Method Article
A Stepwise Approach for Performing Ultrasound Guided Transthoracic Lung Biopsy
In This Article
Summary
Transthoracic ultrasound-guided lung biopsy represents a safe, cost-effective, and efficient approach for patients presenting with subpleural lung lesions suspected of malignancy. Employing a systematic, step-by-step process is crucial to achieve optimal patient selection, minimize complication risks, and maximize diagnostic accuracy.
Abstract
Diagnosing patients with radiological lung lesions, especially those suspected of having primary lung cancer, is a common and critical clinical scenario. When selecting the most suitable invasive procedure to establish a diagnosis in these cases, a delicate balance must be struck between achieving a high diagnostic yield, providing staging information, minimizing potential complications, enhancing the patient experience, and controlling costs. The integration of thoracic ultrasound as a routine clinical tool in respiratory medicine has led to increased awareness and utilization of ultrasound-guided invasive techniques in chest procedures, including transthoracic biopsies. By following a systematic and stepwise approach, transthoracic ultrasound-guided lung biopsy emerges as a safe, cost-effective procedure with a remarkable diagnostic accuracy. These attributes collectively position it as an ideal invasive technique when technically feasible. Consequently, in patients presenting subpleural lung lesions suspected of malignancy, transthoracic ultrasound-guided lung biopsy has become a standard procedure in the realm of modern invasive pulmonology.
Introduction
Establishing a diagnosis in patients with radiological lung lesions is crucial, particularly when malignancy is suspected. Tissue sampling is essential for confirming malignancy, obtaining additional information like genotyping and staging, and diagnosing non-malignant lung lesions (e.g., infection or vasculitis)1,2.
Several invasive procedures are available for tissue sampling in patients with lung lesions, including conventional bronchoscopy, bronchoscopy supplemented with radial endobronchial ultrasound (REBUS), electromagnetic navigation bronchoscopy (ENB), endobronchial ultrasound (EBUS), endoscopic ultrasound (EUS), computed tomography guided transthoracic biopsy (CT-TTNB), and surgical biopsy3,4,5,6. The selection of the optimal procedure involves balancing factors like diagnostic yield, complication risks, patient comfort, and resource allocation3.
Most of these techniques have limitations. For instance, conventional bronchoscopy is less effective for peripheral lesions, CT-TTB carries a higher risk of complications (especially pneumothorax), and procedures like ENB and surgical biopsy can be costly4,6,7.
Ultrasound-guided transthoracic needle biopsy (US-TTNB) is an alternative method for obtaining tissue samples from lung lesions. The technique itself is not new, but its use has significantly increased in patients with peripheral lung lesions of unknown origin, particularly among pulmonologists, who now routinely use thoracic ultrasound (TUS) for point-of-care diagnostics and basic procedural guidance in various clinical scenarios8,9. In addition, ultrasound equipment is now more widely available, with TUS training increasingly formalized and made more accessible to clinicians at an earlier stage9,10,11,12,13,14,15,16.
US-TTNB carries several potential advantages. Complication rates and costs related to the procedure are low, while diagnostic yields remain comparable to other approaches, especially CT-TTB17,18,19,18,19,20,21. Its major limitation, however, is its suitability only for a limited proportion of lung lesions that can be appropriately visualized using TUS. Therefore, it cannot be used for lesions that do not contact the parietal pleura at the chest wall, such as any central tumor; lesions behind structures impenetrable to ultrasound waves, such as the scapula; or lesions with a meaningful air content, which also does not transmit ultrasound, such as 'ground glass' opacities seen on CT8,9,17,18,19,20,22,21,22,23,24,25,26. US-TTNB is also unable to definitively assess the N-stage of lung cancer, meaning in some cases, the procedure must be combined with another invasive procedure to provide a full picture1,2.
Nonetheless, US-TTNB remains an important and increasingly used front-line tool in modern invasive pulmonology practice in selected patients with suspected malignant lung lesions9,18.
