Method Article
Quantification of Biventricular Function and Morphology by Cardiac Magnetic Resonance Imaging in Mice with Pulmonary Artery Banding
* 这些作者具有相同的贡献
摘要
To understand the pathophysiology of right ventricular (RV) adaptation to abnormal loading, experimental models are crucial. However, assessment of RV dimensions and function is complex and challenging. This protocol provides a method to perform cardiac magnetic resonance imaging (CMR) as a noninvasive benchmark procedure in mice subjected to RV pressure load.
摘要
Right ventricular (RV) function and failure are major determinants of outcome in acquired and congenital heart diseases, including pulmonary hypertension. Assessment of RV function and morphology is complex, partly due to the complex shape of the RV. Currently, cardiac magnetic resonance (CMR) imaging is the golden standard for noninvasive assessment of RV function and morphology. The current protocol describes CMR imaging in a mouse model of RV pressure load induced by pulmonary artery banding (PAB). PAB is performed by placing a 6-0 suture around the pulmonary artery over a 23 G needle. The PAB gradient is determined using echocardiography at 2 and 6 weeks. At 6 weeks, the right and left ventricular morphology and function is assessed by measuring both end-systolic and end-diastolic volumes and mass by ten to eleven cine slices 1 mm thick using a 9.4 T magnetic resonance imaging scanner equipped with a 1,500 mT/m gradient. Representative results show that PAB induces a significant increase in RV pressure load, with significant effects on biventricular morphology and RV function. It is also shown that at 6 weeks of RV pressure load, cardiac output is maintained. Presented here is a reproducible protocol for the quantification of biventricular morphology and function in a mouse model of RV pressure load and may serve as a method for experiments exploring determinants of RV remodeling and dysfunction.
引言
Patients with acquired and congenital cardiovascular diseases, including pulmonary hypertension (PH), are at risk of right ventricular (RV) dysfunction and failure1. RV adaptation as a result of increased pressure load is characterized by concentric hypertrophy in early stages and progressive dilatation in end-stage disease. Furthermore, it is associated with disorders in metabolism and the extracellular matrix, processes of inflammation and, eventually, RV failure2,3,4,5,6. Animal models have been developed to explore the underlying processes of the progression towards RV failure. However, optimization of models and adequate assessment of RV function and dimensions has been challenging. For noninvasive assessment of RV function and dimensions, cardiac magnetic resonance (CMR) imaging is the golden standard. This technique creates images of the beating heart by using a strong magnetic field and radiofrequency waves. CMR is available for humans, and for animals such as laboratory rodents. As the latter require higher spatial resolution due to the smaller size of the heart, the magnetic field required to provide adequate images must be higher, compared to humans.
Multiple models mimicking RV pressure overload are available, including models of PH7,8,9,10,11,12,13,14,15,16,17 and models of proximal RV pressure load2,3,10,18,19,20,21,22,23. The choice of either a model of PH or a model of proximal RV pressure load depends on the research question: the effect of an intervention on the pulmonary vasculature and therefore possibly RV afterload modulation (i.e., PH models), or the direct effect on the RV (i.e., proximal RV pressure load models). Several methods for experimental induction of PH are available, including use of monocrotaline (MCT)12,13,14,16,22,24,25,26, MCT combined with an aortocaval shunt9, chronic hypoxia7,27,28,29, and the combination of a vascular endothelial growth factor receptor antagonist, Sugen 5416, with chronic hypoxia8,10,30,31. Such models represent progressive pulmonary models of proximal RV pressure load and are not targeted at the pulmonary vasculature but induce a constant afterload by constriction of the pulmonary artery, with an accompanying increase of RV afterload2,3. This can be performed by a suture-banding (pulmonary artery banding, PAB) or a vascular clip around the pulmonary artery. PAB has been performed in several animal species, and cardiac dimensions and function have been studied in various ways, such as histology, transthoracic echocardiography (including speckle tracking), and heart catheterization2,32,33,34,35,36,37,38,39,40. PAB in small rodents, such as mice, is challenging. This is because subtle differences between the tightness of artery constriction have marked results on the degree of RV pressure load and subsequent functional status and survival. When the constriction is very tight, the animal will die during or shortly after operation, whereas the desired phenotype will not be achieved when the constriction is not tight enough. However, the use of mice has advantages compared to other animals, because of the excellent genetic modification possibilities (i.e., transgenic or knockout models) and fast breeding. This is of added value in the study of diseases and in exploring the contribution of molecular and (epi-) genetic factors.
Animal study designs are shifting towards the investigation of temporal changes during disease2,3,8,13,21. For such studies, noninvasive modalities are necessary, because serial assessments can be performed. Alternatives to CMR in the assessment of cardiac remodeling could be (1) tissue characterization using histopathology, with multiple animals being sacrificed at different time points, (2) invasive functional assessment by pressure-volume analysis, or (3) echocardiography, which allows the researcher to identify cardiac hypertrophy or dilatation noninvasively within the same animal serially. CMR has two major advantages in assessment of the RV: (1) CMR is a noninvasive modality, enabling serial measurements in one animal, hereby contributing to reducing animal numbers needed for studies, and (2) CMR does not rely on a particular geometric shape and visualizes three-dimensionally. CMR-derived RV volumes and function measurements have been shown to be accurate and are considered to be the noninvasive golden standard in different cardiac entities in humans42,43,44,45, but had not yet been translated to a CMR protocol for mice with RV pressure overload.
