactions resulting in platelet-leukocyte aggregates bound to vascular endothelium). Finally, this method can be adapted to a host of imaging systems. Depending upon the needs of the study and the desired dataset, after surgical preparation, the externalized liver can be placed on almost any imaging system desired. We have successfully applied this protocol to imaging using widefield fluorescence microscopy, spinning disk microscopy, laser scanning confocal microscopy using a resonance head scanner, as well as two-photon microscopy. However, there is no reason to believe that this method should be limited to the aforementioned microscopy systems.
This methods paper outlines a protocol for IVM, which was used previously to study platelet-leukocyte-endothelial interactions in the mouse liver. This protocol has been used to compare temporal platelet-endothelial adhesion of platelets, either labeled with fluorescently conjugated antibodies or genetically modified for endogenous fluorescence4. This protocol has been used to evaluate transient platelet-leukocyte-endothelial interactions in the liver sinusoids in an endotoxemic model of liver inflammation and to co-localize P-selectin and recombinant protein. In addition, this protocol made it possible to determine whether the differences seen in neutrophil density using standard histology were a result of differences in red blood cell velocities (as a surrogate for volumetric flow rate)5. Finally, this method has been used to evaluate platelet recruitment by Kupffer cells in the liver sinusoids in an acute model of APAP-induced liver injury6. This body of work would not have been possible using standard histological methods. As stated, this protocol can be adapted for a variety of imaging systems, and, with proper surgical preparation, fluorescent antibody choice, and imaging settings, this protocol is highly reliable and reproducible for the study of liver pathology.
研究方案
All animal protocols were approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine and the Research & Development Committee of the Michael E. DeBakey Veterans Affairs Medical Center. All experiments are terminal, with euthanasia performed at the end under a surgical plane of anesthesia. See the Table of Materials for details related to all materials, reagents, and equipment used in this protocol.
1. Preparation of antibodies and dyes
- Antibody and dye selection
NOTE: The number of simultaneous colors observed will be dependent on the specific microscope setup. In the system used here, four different wavelengths can be visualized simultaneously.- Visualizing vessels:
- Based on the experimental design, label the vasculature with either an endothelium-specific (e.g., CD31) antibody and/or a molecule that is uniformly distributed in the plasma (e.g., dextran or albumin).
NOTE: This article describes the use of Texas Red-labeled dextran (150 kDa; 250 µg/mouse). The benefit of this large molecular weight protein is in its ability to stay within the vasculature during acute inflammation. Care must be taken when using antibody labeling to avoid antibodies that have the potential to interfere with endothelial function.
- Based on the experimental design, label the vasculature with either an endothelium-specific (e.g., CD31) antibody and/or a molecule that is uniformly distributed in the plasma (e.g., dextran or albumin).
- Visualizing platelets:
- To identify platelets, use either genetically modified mice (e.g., R26R-EYFPf/f/PF4-Cre) or platelet-specific (e.g., anti-GPIbβ) antibodies for IVM. For in-depth information regarding the use of fluorescent labeling of platelets, refer to a previous publication4.
NOTE: Here, DyLight488-labeled anti-GPIbβ antibodies (X488; 6 µg/mouse) are used. Care must be taken when using antibody labeling to avoid interference with platelet function or adhesion.
- To identify platelets, use either genetically modified mice (e.g., R26R-EYFPf/f/PF4-Cre) or platelet-specific (e.g., anti-GPIbβ) antibodies for IVM. For in-depth information regarding the use of fluorescent labeling of platelets, refer to a previous publication4.
- Visualizing leukocytes:
- Similar to imaging platelets, visualize the leukocytes of interest with either genetically modified mice (e.g., LysM-EGFP for granulocytes) or leukocyte-specific antibodies (e.g., Ly6G for murine neutrophils). To follow this protocol, use Brilliant Violet 421 (BV421)-labeled anti-Ly6G antibodies (3 µg/mouse). In acute (<6 h) experiments, label neutrophils with 3 µg of anti-Ly6G (clone 1A8) per animal.
NOTE: When using anti-Ly6G antibody (clone 1A8), it should be noted that this antibody has been used to deplete neutrophils (0.05-1 mg antibody/mouse)7-9 after at least 24 h and/or with repeated injections. Anti-Ly6G (1A8) antibodies have also been reported to affect neutrophil adhesion and transmigration at high (but not low) shear stress in certain, but not all, situations 9,10. Therefore, the antibody dosage should be chosen carefully for each case.
- Similar to imaging platelets, visualize the leukocytes of interest with either genetically modified mice (e.g., LysM-EGFP for granulocytes) or leukocyte-specific antibodies (e.g., Ly6G for murine neutrophils). To follow this protocol, use Brilliant Violet 421 (BV421)-labeled anti-Ly6G antibodies (3 µg/mouse). In acute (<6 h) experiments, label neutrophils with 3 µg of anti-Ly6G (clone 1A8) per animal.
- Additional proteins:
- Depending on the number of lasers and filter sets/detectors available on a particular imaging system, add more wavelengths to identify more objects simultaneously while taking care to avoid spectral overlap.
NOTE: This protocol describes the imaging of a 4th object, P-selectin (clone Psel.KO2.3), using a PerCP-eFluor 710-labeled anti-P-selectin antibody (4 µg/mouse). Although the majority of the descriptions and images in this paper are for LPS-induced acute liver injury, other injury models may be of interest and may call for alternative labels. See the discussion for a description of antibodies used for APAP-induced injury6.
- Depending on the number of lasers and filter sets/detectors available on a particular imaging system, add more wavelengths to identify more objects simultaneously while taking care to avoid spectral overlap.
- Visualizing vessels:
- Removal of preservatives and sterilization
NOTE: Although there are commercially available antibodies and dyes ready for injection into animals, most of them will have carriers or preservatives (e.g., sodium azide) that may be harmful to animals or add confounding variables. Antibodies and dyes containing carriers or preservatives should be dialyzed against Dulbecco's phosphate-buffered saline without calcium or magnesium (DPBS-/-; or other physiologic buffers).- Pour 500 mL DPBS-/- (~1,000-fold volume of the antibody/dye to be dialyzed) into a beaker with a magnetic stir bar. Attach a 7,000 MWCO cassette to a float buoy and place it in the dialysis buffer for at least 5 min to precondition the membrane. Remove the cassette and blot the plastic edges dry with lint-free tissue. Avoid touching the dialysis membrane.