Indications and contraindications
US-TTNB is indicated when all of the following criteria are met: (1) subpleural lung consolidation that can be visualized using thoracic ultrasound; (2) clinically warranted lung tissue biopsy (e.g., for establishing a diagnosis, obtaining material for supplementary diagnostic analyses). US-TTNB should not be performed when one or more of the following criteria are met: (1) manifest chronic or acute respiratory failure; (2) hemorrhagic diatheses; (3) ongoing treatment with anticoagulants or platelet aggregation inhibitors; (4) another invasive method for tissue sampling can establish a diagnosis and simultaneously provide N- or M-staging (e.g., EBUS/EUS-B in patients with suspected lung cancer and suspected involvement of mediastinal lymph nodes).
It should be noted, however, that the listed contraindications are generally relative. The physician, in agreement with the patient, should consider the clinical consequences of obtaining a biopsy by US-TTNB and balance this against the risk of complications and other potential invasive or diagnostic methods that could be performed instead of US-TTNB.
Protocol
The described protocol below follows the human care guidelines of Odense University Hospital, Odense, Denmark, and the University of Southern Denmark, Odense, Denmark. The step-wise protocol described below represents a potential, systematic approach for a typical patient with suspected lung cancer for whom US-TTNB was performed in an outpatient or daycase setting. Informed written consent was obtained from the patient. We have taken into account current clinical practices at the authors' institutions, as well as descriptions from previously published papers9,13,17,26, and have sought to achieve a balance between likely diagnostic success, costs, and patient experience, as described in the introduction. However, the approach can and should be modified in line with local differences in available equipment, clinical settings, patient preferences, and specific requirements for the obtained biopsy material, including if a non-malignant lesion is suspected. Any US-TTB should also be performed in accordance with existing local, national, and international guidelines and standards, where applicable. The protocol depends on there being one physician who is trained and competent in performing US-TTNB and one assistant, such as a procedural nurse. Both should have sufficient experience with the procedure and should be confident in the management of possible acute complications.
1. Preprocedural assessment of available clinical information and imaging
- Prior to performing the procedure and informing the patient, assess the available clinical information to ensure that the procedure is indicated and clinically relevant.
- Ensure that there are no absolute contraindications for performing the procedure, such as uncorrected coagulopathy, manifest respiratory failure, known allergies to medications required for the procedure, or a patient who is unable to cooperate with the procedure.
- Assess the presence of any relative contraindications, and if any are present, ensure that the potential clinical impact of performing the procedure outweighs the associated risks. Relative contraindications may include reduced FEV1, the use of certain antiplatelet medications, or underlying lung disease.
- Review the available cross-sectional imaging, especially CT and Positron Emission Tomography (PET), if available. Ensure that the target lesion is in contact with the visceral pleura, located in an area likely to be accessible using TUS, and reachable with a biopsy needle.1
NOTE: If necessary, also consider which lesion(s) to biopsy and whether specific areas of the selected lesions (e.g., non-necrotic regions) should be prioritized.
2. Preprocedural equipment control
- Ensure that all equipment needed for the procedure (see Table of Materials) is available in the procedural room, with spares where appropriate.
- Ensure that equipment needed for handling acute complications, especially pneumothorax, severe pain, and bleeding, is readily available in the procedural room and fully functioning.
- Turn on the US machine and ensure that relevant transducers are available and fully functioning (see Table of Materials).
3. Checking and consenting the patient
- Ensure that it is the correct patient for the given procedure and that relevant materials (e.g., patient chart, imaging) and labels are also correct. In some centers, a pre-procedure checklist may be required depending on local and national guidelines27.
- Provide the patient (and/or proxy, if required) with oral and written information regarding the US-TTNB procedure, covering the individual steps prior to, during, and following the procedure.
- Also, provide oral and written information regarding the most common (e.g., localized pain following the procedure) and rare but severe complications (e.g., pneumothorax, hemothorax).
- Ensure that there are no relevant allergies to any medication/materials used during the procedure.
- Document written informed consent for the procedure from the patient (and/or proxy, where needed).