Many models of PAB are described in the literature, but with high heterogeneity in methods of assessing hemodynamic effects and RV function and adaptation. This protocol outlines the procedure of PAB in mice with validation of the model by measuring the PAB gradient by echocardiography and evaluating cardiac dimensions and function with CMR. While a protocol of CMR in animals subjected to PAB has been published for rats, this combination has not been described for mice until now. While rats are most commonly used for PH models8,12,13,14,15,16,22,24,25,26,27,28,29,30,31,46, mice are most often used for transgenic or knock-out studies and thereby contribute to our understanding of mechanisms in pressure-loaded RV failure. This protocol could form the basis for future studies to unravel signaling pathways involved in the transition towards RV failure.
研究方案
All experiments and animal care are conducted according to the Dutch Animal Experimental Act and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. The Animal Experiments Committee of the University of Groningen, the Netherlands, approved the current experimental protocol (permit number: 2014-041/3005).
1. Housing and acclimatization
- Use 20–30 g wild type C57 black 6 (C57BL/6) mice (institutional breeding line described previously47), male and female, all more than 8 weeks old. House the mice in groups with a maximum of five per cage. In order to get used to human handling, let the mice acclimatize for at least 7 days. Do not perform any procedures during this period.
2. Pulmonary artery banding surgery
- Preparation
- Place the mouse in the induction chamber filled with 5% isoflurane/100% oxygen. Check for the lack of reflexes by giving a pain stimulus (i.e., toe pinch).
- Shave the left hemithorax of the mouse using an electric shaver.
- Gently pull out the tongue and hold with mild tension.
- Illuminate the inner throat by placing a light source on the exterior throat at the level of the glottis.
- Intubate the mouse endotracheally with a 20 G flexible cannula.
- Place the animal on its right side on a heat mat (set temperature at 37 °C).
- Connect the cannula to the miniventilator and start ventilation with 1.5%–2.5% isoflurane/oxygen (180 breaths/min, tidal 250 μL).
- Inject 0.1 mg/kg buprenorphine subcutaneously for postoperative analgesia.
- Prevent dehydration of the eye using eye ointment.
- Pulmonary artery banding surgery by left lateral thoracotomy
- Place the mouse on its right side by placing the right foreleg in a neutral position, the right hind leg extended, and the left foreleg bent back.
- Disinfect the skin on the thorax with chloride-hexidine, swab 2x.
- Use sterile instruments for surgery. Open the skin with small scissors (round handle, 12 mm blades) from the left armpit parallel to the second and third rib.
- Identify the m. pectoralis superficialis (oblique, superficial muscle) and the m. pectoralis profundus (oblique, underlying muscle).
- Using suture loops, pull the m. pectoralis superficialis towards the ventral side and the m. pectoralis profundus towards the dorsal side of the mouse.
- Open the second intercostal space and spread the ribs by using adapted paper clips, allowing the left heart ear, left lung, and the pulmonary artery to become visible.
- Separate the arteria pulmonalis from the aorta. Place a suture loop around the pulmonary artery with a blunt 25 G needle that contains a 6-0 suture and place a lose 2-1-1 ligature around the arteria pulmonalis.
- Place a 23 G needle parallel to the arteria pulmonalis within the 6-0 suture and first fix the most proximal suture knot and then distal knot of the 2-1-1 suture. Remove the 23 G needle. Make sure the knot is adequate.
- Close the thorax with two or three separate sutures with a monofilament polypropylene 5-0 suture. Release the m. pectoralis superficalis and m. pectoralis profundus.
- Suture the skin with a pure polyglycolic acid 5-0 suture. Use a continuous suture technique to minimize scar formation in the tissue; scar tissue will influence the image quality of the echocardiography.
- Turn off the isoflurane while continuing ventilation with oxygen during recovery from anesthesia until the mouse regains its own, spontaneous respiration as can be observed from movement of the abdomen.
- Uncouple the endotracheal tube from the ventilator. Check for spontaneous respiration, extubate only when spontaneous respiratory action is visible. When spontaneous respiration is not seen, connect the tube to the ventilator again and return to step 2.2.12.
- Observe the mouse until it regains consciousness.
- Sham surgery
- Perform the above procedure with the exception of the banding (steps 2.2.2–2.2.6).
- Postsurgical period
- House the mouse individually in an incubator (37 °C) for 24 h.
- Observe the mouse daily during the first 3 postoperative days. In case of any signs of discomfort, inject 0.1 mg/kg buprenorphine subcutaneously 2x daily for postoperative analgesia.
3. Echocardiography
- Preparation
- Perform PAB gradient analysis by means of echocardiography 14 days after PAB surgery.
- Start the echocardiography device. Choose the cardiac package and a 14.0 MHz transducer.
- Anesthesia
- Place the mouse in the induction chamber filled with a mixture of 5% isoflurane and 100% oxygen.
- Shave the thorax of the mouse.
- Place the mouse on its back on the heat mat (temperature 37 °C) and place the snout in the ventilation mask.
- Ventilate with a mixture of 1.5%–2.5% isoflurane and 100% oxygen (0.15 L/min) and room air (0.3 L/min).
- Check the depth of the anesthesia by performing a toe pinch and adjust the anesthesia accordingly.
- Prevent dehydration of the eye by using eye ointment.
- Determination of PAB gradient by echocardiography
- Place pediatric electrocardiogram-stickers on each foreleg and one on both hind legs. Use the stickers to hold the animal.
- Apply ultrasound gel to the shaved part of the mouse’s thorax.