- Inject the antibody/dye into the cassette through one of the four ports using an 18-20 G needle. Place the cassette with the antibody/dye into the dialysis buffer. Place the beaker on a magnetic stirrer and dialyze for at least 1 h at 4 °C. Change the dialysis buffer.
- Repeat the dialysis and changing of the dialysis buffer in step 1.2.2 for a minimum of two more times (at least three exchanges).
- Remove the cassette and blot the plastic edges dry with lint-free tissue. Avoid touching the dialysis membrane. Aspirate the antibody/dye from the cassette through an unused port using an 18-20 G needle.
- In a tissue culture hood, sterilize the preparations using a 0.2 µm filter prior to use in animals. Store unused portions in sterile tubes at 4 °C.
- Controls
- While the exact controls necessary for each experiment will vary depending upon the design of the study, be sure to include proper sham cohorts, vehicle-treated and non-vehicle-treated groups, as well as isotype controls in order to exclude non-specific labeling and biological effects when analyzing the study results.
2. Intraperitoneal endotoxin injection
NOTE: To evaluate platelet-neutrophil-endothelial interactions in the liver sinusoids in an experimental model of murine sepsis, endotoxin injection can be used.
- Weigh the mice to determine the amount of endotoxin to inject (5 mg/kg; Escherichia coli serotype O111:B4; endotoxin content 1 x 106 EU/mg).
NOTE: Endotoxin potency and effect are species- and strain-dependent and may vary from lot to lot11. Therefore, to maximize the consistency of results, experiments should be conducted with the same lot of endotoxin12. - Draw up endotoxin diluted with normal saline (0.9% sodium chloride) into a syringe with a 27 G needle attached. In the non-dominant hand, hold the mouse by the scruff using the thumb and index finger. Using the other hand, put traction on the tail to expose the abdomen and place the tail between the fifth digit and the palm of the non-dominant hand.
- Clean the abdomen with 70% ethanol. Insert the needle into the lower-left (or right) abdomen and inject the endotoxin.
NOTE: Care should be taken to avoid inserting the needle too deeply because there is a risk of organ injury that may confound the experiments (e.g., injury to the intestines, bowel, kidneys, etc.). - Perform steps 2.1-2.3 in control animals using normal saline (or vehicle).
NOTE: APAP-induced liver injury can be accomplished using a similar methodology as above, using APAP rather than endotoxin6. See the discussion for details related to the APAP-induced liver injury model.
3. Induction and maintenance of anesthesia of the animals and euthanasia
- Place the animals under a surgical plane of anesthesia. Maintain the anesthesia using inhaled isoflurane via a nosecone or a tracheostomy tube. Assess and adjust the level of anesthesia by monitoring for movement after a toe pinch.
- Perform euthanasia at the end of all experiments by exsanguination under a surgical plane of anesthesia followed by bilateral pneumothoraces.
4. Catheterization of the jugular vein and trachea
- Perform a tracheotomy to facilitate breathing and the placement of vascular catheters for the injection of labeled antibodies/dyes.
NOTE: A surgical dissection microscope or loupes are helpful in catheterization. - Shave the fur from the neck and abdomen; remove excess hair. Place the animal on a warming pad to maintain the body temperature at 37 °C, using a rectal probe to monitor core temperature. Scrub the neck with betadine followed by 70% ethanol.
- Make a vertical midline incision in the neck and expose the trachea using blunt dissection.
- Tracheostomy
- Prepare a tracheostomy tube PE90 tube with a 21 G blunt needle inserted (Figure 1A).
NOTE: Adding a ~45° bevel to the end of the tracheostomy tube may help facilitate its insertion into the trachea. Alternatively, an 18-20 G peripheral intravenous catheter may also be substituted for PE90 tubing. - Once the skin and fascia are incised, reflect the salivary glands away from the center.
- Using blunt dissection, separate the sternothyroid muscle to give better access to the trachea.
NOTE: Take care to avoid the supplying vessels and the carotid arteries that are adjacent to the trachea. - Make a small (~1-2 mm) horizontal incision between the tracheal rings on the ventral/anterior side of the trachea (Figure 1B).
NOTE: The use of Vannas scissors will facilitate proper dissection. Ensure that only the anterior face of the trachea is incised; complete transection of the trachea will make it difficult to cannulate the trachea and risks damaging nearby vascular structures. - Pass a tracheostomy tube into the hole. Ensure that the tip of the tube is not inserted past the carina.
- Secure the tracheostomy tube within the trachea by tying a #4-0 silk suture around the trachea and tube, using the tracheal rings as support (Figure 1C).
- Prepare a tracheostomy tube PE90 tube with a 21 G blunt needle inserted (Figure 1A).
- External jugular cannulation
- Prepare a jugular catheter (Figure 2) using PE50 tubing (OD 0.97 mm, ID 0.58 mm) with PE10 tubing (OD 0.61 mm, ID 0.28 mm) inserted into one end (Figure 2 inset) and a 22 G or 23 G blunt needle inserted into the other. Use adhesive to ensure that the tubing does not slip or leak. Attach a syringe filled with sterile normal saline to flush the catheter.
- Expose the external jugular vein (EJV) after salivary gland reflection. Using blunt dissection, free the EJV from the surrounding connective tissue (Figure 3A).
- Using #4-0 silk sutures, ligate the cranial end of the visualized EJV and place a loose loop around the caudal end of the EJV (Figure 3B). Clamp the ends of each suture together with hemostats and gently retract the two hemostats away from each other to help expose the EJV.
NOTE: The optimal region to cannulate is between the branches of the anterior jugular vein cranially and the internal jugular vein caudally. To ease suture placement, fold a piece (~15 cm) of suture in half and pass the bent end underneath the EJV with one pair of forceps while raising the EJV with a separate pair of forceps to prevent rolling of the vessel. Then, cut the suture to make two separate lengths. - Using Vannas scissors, make a small (~1 mm) incision in the anterior surface of the EJV. Insert a bent 30 G blunt needle (augmented with a 0.15 mm minutien pin bluntly filed and inserted into the needle tip) into the EJV through the incision and gently lift it up to facilitate passage of the catheter containing normal saline into the EJV.