- To obtain the images of the pulmonary artery, two views can be used: the parasternal long axis (PLAX) or the parasternal short axis (PSAX) view. Obtain both and use the view that gives the best quality measurements and highest velocities for analysis.
- Obtain PLAX and PSAX views.
- Press the color-Doppler button to visualize blood flow.
- Place the ultrasound probe at a 30° angle to the parasternal line to obtain PLAX (for detailed description see Cheng, et al.48), visualizing the ascending aorta.
- Sweep the probe minimally towards the left so the ascending aorta disappears behind the pulmonary artery. The appropriate PLAX is identified when the pulmonary artery is visualized, with blood flowing vertically.
- Place the cursor in line with the pulmonary artery. Press the continuous wave (CW) Doppler button to derive velocity time integral measurements during three cardiac cycles. Press Save.
- Rotate the probe 90° clockwise from the PLAX to obtain PSAX, then tilt the probe slightly towards the cranial/ventral direction to derive the PSAX at the aortic level. The appropriate PSAX view is identified if the RV outflow tract is situated between the aorta and the probe. This continues in the pulmonary artery, with blood flowing vertically. For a detailed description see Cheng et al.48
- Place the cursor in line with the pulmonary artery. Press the continuous wave (CW) Doppler button to derive velocity time integral measurements during three cardiac cycles. Press Save.
- Measure the three maximum velocities of the best view (PSAX or PLAX) and calculate the mean. Use the simplified Bernoulli’s principle to derive the PAB gradient in millimeters mercury (mmHg).
4. Cardiac magnetic resonance imaging
- Preparation
- Perform CMR analysis 6 weeks (i.e., 42 days) after PAB surgery.
NOTE: Additionally, earlier timepoints after PAB surgery may be chosen when multiple timepoints are to be included, depending on the research question. Later timepoints could be considered; however, RV failure and death may increasingly occur. - Use a sufficiently powerful magnet (typically, >7 T is used for rodent CMR scanning). For the current protocol, a 9.4 T vertical system, with 1,500 mT/m gradient set and 89 mm bore size is used.
- Install CMR postprocessing software for analyzing volumes and masses in the derived images. The software is deemed appropriate if it allows manual segmentation to determine end-diastolic (ED) and end-systolic (ES) volumes (EDV and ESV, respectively) and ventricular mass (measured both ED and ES).
- Perform CMR analysis 6 weeks (i.e., 42 days) after PAB surgery.
- Anesthesia and fixation
- Place the mouse in the induction chamber filled with a mixture of 5% isoflurane and 100% oxygen. Verify the effect of the anesthesia by giving a pain stimulus by a toe pinch.
- Put eye ointment on the eyes of the mouse to keep them moist during scanning.
- Place the mouse in the scanner's animal bed with integrated air supply, a warmed (37 °C) mixture of 1.5%–2.5% isoflurane, 100% oxygen (0.15 L/min), and room air (0.3 L/min), and a pressure pad that enables observation of heart rate (aim for 400–500 bpm) and respiratory rate (aim for ~35 breaths per min) during scanning. Regulate the anesthesia based those two parameters. Make sure the bed is made of plastic, without any magnetic material.
- Place the animal bed with the mouse into the scanner.
- Performing cardiac magnetic resonance imaging
- Make preacquisition adjustments by tuning the radiofrequency (RF) birdcage coil on 1 Hydrogen (1 H) resonance frequency.
- Then set the magnetic field as homogeneous as possible using the automatic shimming procedure.
NOTE: The computerized shimming is done by the so-called Tuning method, which uses the area under the 1 H FID as a quality parameter. In this Tuning procedure a user-defined group of shims (Z, Z2, X, Y, XZ, and YZ) is examined in an iterative cycle. Each shim in succession is adjusted individually to maximize the area under the FID. This is essentially a linear procedure that works well quickly. - Optimize the RF pulse by maximizing the one-dimensional image profile with adjustment of RF pulse power.
- Assign the exact position of the heart in the scanner by making scout scans using a tripilot sequence. Use a fast gradient echo sequence to acquire the scout images through the thorax: a transversal, coronal, and sagittal slice. (Figure 1A,B,C)
- Adjust the axes to the actual axes of the axial, two-chamber, and four-chamber view (Figure 1D,E).
- Subsequently, position the cine slices perpendicular to an imaginary axis between the RV outflow tract and the utmost apical part of the RV.
- Derive ten to eleven 1 mm-thick cine slices without a slice gap to cover the entire top to base imaging of the RV (Figure 1F) by means of the Self-gated IntraGate-fast low-angle shot (FLASH) method, which obviates the need for an electrocardiogram (ECG) and respiratory gating. The acquisition parameters are displayed in Table 1. Save the images in DICOM format.
- Performing analyses on acquired images
- Double click on the software to open the program.
- Open images in the CMR postprocessing software by using the import button.
- Identify the end-systolic phase (defined as the phase with the visually smallest RV cavity) and the end-diastolic phase (defined as the phase with the visually largest RV cavity).
- According to guidelines from the Society for Cardiovascular Magnetic Resonance49, draw the epicardial contours manually in end-diastole and end-systole from apex to base, by marking several points at the epicardial border of each image. At the last point, double click to complete the epicardial contour.
- Do the same for the endocardial contours. (Figure 2). The left ventricular and right ventricular EDV, ESV, ED mass, and ES mass are now automatically calculated by the software.
NOTE: Mass is defined as myocardial volume times myocardial density (i.e., 1.05). - Depending on the research question and population under study, index these variables for subject size by means of tibia length or body weight, according to previously published formulas50.
- Calculate the eccentricity index (EI) both in end-diastole and end-systole, by dividing the diameter of the LV cavity parallel to the intraventricular septum (IVS) by the diameter of the LV cavity perpendicular to the IVS, derived from the short axis at midpapillary level.
- The software calculates the stroke volume (SV) in mL as
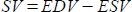 , and ejection fraction (EF, %) as
, and ejection fraction (EF, %) as  .
. - Calculate the cardiac output (CO) in ml/min as
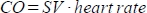 . The heart rate is measured manually by the pressure pad embedded in the animal bed as described above, because the scanner is not able to register the high frequent heart rate adequately.
. The heart rate is measured manually by the pressure pad embedded in the animal bed as described above, because the scanner is not able to register the high frequent heart rate adequately. - Depending on the research question and population under study, index the CO and SV for subject size by means of tibia length or body weight, according to previously published formulas50.
5. Statistical analyses
- Open the software used for data visualization and statistical analyses.
- Sort the data per group (PAB and sham) with every group in a separate column.
- Use the Mann-Whitney test to compare PAB versus sham for every variable.
结果
Mortality rate of the PAB surgical procedure is around 10%. The presented results show characteristics of mice in the sham (n = 5) and PAB (n = 8) groups. As shown in Figure 3, PAB gradient values significantly increased compared to sham animals at 2 and 6 weeks after PAB. This increase of loading caused RV dilatation expressed as increased RV, EDV, and RV ESV (Figure 4A,B). RV dysfunction occurred as RV EF decreased (Figure 4C). RV SV remained unaffected (Figure 4D). RV ED and RV ES mass increased, indicating right ventricular hypertrophy (Figure 4E,F). LV EDV and LV ESV decreased (Figure 4G,H). LV function in terms of LV EF and LV SV was unaffected (Figure 4I,J). Neither LV ED or LV ES mass changed (Figure 4K,L). Septal flattening at both end-diastole and end-systole occurred, reflected by significant decreases of both eccentricity indexes (Figure 4M,N). Heart rate and SV were not different between PAB and sham animals and thus CO was unaffected (Figure 4P,Q). Figure 4O shows representative CMR images at midpapillary level, in end-diastole (top) and end-systole (below) in sham (left) and PAB (right).
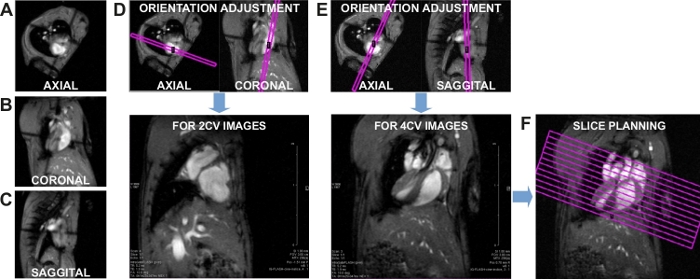
Figure 1: Slice orientation and planning. (A) Axial scout image, (B) coronal scout image, and (C) sagittal scout image. (D) Adjustment of the orientation slice for a two-chamber view (2CV) image. (E) Adjustment of the orientation slice for a four-chamber view (4CV) image. (F) Slice planning for cardiac cine imaging. Please click here to view a larger version of this figure.

Figure 2: CMR quantification. For quantification, the endocardial (red for LV, yellow for RV) and epicardial (green for LV, blue for RV) contours were delineated in end-diastole (ED, top) and end-systole (ES, bottom) in a stack of short axis slices that covered both ventricles. These are shown in a sham and a pulmonary artery banding (PAB) mouse. Please click here to view a larger version of this figure.
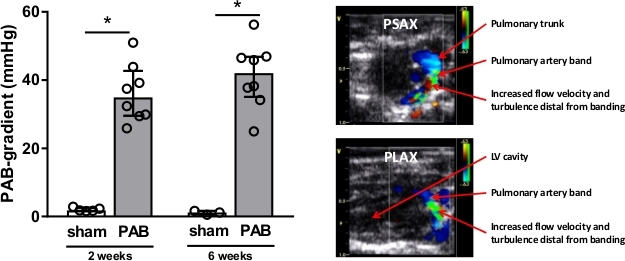
Figure 3: PAB gradient measured by Doppler echocardiography. Measurements were performed at 2 and 6 weeks respectively in the sham (n = 5) and pulmonary artery banding (PAB, n = 8) groups. The statistical analyses were performed using the Mann-Whitney test. Values are presented as median and interquartile range. * = p < 0.05 compared to sham. ○ = individual animal. PSAX = parasternal short axis. PLAX = parasternal long axis. LV = left ventricle. Please click here to view a larger version of this figure.