NOTE: Ensure that the catheter and hub do not have any air bubbles before insertion and flushing into the mouse. An air embolus may be lethal to the animal. - Once the catheter is within the EJV, loosen the caudal loop just enough to allow passage of the catheter deeper into the vein. Check for placement of the catheter by aspirating blood and flushing the catheter to check for leaks (Figure 3C).
- Using the caudal loop, secure the catheter within the EJV. Using the cranial suture, secure the catheter to the cranial end of the EJV to minimize accidental decannulation (Figure 3D).
- Close the neck skin incision with #4-0 silk suture.
- Tracheostomy
5. Exteriorization of the liver
- Clean the abdomen with a betadine scrub followed by 70% ethanol.
- Make a ~1 cm vertical incision in the skin of the upper abdomen beginning at the base of the sternum. Using blunt dissection, separate the skin from the abdominal muscles. Cauterize the large feeding vessels of the skin and muscles to minimize bleeding during the following steps (Figure 4A).
- Make a ~1-2 cm horizontal incision of the skin followed by the abdominal muscle, bisecting the vertical incision in step 5.2 approximately at the top of the visible liver; use a cautery tool to assist in hemostasis of the skin (Figure 4B).
NOTE: The size of the abdomen incision is critical for optimal imaging of the liver. If the opening is too small, there is a risk of liver ischemia due to overstretching or impingement of the hepatic artery and/or portal vein. If the opening is too large, then other organs, such as the intestines, may also be exteriorized and interfere with imaging. - Cut an approximately 1 cm circle in the center of a 2 in x 2 in gauze and moisten it with warm normal saline. Position the gauze so the opening is centered over the incision. Gently pull the edges of the incision apart and apply light, upward pressure on the abdomen to exteriorize the liver so that the liver rests easily on top of the moistened gauze (Figure 4C).
- Place the mouse onto the imaging tray in the prone position, with the liver centered over the coverslip (Figure 4D).
NOTE: In a control animal, the liver should appear maroon. An inverted, laser scanning confocal microscope with a two-way resonance head scanner is used for IVM in this protocol. Therefore, a 3D printer was used to create a custom IVM tray (Figure 5). The .stl files created for 3D printing are provided (Supplemental File 1); adjustments may be necessary to fit individuals' needs. - Transfer the animal to the inverted microscope.
6. Liver intravital microscopy imaging
NOTE: This protocol uses a laser scanning confocal microscope with a two-way resonance head scanner for intravital microscopy. To stabilize the resonance head frequency, turn on the system for at least 30 min prior to imaging. Different systems may have different capabilities. Discussion of these is outside the scope of this article. Contact the equipment representatives for the technical details of specific systems.
- To maintain a surgical plane of anesthesia, connect the animal to the isoflurane delivery system.
NOTE: Depending on the experimental protocol, mechanical ventilation may be necessary (vs. spontaneous ventilation). - Place a radiant warming pad over the animal, using the rectal probe to maintain a core temperature of 37 °C.
NOTE: In this protocol, a rectangular piece of foam that fits around the IVM tray acts as an insulator and a spacer on which to place the radiant warming pad (Figure 4D). - Set the objective turret to the desired magnification, applying immersion oil as necessary. To follow this protocol, use the 60x/NA1.30 silicone oil objective with 1x optical zoom.
- Once the focal plane is identified, identify ≥10 random fields of view, avoiding the areas near the edges of the liver.
NOTE: As the liver exhibits autofluorescence in the fluorescein isothiocyanate (FITC) channel, use epifluorescence to quickly identify the general focal plane.- Depending on the system, identify the edges of the visible liver using epifluorescence in the FITC channel and record the (x,y) stage coordinates.
- Use the (x,y) boundaries of the liver and a random number generator to identify random (x,y) coordinates. For an acceptable field of view, look for (a) the presence of vessels dispersed throughout the field of view; (b) ≥80% of the vessels in the same focal plane; and (c) ≥1 field of view away from any liver edges (example in Figure 6). If the focus of image analysis is on platelet-leukocyte-endothelial interactions in the hepatic sinusoids, exclude fields with vessels whose diameters are ≥15 µm (murine sinusoids are typically ≤10 µm in diameter).
7. Image analysis
NOTE: ImageJ/FIJI was used to process all of the images in this paper. FIJI (fiji.sc) is an open-source version of Image J (imagej.nih.gov) that has more functionality through the use of additional plugins and macros13.
- Open the multichannel, time-lapsed files in ImageJ/FIJI.
NOTE: Depending on the platform, additional programs/plugins may be required. ImageJ/FIJI has a Bio-Formats Importer that can open multiple different types of files, such as the .oir files by Olympus. Here, the files have been converted to .tif because it is a more widely used format. - Make sure that the correct scale is set; look for the pixel/µm ratio and the time interval in the metadata/properties.
- Use different filters to remove background noise (e.g., a median filter with a 2 pixel radius used in this protocol). Ensure that the processing is similar amongst all the images to minimize variation in post-processing. As counts and lengths are being compared in these experiments, instead of mean fluorescence intensity, filter to improve clarity and the identification and measurement of cell-cell interactions.
NOTE: The use and magnitude of filters will be dependent on the size of the target(s) of interest and the signal-to-noise ratio in the images. - As there is heterogeneity between fields of view in vascular density, measure the total vascular area in each field using the selection brush (Figure 7).
- Right-click the oval selection tool and pick the selection brush tool.
- Adjust the size of the brush by double-clicking the selection brush tool icon.
- Trace over the vessels to highlight/select them. To delete parts of the selection, use [alt]-left click and add parts by using [shift]-left click.
- Press [M] to measure the selected area.
NOTE: If the image scale has been properly set, then the output should be in µm2, which is converted to mm2.
- Count the number of platelets and leukocytes in the field. Consider cells to be adhered cells if they moved <1 cell body over 30 s. Calculate the number of cells (platelets and/or leukocytes) per mm2.
- To estimate the relative blood flow within each vessel, measure the mean red blood cell (RBC) velocity as a surrogate using the MTrackJ plugin in ImageJ/FIJI (imagescience.org/meijering/ software/mtrackj)14, as described previously5. Identify RBCs as round or disc-shaped filling defects in the dextran channel. Save the tracks for record keeping, asynchronous analysis, and verification.
NOTE: An example of this is shown in Figure 8 and Supplemental Video S1.
结果
Assessing the effect of vimentin rod domain in leukocyte adhesion to inflamed endothelium
Leukocyte P-selectin glycoprotein ligand-1 (PSGL-1) binding to endothelial and platelet P-selectin occurs during the acute phase of sepsis-induced liver injury inflammation. However, the recombinant human rod domain of vimentin (rhRod) has been shown to bind to P-selectin and block leukocyte adhesion to both endothelium and platelets. This protocol was utilized in a mouse model of sepsis to visualize the real-time effect of rhRod on PSGL-1-dependent leukocyte adhesion in the liver. It was found that rhRod co-localizes with P-selectin in the liver sinusoids and does, in fact, decrease leukocyte adhesion in the liver5.
Hepatic platelet recruitment in acetaminophen-induced liver injury
The accumulation of platelets in the liver contributes to APAP-induced liver injury. This protocol was used to assess the contribution of Chitinase 3-like-1 (Chi3l1) in the recruitment of platelets within liver sinusoids. The application of this protocol allowed for the visualization of platelet recruitment in the acute phase of APAP-induced liver injury, where it was found that APAP administration results in increased Chi3l1 and platelet accumulation in the liver, which was attenuated in Chi3l1-/- mice6.
Validating the fluorescent labeling of platelets for intravital microscopy
To utilize this protocol for the assessment of platelet interactions in vivo, it was necessary that the fluorescent labeling method utilized for the visualization of platelets be thoroughly validated. We originally assessed two different sources of fluorescence: X488 antibody-labeled platelets and mice containing endogenous platelet fluorescence. Using this protocol, it was found that both approaches achieve platelet-specific fluorescence, and similar levels of platelet aggregation and adhesion were found in mice with endogenous platelet fluorescence and their littermates. However, mice with endogenous platelet fluorescence exhibited significantly higher platelet aggregation and adhesion when compared to C57BL/6 mice with X488-labeled platelets. As such, X488 antibody labeling of platelets was preferred over endogenously fluorescent platelets for the intravital functional assessment of platelets in mice4.
Attenuating liver movement during intravital imaging
The optimal viewing window is one where there is minimal movement of the liver. However, the liver may move due to its adjacency to the diaphragm (Supplemental Video S2). Several factors may influence the degree to which the liver moves, such as the size of the animal compared to the geometry of the imaging stage. If the stage is too flat, then the liver may be pressed against the coverslip and have areas of ischemia due to excess pressure and/or impingement of the vessels into the liver (i.e., hepatic artery and/or portal vein). In our custom stage, we have incorporated a concavity to minimize the external pressure on the liver. Additional methods of minimizing movement include adjusting the size of the abdominal incision to allow the liver to be further away from the diaphragm. Care must be taken to prevent other internal organs (e.g., intestines and kidneys) from falling out and obscuring the view. To further reduce motion, some groups utilize a small piece of plastic wrap draped over the liver lobe and imaging tray15,16. In addition, the falciform ligament can be cut to further isolate the liver from abdominal movement17,18. Finally, if liver movement is still present, software stabilization (also known as registration; examples in Supplemental Video S3) may be used to stabilize the image for analysis. One should be aware that this can be a computationally taxing process and may not be necessary, depending on the desired outcome. If videos contain unwanted noise, median filters are recommended. Examples of median-filtered videos can be seen in Supplemental Video S4 and Supplemental Video S5.
Vessel identification and platelet-Kupffer-endothelial interaction
Supplemental Video S6 and Supplemental Video S7 give examples of larger veins that may be visualized with this technique. Using this liver IVM methodology, we are also able to evaluate platelet-Kupffer-endothelial interactions after APAP-induced acute liver injury (Supplemental Video S8). To compare adherent cells between experimental groups, the vascular area is first measured (step 7.4). Then, adherent cells can be counted and (step 7.5) normalized to vascular density (Figure 7).
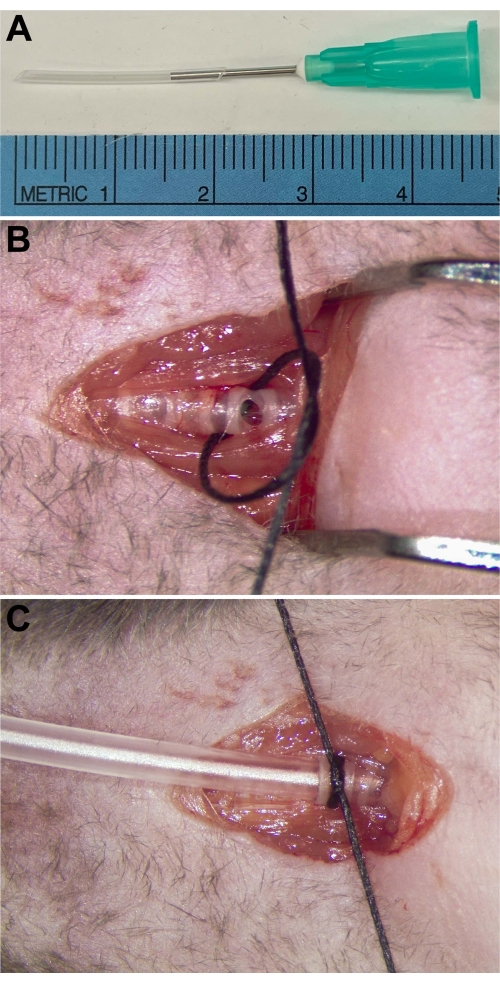
Figure 1: Tracheostomy tube placement. (A) The tracheostomy tube consists of PE90 tubing with a 21 G blunt needle inserted to allow connection to a ventilator. (B) The anterior surface of the trachea is cut caudal to the larynx. A suture is passed underneath to secure the tracheostomy tube after it is inserted. (C) Tracheostomy tube inserted and secured within the trachea. Please click here to view a larger version of this figure.