Figure 4: Representative results of morphological and functional changes. The first panel shows RV parameters: RV EDV (A), RV ESV (B), RV EF (C), RV ED mass (E), and RV ES mass (F). The LV parameters are shown in the second panel: LV EDV (G), LV ESV (H), LV EF (I), LV SV (J), LV ED mass (K), and LV ES mass (L). Septal deviation is represented by the eccentricity index ED (M) and ES (N). Cardiac dimensions are shown in representative images (O). Heart rate (P) and cardiac output (Q) are also shown. The changes were observed due to 6 weeks of PAB measured by CMR in the sham (n = 5) and pulmonary artery banding (PAB, n = 8) groups. The statistical analyses were performed using the Mann-Whitney test. Values are presented as median and interquartile range. * = p < 0.05 compared to sham. ○ = individual animal. RV = right ventricle. LV = left ventricle. ED = end diastolic. ES = end systolic. EDV = ED volume. ESV = ES volume. SV = stroke volume. CO = cardiac output. EF = ejection fraction. Please click here to view a larger version of this figure.
| Echo time (ms) | 1.286 |
| Repetition time (ms) | 9.226 |
| Radiofrequency pulse (ms) | 0.300 |
| Flip angle (degrees) | 10 |
| Spectral width (Hz) | 75,757 |
| Echo position (%) | 20 |
| Acquisition m atrix | 256 x 128 |
| Reconstructed matrix | 256 x 256 |
| In-plane resolution (µm) | 117x117 |
| Averages | 8 |
| Frames per heart beat | 15 |
| Slice thickness (mm) | 1 |
| Navigator points | 256 |
| Acquisition time per slice (s) | 120 |
Table 1: Acquisition parameters of the CMR protocol.
讨论
This protocol provides a reproducible method for PAB in mice and the subsequent assessment of cardiac remodeling and functional adaptation using CMR.
PAB differs from other models of increased RV pressure load because it involves absolute and static increase of afterload without the presence of other triggers. RV pressure load in models of hypoxia, monocrotaline, shunt, or a combination of these inducers are based on remodeling of the pulmonary vasculature. This remodeling is driven by endothelial damage, inflammation, cytokine migration, and vasoconstriction. The degree of these processes differs per model, therefore the degree of pressure load differs subsequently. In contrast to these models, PAB induces fixed RV afterload and is therefore reproducible and not affected by therapeutic interventions. This allows for the study of interventions targeting the pressure-loaded RV without affecting the RV afterload. This model of PAB in mice shows a significant gradient across the PAB and enables evaluation of this substantial pressure load.
Dimensional evaluation by echocardiography is challenging due to the triangular shape of the RV wrapped around the LV, and its position immediately behind the sternum41,42. Echocardiography, both 2D and 3D, has shown to be inferior compared to CMR51,52. In pediatric cohorts with congenital heart diseases, echocardiographic volumetry shows lower reliability and systematic underestimation compared to CMR53,54. Results regarding myocardial deformation measurements are still preliminary in this specific group of patients55,56,57. Of course, in clinical practice, echocardiography is a very accessible tool to identify abnormal loading conditions by recognition of shunts and valve insufficiencies in case of volume load, and stenosis and pulmonary hypertension by increased gradients and septal flattening in case of pressure load. Pressure-volume analysis by means of invasive heart catheterization is an available alternative for invasive hemodynamic and functional assessments. This technique is widely regarded indicative for load-independent ventricular function, but also comes with limitations that currently hamper the theoretical benchmark status of PV-loops in RV in small animals. For example, deriving reproducible and accurate volume and flow measurements is challenging in these small animals and many procedures require open-chest measurements. Furthermore, serial assessments are difficult if not unfeasible due to the invasive nature of the technique. Compared to both echocardiography and catheterization, assessment of volumes and function will be more accurate with CMR. In research, it is important for translatability to obtain results using modalities that can also be used for clinical practice. Therefore, development of standardized methods and optimization in experimental protocols is highly relevant.
The current protocol describes the use of self-gated CMR, which obviates the need for ECG triggering and respiratory gating. This method has been described previously in a report from the same institution, demonstrating good intra- and interobserver variability58. Another method that could be used if the self-gated method is unavailable, is prospective ECG triggering. However, a previous report from this institution demonstrated that the self-gated method provides less variability, better signal and contrast-to-noise ratios, and less arrhythmia-induced artifacts. Therefore, we recommend using the self-gated method, as stated in the current protocol.
Accurate assessment of RV pressure load is crucial in order to validate the PAB model. This can be performed by means of invasive heart catheterization, for example. However, disadvantages of such invasive procedures are that they are very hazardous and complex to perform serially or during the follow-up time of the study and are therefore generally performed just before termination. However, at termination, RV pressure is not only dependent on the tightness of the banding but becomes increasingly dependent on RV function. Whenever RV failure occurs within the duration of PAB, as measured by decreased cardiac output, RV systolic pressure will decrease, biasing results. Such biases can be avoided or minimized by assessing RV pressure load at 2 weeks after PAB surgery, instead of at termination. By means of echocardiography, assessment of RV afterload at this time point can be performed reliably and safely. This allows grouping of the mice into groups with equal pressure load, which could be helpful for intervention studies. Also, repeated measurements are easily feasible.
The most critical step in the surgical protocol is the separation of the arteria pulmonalis from the aorta and the subsequent placement of the suture loop. This has to be performed gently in order not to cause any rupture, because this would result in fatal bleeding. PAB in mice requires that well-trained microsurgeons perform the actual banding, including knotting the suture, which should be done very carefully.
The current model aims to generate chronic RV pressure load, resulting in RV remodeling, RV dysfunction, and eventually RV failure. Therefore, adequate tightening of the PAB is important. During the development of the model, it has become apparent that small differences in tightness of the banding significantly affected the profile of RV adaptation: e.g., the use of a 25 G needle appeared to be “too tight”, as it induced high rates of mortality during surgery. Needles <23 G were “too loose”, as they did not induce the desired phenotype of RV remodeling and dysfunction.
The most critical step in the echocardiographic examination is adequate measurement of pulmonary flow velocity (step 3.3.7). One has to make sure that the angle of the probe is correct: the pulmonary artery has to be exactly vertically visible within the image. Otherwise, flow velocity, and therefore the PAB gradient, are underestimated.