Figure 2: Vascular catheter. PE50 tubing with a 23 G blunt needle inserted on one end and PE10 tubing inserted in the other. (Inset) Enlarged view of the PE10 tubing within the PE90 tubing. Adhesive glue may be needed to prevent dislodgement of the PE10 tubing and/or leakage. Please click here to view a larger version of this figure.

Figure 3: Identification and cannulation of the external jugular vein. (A) The EJV (arrowhead) should be visible after the tracheostomy tube is placed if the salivary glands are reflected away from midline. (B) The cranial end of the exposed EJV is ligated. A loose loop of suture is placed around the caudal end of the exposed EJV. (C) After the EJV is isolated, a small incision is made, and the catheter is inserted into the vessel, past the caudal suture. (D) After the catheter placement is confirmed by aspiration of blood, it is flushed with saline and secured to the vessel. Abbreviation: EJV = external jugular vein. Please click here to view a larger version of this figure.

Figure 4: Exteriorization of the liver. (A) A small vertical incision is made in the center of the upper abdomen. Blunt dissection is used to separate the skin from the muscle. (B) A cautery is used to cauterize the feeding vessels of the skin and muscle to prevent bleeding with dissection. (C) After cauterization, a horizontal incision is made, bisecting the vertical incision. Light abdominal pressure allows for the liver to be exteriorized onto a piece of moist gauze. (D) A view of the externalized liver from below the custom liver IVM tray. (E) The custom liver IVM tray is shown without an animal, highlighting the ventilator tubing, the foam insulation, and the viewing window. (Inset) The mouse is placed supine on the custom stage. Abbreviation: IVM = intravital microscopy. Please click here to view a larger version of this figure.
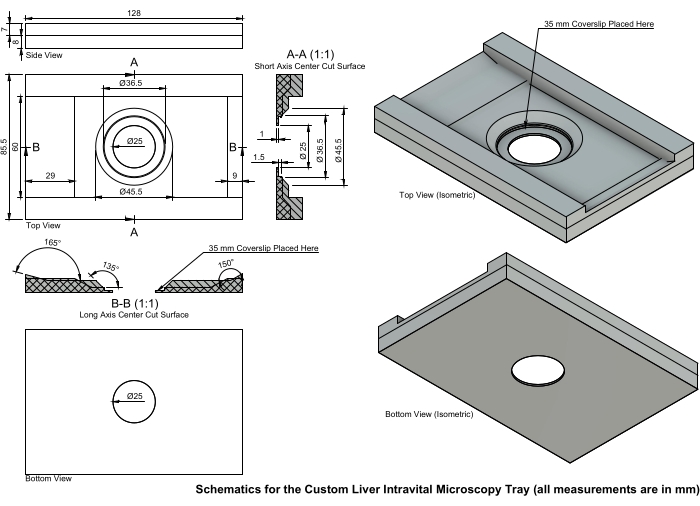
Figure 5: Schematics for the custom liver IVM tray. The IVM tray is printed with a recessed center to minimize pressure on the liver. A 35 mm #1 or #1.5 coverslip is placed in the center and secured with clear nail polish. This tray was designed for the microscope stage, and individual dimensions may vary depending on the investigator's equipment. Abbreviation: IVM = intravital microscopy. Please click here to view a larger version of this figure.

Figure 6: Edge of the liver. The arrow points to the edge of the liver, which should not be used for image analysis. Scale bar = 50 µm. Please click here to view a larger version of this figure.
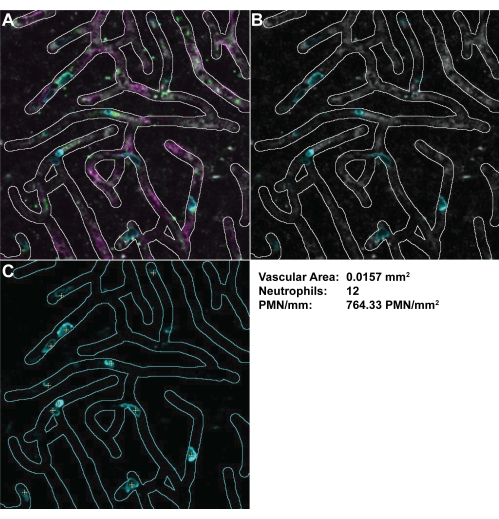
Figure 7: Measuring the vessel density and calculating the PMN/mm2. Vessels were highlighted with the selection brush tool with (A) all channels and (B) only the PMNs and dextran channels selected. (C) PMNs can be more easily identified with the dextran channel removed. PMNs that were firmly adhered were marked (yellow crosses). These calculations can then be used to measure the vascular area and PMN/mm2 in the field of view. Abbreviation: PMN = polymorphonuclear neutrophil. Please click here to view a larger version of this figure.

Figure 8: Using MTrackJ to measure red blood cell velocity as an estimate of blood flow. The MTrackJ plugin in ImageJ/FIJI was used to select and follow RBCs to measure the RBC velocity. After selecting an RBC, the stack automatically advances to allow the user to select the new position of the selected RBC. Once the tracks are selected, the mean velocity of each track can be determined. Abbreviation: RBC = red blood cell. Please click here to view a larger version of this figure.
Supplemental Video S1: Video of RBC tracking using MTrackJ. Video of the RBC tracks in real time. Abbreviation: RBC = red blood cell. Please click here to download this File.
Supplemental Video S2: Liver IVM field of view with movement due to diaphragm movement with respiration. Abbreviation: IVM = intravital microscopy. Please click here to download this File.
Supplemental Video S3: Image stabilization using ImageJ/FIJI. Image-stabilized liver IVM field using the descriptor-based registration plugin. Abbreviation: IVM = intravital microscopy. Please click here to download this File.
Supplemental Video S4: Montage video of liver sinusoids in a control (normal saline) mouse. In control mice, there are relatively few adherent neutrophils (cyan) and platelets (green). P-selectin (magenta) lines the sinusoids (gray). Please click here to download this File.
Supplemental Video S5: Montage video of liver sinusoids in an endotoxemic mouse. As compared to the control mouse, endotoxemic mice have more adherent neutrophils (cyan) and platelets (green) in the liver sinusoids (gray). P-selectin (magenta) is seen lining the sinusoids. Please click here to download this File.
Supplemental Video S6: Montage video of liver sinusoids draining into a central vein in a control mouse. This is an example of a field of view containing a larger vessel that may or may not be of interest to an investigator. Of note, there is decreased P-selectin staining (magenta) in the larger, central vein as compared to the sinusoids. The other colors indicate neutrophils (cyan), platelets (green), and intravascular space (gray). Please click here to download this File.
Supplemental Video S7: Montage video of a branch of the portal vein giving rise to the liver sinusoids in an endotoxemic mouse. In this field of view, a branch of the portal vein is seen giving rise to the sinusoids. Note the increased number of adhered neutrophils (cyan) and platelets (green). There is also P-selectin expression (magenta) in the larger vessel. The intravascular space is labeled with TRITC-dextran (gray). Abbreviation: TRITC = tetramethylrhodamine. Please click here to download this File.
Supplemental Video S8: Video of acute liver injury in an acetaminophen-induced liver injury model. This is an example of using liver IVM to study platelet-Kupffer-endothelial interactions in an acute model of APAP-induced liver injury. (Left) There are multiple Kupffer cells (cyan) and platelets (white) seen within the liver sinusoids (red) 150 min after APAP overdose. (Right) Using the same objective (60x), we performed a 3x zoom on the upper Kupffer cell to evaluate Kupffer-platelet interactions. Abbreviations: IVM = intravital microscopy; APAP = acetaminophen. Please click here to download this File.
Supplemental File 1: .stl file for the custom liver IVM microscopy tray. Depending on the microscope used, alteration of the .stl file may be required to adapt the tray to the investigator's specific microscopy system. Please click here to download this File.
讨论
The purpose of this methods paper is to outline the necessary steps required to reliably capture high-resolution intravital images and videos of the mouse liver under homeostatic conditions and following the administration of endotoxin or APAP. While this protocol has allowed for the consistent production of data on platelet-leukocyte-endothelial interactions in the liver, there are a number of critical steps required for success, as well as potential pitfalls that are important to avoid when using this imaging paradigm. Success using this protocol requires thorough and extensive planning during antibody selection to avoid interfering with cell/protein function, proper surgical technique to avoid tissue damage and preserve homeostatic hemodynamics, care during liver externalization to avoid liver ischemia or the externalization of additional organs, and proper imaging settings to avoid light/dye-induced tissue damage.
With practice, this protocol allows for high-quality, reproducible imaging of liver microvasculature in vivo and has significant benefits when compared to existing and alternative methods of study. Although two models of liver pathology are outlined here, namely endotoxin and APAP-induced liver injury, the basis of this protocol has the potential to be applied to a myriad of additional injury models. Furthermore, while antibodies and analysis techniques have been outlined for the study of platelet-leukocyte-endothelial interactions, with proper planning and antibody selection, this protocol can be adapted for the study of additional cells/proteins of interest. For example, for APAP-induced injury6, the following antibodies can be used: BV421-labeled F4/80 antibody (0.75 µg/mouse); TRITC-labeled bovine serum albumin (500 µg/mouse); and DyLight649-labeled anti-GPIbβ antibody (3 µg/mouse). There are some P-selectin antibody clones with blocking/neutralizing ability (e.g., clones RB40.3 and AK-420). The P-selectin antibody clone we use is Psel.KO2.3. To our knowledge, this clone does not have blocking ability. APAP-induced liver injury can be accomplished using a similar methodology as above, using APAP rather than endotoxin6. Animals should be fasted overnight21, and there is a noted sex difference in response22. A dose of 210 mg/kg of APAP is administered to males and 325 mg/kg to females. Administration should be performed in the same manner as protocol steps 2.1-2.3. Imaging occurs 2-3 h post injection for acute injury and 24 h post injection for delayed injury.
Most studies involving fluorescence imaging of liver tissue utilize standard histological practice. As mentioned earlier, while this has the potential to produce accurate and high-quality data, there are a number of assumptions and limitations inherent to the process. Fixation alone can lead to increased autofluorescence (an issue particularly relevant to the liver, as even freshly isolated liver tissue has significant autofluorescence23), as well as tissue shrinkage and distortion. Care must be taken when studying the liver using standard histological practices to ensure that what is captured is similar to what is seen in vivo. Tissue removal comes with its own considerations as well, especially when studying hemopoietic cells and vascular endothelium. The process of tissue dissection is in and of itself an injury and has the potential to introduce artifacts not found in vivo. It must also be considered when studying vasculature that removal of the tissue results in the loss of physiological pressure within vessels and an accompanying vessel collapse. Perfusion fixation will also wash away the majority of cells within the vasculature.
The intravital imaging modality described in this paper completely avoids the potential for fixation artifacts or tissue degradation and maintains tissue integrity as the liver is merely externalized and not removed from the living system. While this is in and of itself a noteworthy advantage over standard histological methods, intravital imaging also allows for the imaging and identification of sequential processes (e.g., platelet- vs. leukocyte-initiated processes), as well as ephemeral and transitory processes. Whereas histological methods only allow a single snapshot of the tissue at the moment of fixation, IVM affords the investigator the possibility for videomicroscopy of the living system and a temporal appreciation of biological functions. For example, using appropriately sized fluorescent molecules such as TRITC-dextran or TRITC-albumin, one can study the temporal and spatial aspects of vascular permeability and leakage. It should be noted, however, that the tissue depth that can be effectively imaged is dependent upon the imaging system being used and the maximum beam intensity allowable before light/dye-induced tissue damage occurs. In this respect, tissue sectioning in standard histological practices allows for imaging at any depth within the liver, albeit with a loss of temporality.
Although liver IVM is a powerful tool, there are limitations that should be considered when planning experiments. First and foremost, this protocol does not allow for repeated measurements; instead, the experiments are all terminal. Although this is the case, there are methods to allow for repeat imaging over a series of days or weeks, such as the use of implanted abdominal imaging windows24. Another limitation is in the movement of the liver. This is due to the organ directly abutting the diaphragm, which pushes the liver caudally with each breath. This can be mitigated with positioning and the use of moist gauze to keep the liver in place on the slide, but it may not always be possible. Depending on the goal of imaging, liver movement may still allow for analysis if the field of view returns to the original location. For example, when using the vascular network as a map to identify unique fields, since we are evaluating for adherent cells, the first and last time-lapsed images can be compared to determine whether a cell moved or not. For rapidly moving objects, because liver movement may make data analysis difficult, image stabilization (also known as registration in ImageJ) may be required. Of note, this process is often computationally taxing and may not work if the movement is non-rhythmic. While examining the liver for epifluorescence, it is imperative that the microscopist is blinded to the experimental group to which each animal is assigned. This will decrease observer bias in the selection of fields of view. Additionally, picking random (x,y) coordinates will also reduce confirmation and selection bias. This and other tips to improve rigor in microscopy are reviewed by Jost and Waters25.