It is important to try to limit the length of time of the procedures during the experiment, especially CMR. Furthermore, when analyzing the CMR images with postprocessing software, the researcher must become familiar with the manual segmentation and postprocessing guidelines before reproducible results can be obtained.
Using CMR as in the current protocol does not enable assessment of flow velocities over the PAB. Therefore, additional echocardiographic measurements using the Doppler mode are inevitable. Due to the PAB and the subsequent marked increase in PA flow, the signal is very clear, making determination of the PAB gradient by echocardiography convenient and reproducible. Notwithstanding, the extra echocardiographic measurements may involve more logistical arrangements. In general, inclusion or exclusion of papillary muscles and trabeculae affects volumes and subsequent functional parameters. Here, we chose to include papillary muscles and trabeculae in blood volumes (and thus exclude from myocardial mass) which may underestimate the ejection fraction. Furthermore, the current protocol focuses on parameters used in clinical practice, representing global function. Parameters such as the tricuspid annular plane systolic excursion (TAPSE), fractional septum to free wall distance at the middle of the RV (fSFD), and fractional tricuspid annulus-apex distance change (fTAAD) were not analyzed.
A major advantage of CMR is the ability to perform noninvasive, serial testing within one subject with a relatively high accuracy of volumetric and functional measurements. Because it is a measurement after which the animal can survive, unlike open-chest pressure-volume analysis, for example, it allows for a follow-up after the measurements. Although we have focused on cardiac dimensions and function, future uses of this technique include CMR-derived tissue characterization or scar tissue assessment by means of late gadolinium enhancement. This enables reduction of histopathological assessments, which will lead to a reduction in animals required for studies. More CMR research may optimize tissue characterization in humans and reduce iatrogenic damage due to biopsies.
In conclusion, this protocol was created to provide guidance in the assessment of cardiac morphology and function in mice exposed to increased RV pressure load. The combination of PAB with CMR improves standardization and reproducibility. This makes it a very valuable technique for the study of signaling pathways involved in the failure of the pressure-loaded RV by the use of transgenic or knockout mice.
披露声明
The University Medical Center Groningen has contracted with Actelion and Lilly for consultancy activities of R.M.F. Berger outside the content of this manuscript. The other authors declare that they have no competing interests.
致谢
We would like to thank P. Da Costa-Martins for her support with the animal experiments in this study.
材料
| Name | Company | Catalog Number | Comments |
| 14.0 MHz i13L-echocardiography transducer | GE Healthcare, Waukesha, WI, USA | ||
| 20G cannula | |||
| 23G needle | |||
| 9.4T magnetic resonance scanner with 1,500 mT/m gradient set | Bruker BioSpin, Ellingen, Germany | ||
| Anesthesia induction chamber | |||
| Blunt 25G needle | |||
| Buprenorphine | |||
| Chloride-hexidine | |||
| CMR post-processing software | Medis Medical Imaging Systems, Leiden, The Netherlands | Qmass version 7.6 | |
| Data visualisation and statistical software | GraphPad Prism Inc, La Jolla, CA, USA | software version 7.02 | |
| Echocardiography machine | GE Healthcare, Waukesha, WI, USA | Vivid Dimension 7 | |
| Eye ointment | |||
| Heat mat | |||
| Incubator (37°C) | |||
| Isoflurane | |||
| Isoflurane evaporator | |||
| Miniventilator for rodents | Hugo Sachs | model 687 | |
| monofilament polypropylene 5-0 sutures | |||
| monofilament polypropylene 6-0 sutures | |||
| Needle and syringe for subcutaneous injections | |||
| Pediatric electrocardiogram-stickers | |||
| pure polyglycolic acid 5-0 sutures | |||
| Sterile surgical instruments | |||
| Ventilation mask |
参考文献
- Norozi, K., et al. Incidence and Risk Distribution of Heart Failure in Adolescents and Adults With Congenital Heart Disease After Cardiac Surgery. The American Journal of Cardiology. 97 (8), 1238-1243 (2006).
- Borgdorff, M. A. J., et al. Clinical symptoms of right ventricular failure in experimental chronic pressure load are associated with progressive diastolic dysfunction. Journal of Molecular and Cellular Cardiology. 79, 244-253 (2015).
- Koop, A. M. C., et al. Right ventricular pressure overload alters cardiac lipid composition. International Journal of Cardiology. , (2019).
- Faber, M. J., et al. Right and left ventricular function after chronic pulmonary artery banding in rats assessed with biventricular pressure-volume loops. American Journal of Physiology and Heart Circirculation Physiology. 291 (4), 1580-1586 (2006).
- Bogaard, H. J., et al. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 120 (20), 1951-1960 (2009).
- Samson, N., Paulin, R. Epigenetics, inflammation and metabolism in right heart failure associated with pulmonary hypertension. Pulmonary Circulation. 7 (3), 572-587 (2017).
- Rumsey, W. L., et al. Adaptation to hypoxia alters energy metabolism in rat heart. American Journal of Physiology Heart and Circulatory Physiology. 276 (1), 71-80 (1999).
- Drozd, K., et al. Effects of an endothelin receptor antagonist, Macitentan, on right ventricular substrate utilization and function in a Sugen 5416/hypoxia rat model of severe pulmonary arterial hypertension. Journal of Nuclear Cardiology. 24 (6), 1979-1989 (2017).