The image acquisition rate and duration will largely depend on the end user's target of interest. In our studies, we study platelet-leukocyte-endothelial interactions in the circulation. These studies used an acquisition rate of 30 frames per second (fps), which allows for this system to capture a 512 pixel x 512 pixel image using the resonance scanner. The system's (Olympus FV3000) resonance scanner is capable of imaging up to four simultaneous colors in a roundtrip pattern (left to right on one line, then right to left on the next line). This allows for a 512 pixel x 512 pixel scan size to be imaged in 33.3 ms (~0.063 ms per line). This has been adequate to evaluate erythrocyte velocity and platelet and leukocyte adhesion and/or rolling along to the vessel walls. For investigators interested in more rapidly or ephemeral processes, higher acquisition rates may be necessary. On this system, we are able to capture more rapidly if we narrow the scanning area. For our purposes, because we wanted a larger vascular area to analyze, we used a full-frame capture at 30 fps. Similarly, the duration of image acquisition will also depend on the end user's endpoint. For all studies, we use standardized capture durations. Although a longer duration may provide more data, the main balancing measure is file size, which can be quite large with multi-channel, time-lapsed images. We are interested in firmly adherent leukocytes and/or platelets in flowing vessels and a priori define a firmly adhered leukocyte as one that moves <1 cell body for at least 30 s. For investigators interested in slower processes, such as leukocyte crawling, a longer duration of time may be necessary. Finally, with more sophisticated systems, it is also possible to combine multiple dimensions to visualize certain processes. For example, synchronized platforms can be used to capture 3D stacks in a time-lapsed fashion. These types of studies will require more preparation but can provide more insight into certain physiologic mechanisms.
IVM is an incredibly powerful tool in the study of liver vasculature in homeostatic and pathological conditions. It allows one to visualize cell-cell and cell-molecule interactions as they occur in the biological system, and, as such, its potential application in the study of liver pathogenesis cannot be overstated. Imaging can occur prior to the initiation of pathological models, throughout the acute phase of pathology, or anytime thereafter for the study of chronic symptoms or secondary insults. Although we study platelet-leukocyte-endothelial interactions, this protocol has incredible potential for adaptation to suit the needs of investigators. It must be noted that any variations from the protocol as described herein necessitate thorough verification that the antibodies/fluorescent solutions do not result in loss of function or alteration of the target cells/molecules. This can be verified through preliminary studies using the new antibodies under homeostatic and pathological conditions, using proper controls, and with a thorough understanding of the typical cell-cell or cell-molecule interactions being studied. While proper surgical technique, antibody consideration, and imaging configurations are paramount for the success of this imaging modality, once proficiency is achieved, this technique allows for repeatable, high-quality imaging of the liver as it exists in vivo. It is our hope that the use of this protocol for future studies will lead to a greater understanding of liver biology and pathogenesis and in so doing, propel us closer to the goal of producing novel therapeutics for those suffering from primary or secondary liver injury.
披露声明
The authors have no conflicts of interest to disclose. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
致谢
This work was supported by NIH/NIGMS GM-123261 (FWL) and NIH/NHLBI HL139425 (JC). Research support was also funded by NIH/NHLBI HL116524.
材料
| Name | Company | Catalog Number | Comments |
| Surgical Supplies | |||
| 2" x 2" non-woven sponges | McKesson Med. Surg | 92242000 | For liver isolation |
| #4-0 silk braided suture with needle | SOFSILK | N/A | 4-0 Softsilk coated braided black, nonabsorbable: C-1 cutting needle |
| #4-0 silk braided suture without needle | Ethicon | N/A | 4-0 Black braided silk, nonabsorbable |
| 21 G blunt needle (0.5 inch) | SAI Infusion Technologies | B21-50 | This is used to attach to the end of the tracheostomy tube to allow for connection to the ventilator. An alternative source is Instech |
| 23 G blunt needle (0.5 inch) | SAI Infusion Technologies | B23-50 | This is used for the vascular catheter to allow for connection to a syringe. An alternative source is Instech |
| Dissecting Scissors (Pointed Tip) | Kent Scientific | INS600393-G | Micro Dissecting Scissors; Carbide Blades; Straight; Sharp Points; 24 mm Blade Length; 4 1/2" Overall Length |
| McPherson-Vannas Micro Scissors (Vannas) | Kent Scientific | INS600124 | These are useful for creating the openings in the trachea and vessels |
| Polyethylene tubing 10 | Instech | BTPE-10 | This is used to make the intravascular portion of the catheter. An alternative source is BD Intramedic |
| Polyethylene tubing 50 | Instech | BTPE-50 | This is used to make the extravascular portion of the catheter. An alternative source is BD Intramedic |
| Polyethylene tubing 90 | Instech | BTPE-90 | This is used to make the tracheostomy tube. An alternative source is BD Intramedic |
| USP grade sterile normal saline | Coviden | 8881570121 | Hospira 0.0% Sodium Chloride Injection, USP |
| Microscopy Supplies | |||
| Isoflurane delivery system and ventilator | Kent Scientific | Somnosuite | Combination rodent ventilator and volatile anesthetic delivery system |
| Foam spacer for warming pad during microscopy | N/A | N/A | This spacer should be cut from high quality foam, should fit around the liver microscope tray and specific height dimensions are dependent upon the microscope system |
| Laser scanning confocal microscope system with resonance head scanner | Olympus | FV3000 | Although we describe the use of an Olympus FV3000 using a resonance head scanner, this protocol with work with most imaging systems |
| Liver Microscope Tray | N/A | N/A | The liver microscope tray was designed for an inverted microscope |
| Antibodies & Related Reagents | |||
| Brilliant Violet 421/anti-mouse Ly6G antibody | BioLegend | 127628 | 3 µg/mouse. To label neutrophils |
| BV421/F4/80 antibody | BioLegend | 123132 | 0.75 mg/kg. To label Kupffer cells |
| Dulbecco's phosphate buffered saline w/o calcium or magnesium | Gibco/ThermoFisher Scientific | 14190144 | Used as dialysate to remove sodium azide from antibodies |
| DyLight649/anti-GPIbβ antibody | emfret Analytics | X649 | 3 µg/mouse. To label platelets |
| DyLight488/anti-mouse GPIbβ antibody | emfret Analytics | X488 | 6 µg/mouse. To label platelets |
| Endotoxin from Escherichia coli serotype O111:B4 | Sigma-Aldrich | L3024 | 5 mg/kg; Potency of endotoxin may vary from lot to lot. Therefore, the same lot should be used for a series of experiments to minimize variation due to endotoxin lot |
| PerCP-eFluor 710/anti-mouse P-selectin antibody | Invitrogen | 46-0626-82 | 4 µg/mouse. To label P-selectin |
| Slide-a-Lyzer 7,000 MWCO cassette | Thermo Scientific | 66370 | Used to dialyze antibodies to remove sodium azide |
| Texas Red-labeled dextran | Sigma-Aldrich | T1287 | ~150 kDa; 250 µg/mouse |
| TRITC/bovine serum albumin | Sigma-Aldrich | A2289 | 500 µg/mouse. Dilute to a stock concentration of 50 mg/mL (5%) in normal saline. Used to label the vasculature. It may leak into the interstitial space more readily than high molecular weight dextran during inflammation |
参考文献
- Deitch, E. A. Rodent models of intra-abdominal infection. Shock. 24, 19-23 (2005).
- Nolan, J. P. The role of intestinal endotoxin in liver injury: A long and evolving history. Hepatology. 52 (5), 1829-1835 (2010).
- Miyakawa, K., et al. Platelets and protease-activated receptor-4 contribute to acetaminophen-induced liver injury in mice. Blood. 126 (15), 1835-1843 (2015).
- Da, Q., Derry, P. J., Lam, F. W., Rumbaut, R. E. Fluorescent labeling of endogenous platelets for intravital microscopy: Effects on platelet function. Microcirculation. 25 (6), 12457 (2018).
- Lam, F. W., et al. The vimentin rod domain blocks P-selectin-P-selectin glycoprotein ligand 1 interactions to attenuate leukocyte adhesion to inflamed endothelium. PLoS One. 15 (10), 0240164 (2020).
- Shan, Z., et al. Chitinase 3-like-1 contributes to acetaminophen-induced liver injury by promoting hepatic platelet recruitment. eLife. 10, 68571 (2021).
- Daley, J. M., Thomay, A. A., Connolly, M. D., Reichner, J. S., Albina, J. E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. Journal of Leukocyte Biology. 83 (1), 64-70 (2008).
- Pollenus, E., et al. Limitations of neutrophil depletion by anti-Ly6G antibodies in two heterogenic immunological models. Immunology Letters. 212, 30-36 (2019).
- Wang, J. X., et al. Ly6G ligation blocks recruitment of neutrophils via a β2-integrin-dependent mechanism. Blood. 120 (7), 1489-1498 (2012).
- Cunin, P., et al. Differential attenuation of β2 integrin-dependent and -independent neutrophil migration by Ly6G ligation. Blood Advances. 3 (3), 256-267 (2019).
- Weary, M. E., Donohue, G., Pearson, F. C., Story, K. Relative potencies of four reference endotoxin standards as measured by the Limulus amoebocyte lysate and USP rabbit pyrogen tests. Applied and Environmental Microbiology. 40 (6), 1148-1151 (1980).
- Kiers, D., et al. Comparison of different lots of endotoxin and evaluation of in vivo potency over time in the experimental human endotoxemia model. Innate Immunity. 25 (1), 34-45 (2019).
- Schindelin, J., et al. Fiji: An open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Meijering, E., Dzyubachyk, O., Smal, I. Methods for cell and particle tracking. Methods in Enzymology. 504, 183-200 (2012).
- Sierro, F., et al. A liver capsular network of monocyte-derived macrophages restricts hepatic dissemination of intraperitoneal bacteria by neutrophil recruitment. Immunity. 47 (2), 374-388 (2017).
- Guidotti, L. G., et al. Immunosurveillance of the liver by intravascular effector CD8+ T cells. Cell. 161 (3), 486-500 (2015).
- Davis, R. P., et al. Optimization of in vivo imaging provides a first look at mouse model of non-alcoholic fatty liver disease (NAFLD) using intravital microscopy. Frontiers in Immunology. 10, 2988 (2020).
- Reif, R., et al. In vivo imaging of systemic transport and elimination of xenobiotics and endogenous molecules in mice. Archives of Toxicology. 91 (3), 1335-1352 (2017).
- Sloboda, D. D., Brooks, S. V. Treatment with selectin blocking antibodies after lengthening contractions of mouse muscle blunts neutrophil accumulation but does not reduce damage. Physiological Reports. 4 (1), 12667 (2016).
- Lam, F. W., Da, Q., Guillory, B., Cruz, M. A. Recombinant human vimentin binds to P-selectin and blocks neutrophil capture and rolling on platelets and endothelium. Journal of Immunology. 200 (5), 1718-1726 (2018).
- Mossanen, J. C., Tacke, F. Acetaminophen-induced acute liver injury in mice. Laboratory Animals. 49, 30-36 (2015).
- Guerrero Munoz, F., Fearon, Z. Sex related differences in acetaminophen toxicity in the mouse. Journal of Toxicology. Clinical Toxicology. 22 (2), 149-156 (1984).
- Larsen, A. K., et al. Autofluorescence in freshly isolated adult human liver sinusoidal cells. European Journal of Histochemistry. 65 (4), 3337 (2021).
- Ritsma, L., et al. Intravital microscopy through an abdominal imaging window reveals a pre-micrometastasis stage during liver metastasis. Science Translational Medicine. 4 (158), (2012).
- Jost, A. P., Waters, J. C. Designing a rigorous microscopy experiment: Validating methods and avoiding bias. Journal of Cell Biology. 218 (5), 1452-1466 (2019).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。