- Van Der Feen, D. E., et al. Shunt surgery, right heart catheterization, and vascular morphometry in a rat model for flow-induced pulmonary arterial hypertension. Journal of Visualized Experiments. (120), e55065 (2017).
- Gomez-Arroyo, J., et al. Metabolic gene remodeling and mitochondrial dysfunction in failing right ventricular hypertrophy secondary to pulmonary arterial hypertension. Circulation: Heart Failure. 6 (1), 136-144 (2013).
- Bruns, D. R., Dale Brown, R., Stenmark, K. R., Buttrick, P. M., Walker, L. A. Mitochondrial integrity in a neonatal bovine model of right ventricular dysfunction. American Journal of Physiology - Lung Cellular and Molecular Physiology. 308 (2), 158-167 (2015).
- Zhang, W. H., et al. Up-regulation of hexokinase1 in the right ventricle of monocrotaline induced pulmonary hypertension. Respiratory Research. 15 (1), 119 (2014).
- Paulin, R., et al. A miR-208-Mef2 axis drives the decompensation of right ventricular function in pulmonary hypertension. Circulation Research. 116 (1), 56-69 (2015).
- Sutendra, G., et al. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. Journal of Molecular Medicine. 91 (11), 1315-1327 (2013).
- Balestra, G. M., et al. Increased in vivo mitochondrial oxygenation with right ventricular failure induced by pulmonary arterial hypertension: Mitochondrial inhibition as driver of cardiac failure. Respiratory Research. 16, 6 (2015).
- Piao, L., et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: Resuscitating the hibernating right ventricle. Journal of Molecular Medicine. 88 (1), 47-60 (2010).
- Piao, L., et al. FOXO1-mediated upregulation of pyruvate dehydrogenase kinase-4 (PDK4) decreases glucose oxidation and impairs right ventricular function in pulmonary hypertension: therapeutic benefits of dichloroacetate. Journal of Molecular Medicine. 91, 333-346 (2013).
- Sheikh, A. M., et al. Right ventricular hypertrophy with early dysfunction: A proteomics study in a neonatal model. Journal of Thoracic and Cardiovascular Surgery. 137 (5), 1146-1153 (2009).
- Olivetti, G., et al. Cellular basis of wall remodeling in long-term pressure overload-induced right ventricular hypertrophy in rats. Circulation Research. 63 (3), 648-657 (1988).
- Lauva, I. K., et al. Control of myocardial tissue components and cardiocyte organelles in pressure-overload hypertrophy of the cat right ventricle. The American Journal of Anatomy. 177 (1), 71-80 (1986).
- Fang, Y. H., et al. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: Exploiting Randle's cycle. Journal of Molecular Medicine. 90 (1), 31-43 (2012).
- Piao, L., et al. Cardiac glutaminolysis: A maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. Journal of Molecular Medicine. 91 (10), 1185-1197 (2013).
- Sack, M. N., Disch, D. L., Rockman, H. A., Kelly, D. P. A role for Sp and nuclear receptor transcription factors in a cardiac hypertrophic growth program. Proceedings of the National Academy of Sciences of the United States of America. 94 (12), 6438-6443 (1997).
- Broderick, T. L., King, T. M. Upregulation of GLUT-4 in right ventricle of rats with monocrotaline- induced pulmonary hypertension. Medical Science Monitor. 14 (12), 261-264 (2008).
- Enache, I., et al. Skeletal muscle mitochondrial dysfunction precedes right ventricular impairment in experimental pulmonary hypertension. Molecular and Cellular Biochemistry. 373 (1-2), 161-170 (2013).
- Sun, X. Q., et al. Reversal of right ventricular remodeling by dichloroacetate is related to inhibition of mitochondria-dependent apoptosis. Hypertension Research. 39 (5), 302-311 (2016).
- Adrogue, J. V., Sharma, S., Ngumbela, K., Essop, M. F., Taegtmeyer, H. Acclimatization to chronic hypobaric hypoxia is associated with a differential transcriptional profile between the right and left ventricle. Molecular and Cellular Biochemistry. 278 (1-2), 71-78 (2005).
- Sharma, S., et al. Dynamic changes of gene expression in hypoxia-induced right ventricular hypertrophy. American Journal of Physiology - Heart and Circulatory Physiology. 286 (3), 1185-1192 (2004).
- Nouette-Gaulain, K., et al. Time course of differential mitochondrial energy metabolism adaptation to chronic hypoxia in right and left ventricles. Cardiovascular Research. 66 (1), 132-140 (2005).
- Graham, B. B., et al. Severe pulmonary hypertension is associated with altered right ventricle metabolic substrate uptake. American Journal of Physiology - Lung Cellular and Molecular Physiology. 309 (5), 435-440 (2015).
- Liu, A., et al. Estrogen maintains mitochondrial content and function in the right ventricle of rats with pulmonary hypertension. Physiological Reports. 5 (6), 1-12 (2017).
- Kobr, J., et al. Right Ventricular Pressure Overload and Pathophysiology of Growing Porcine Biomodel. Pediatric Cardiology. 37 (8), 1498-1506 (2016).
- Yerebakan, C., et al. Acute and chronic response of the right ventricle to surgically induced pressure and volume overload - an analysis of pressure-volume relations. Interactive CardioVascular and Thoracic Surgery. 10 (4), 519-525 (2010).
- Gufler, H., et al. Right Ventricular Function After Pulmonary Artery Banding: Adaptive Processes Assessed by CMR and Conductance Catheter Measurements in Sheep. Journal of Cardiovascular Translational Research. 12 (5), 459-466 (2019).
- Baicu, C. F., et al. Time course of right ventricular pressure-overload induced myocardial fibrosis: relationship to changes in fibroblast postsynthetic procollagen processing. American Journal of Physiology-Heart and Circulatory Physiology. 303 (9), 1128-1134 (2012).
- Manohar, M., et al. Regional myocardial blood flow and coronary vascular reserve in unanesthetized young calves exposed to a simulated altitude of 3500 m for 8-10 weeks. Circulation Research. 50 (5), 714-726 (1982).
- Fávaro, G. A. G., et al. Reversible pulmonary trunk banding: VII. Stress echocardiographic assessment of rapid ventricular hypertrophy in young goats. Journal of Thoracic and Cardiovascular Surgery. 145 (5), 1345-1351 (2013).
- Nielsen, E. A., et al. Regional septal hinge-point injury contributes to adverse biventricular interactions in pulmonary hypertension. Physiological Reports. 5 (14), 1-13 (2017).
- Borgdorff, M. A., et al. Sildenafil enhances systolic adaptation, but does not prevent diastolic dysfunction, in the pressure-loaded right ventricle. European Journal of Heart Failure. 14 (9), 1067-1074 (2012).
- Gold, H., Prindle, K., Levey, G., Epstein, S. Effects of experimental heart failure on the capacity of glucagon to augment myocardial contractility and activate adenyl cyclase. The Journal of Clinical Investigation. 49 (5), 999-1006 (1970).
- Brittain, E. L., et al. Right ventricular plasticity and functional imaging. Pulmonary Circulation. 2 (3), 309-326 (2012).
- Jiang, L., et al. Three-dimensional Echocardiography In Vivo Validation for Right Ventricular Volume and Function. Circulation. 89, 2342-2350 (1994).
- Markiewicz, W., Sechtem, U., Higgins, C. B. Evaluation of the right ventricle by magnetic resonance imaging. American Heart Journal. 113 (1), 8-15 (1987).
- Pattynama, P. M. T., et al. Reproducibility of MRI-derived measurements of right ventricular volumes and myocardial mass. Magnetic Resonance Imaging. 13 (1), 53-63 (1995).
- Wiesmann, F., et al. Comparison of fast spiral, echo planar, and fast low-angle shot MRI for cardiac volumetry at .5T. Journal of Magnetic Resonance Imaging. 8 (5), 1033-1039 (1998).
- Van der Feen, D. E., et al. Multicenter Preclinical Validation of BET Inhibition for the Treatment of Pulmonary Arterial Hypertension. American Journal of Respiratory and Critical Care Medicine. 200 (7), 910-920 (2019).
- da Costa Martins, P. A., et al. MicroRNA-199b targets the nuclear kinase Dyrk1a in an auto-amplification loop promoting calcineurin/NFAT signalling. Nature Cell Biology. 12 (12), 1220-1227 (2010).
- Cheng, H. W., et al. Assessment of right ventricular structure and function in mouse model of pulmonary artery constriction by transthoracic echocardiography. Journal of Visualized Experiments. (84), e51041 (2014).
- Schulz-Menger, J., et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) Board of Trustees Task Force on Standardized Post Processing. Journal of Cardiovascular Magnetic Resonance. 15 (1), 1-19 (2013).
- Hagdorn, Q. A. J., et al. A novel method optimizing the normalization of cardiac parameters in small animal models: The importance of dimensional indexing. American Journal of Physiology - Heart and Circulatory Physiology. 316 (6), 1552-1557 (2019).
- Scherrer-Crosbie, M., et al. Determination of Right Ventricular Structure and Function in Normoxic and Hypoxic Mice. Circulation. 98 (10), 1015-1021 (2012).
- Wiesmann, F., et al. Analysis of right ventricular function in healthy mice and a murine model of heart failure by in vivo MRI. American Journal of Physiology-Heart and Circulatory Physiology. 283 (3), 1065-1071 (2002).
- Lu, X., et al. Accuracy and Reproducibility of Real-Time Three-Dimensional Echocardiography for Assessment of Right Ventricular Volumes and Ejection Fraction in Children. Journal of the American Society of Echocardiography. 21 (1), 84-89 (2008).
- Soriano, B. D., et al. Matrix-array 3-dimensional echocardiographic assessment of volumes, mass, and ejection fraction in young pediatric patients with a functional single ventricle: A comparison study with cardiac magnetic resonance. Circulation. 117 (14), 1842-1848 (2008).
- Damy, T., et al. Prevalence of, associations with, and prognostic value of tricuspid annular plane systolic excursion (TAPSE) among out-patients referred for the evaluation of heart failure. Journal of Cardiac Failure. 18 (3), 216-225 (2012).
- Kowalik, E., Kowalski, M., Rózański, J., Kuśmierczyk, M., Hoffman, P. The impact of pulmonary regurgitation on right ventricular regional myocardial function: An echocardiographic study in adults after total repair of tetralogy of fallot. Journal of the American Society of Echocardiography. 24 (11), 1199-1204 (2011).
- Koestenberger, M., et al. Systolic right ventricular function in pediatric and adolescent patients with tetralogy of Fallot: Echocardiography versus magnetic resonance imaging. Journal of the American Society of Echocardiography. 24 (1), 45-52 (2011).
- Bovens, S. M., et al. Evaluation of infarcted murine heart function: Comparison of prospectively triggered with self-gated MRI. NMR in Biomedicine. 24 (3), 307-315 (2011).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。