4. Patient positioning
- Use the chosen biopsy site (see step 1.4) to determine the optimal patient positioning for the procedure.
NOTE: Generally, a supine position is chosen for lesions located anteriorly or anterior-laterally. The patient can be placed on their side, with the hemithorax to be biopsied facing upward, for lesions located posteriorly or posterior-laterally. - Assess whether the patient will be able to lie in and maintain the position deemed optimal for the US-TTNB procedure (see step 1.4) for at least 30 min.
NOTE: If the answer to step 4.2 is yes, place the patient in the given position. If the answer is no, place the patient in an alternate position (e.g., sitting), assuming there is still an acceptable position for the procedure from the perspective of the operator and the patient. If holding the position is still not possible, consider alternative solutions to address patient comfort (e.g., intravenous opioids in case of localized pain in a given position). Occasionally, a switch to an alternative modality, such as CT-TTNB, may be necessary (Figure 1).
5. Preprocedural thoracic ultrasound
- Enter the patient ID into the ultrasound machine to enable the storage of US images/clips, which should be saved frequently during the procedure.
- Choose the optimal transducer based on the placement and size of the lesion. Generally, a low-frequency curved (abdominal) transducer is optimal. A higher-frequency linear transducer is an alternative for very slim patients and/or very small lesions.
- Select a general abdominal or lung preset.
- Identify the target lesion using conventional 2D US and optimize the US image quality and position using depth, gain, and focus.
- Assess the target lesion using a simple "eye-balling" method and determine: (1) the appearance (e.g., hypo/hyperechoic, mixed pattern), (2) its demarcation (e.g., well/diffusely demarcated), (3) signs of obvious invasive growth (e.g., visible growth into the chest wall), and (4) indirect signs of possible invasive growth (e.g., absence of lung sliding without visible invasive growth)9 (Figure 2).
- Measure the target lesion in at least two planes and measure the length of the pleural contact (Figure 2).
- Perform supplementary color Doppler assessment of the target lesion and the more superficially located structures (e.g., chest wall) and note the presence of: (1) signs of neovascularization, (2) larger vessels (e.g., intercostal arteries) to avoid during the biopsy procedure9,13.
- Perform a focused assessment of the affected hemithorax to determine whether lung sliding, a pleural effusion, or other obvious pathology is present prior to the biopsy procedure.
- Based on steps 1.4 and steps 5.5-5.7, choose the area within the target lesion to biopsy.
- Use the biopsy guidance system on the US machine to assist in choosing the optimal needle angle. Note the likely safe biopsy size (most cutting needles come with an option for either a 2 cm or 1 cm length biopsy, with the latter more appropriate in smaller lesions or those adjacent to vital structures).
- Reconfirm image optimization once the optimal biopsy angle has been chosen.
- Fix the transducer (see Table of Materials) in the optimal position for obtaining a biopsy and mark the placement on the patient's skin.
- If the lesion is moving in synchrony with the patient's breathing, ask the patient to perform a breath hold to ensure that the patient can hold their breath for a sufficient time to obtain a biopsy (around 10-20 s).
6. Patient monitoring and safety
- Attach the patient to physiological monitoring system (see Table of Materials) to typically allow for continuous or regular assessment of: (1) saturation, (2) pulse including ECG, and (3) blood pressure.
- Insert and flush a peripheral venous catheter (usually of a minimum size of 20 G).
7. Biopsy equipment preparation
- Attach the biopsy guidance system to the transducer selected for the procedure. Set the angle of the biopsy guidance system to match the angle previously selected on the US machine (step 5.10).
- Apply ultrasound gel (see Table of Materials) to the transducer and secure it in the "transducer holder" on the US machine.
- Prepare a sterile trolley and arrange the equipment necessary for the biopsy procedure (Figure 3).
- Ensure that the operator wears sterile gloves (with or without a gown).
- Disinfect the biopsy area and the surrounding skin area twice.
- Attach a surgical drape (see Table of Materials) with a hole to the patient's skin, using the mark made in step 5.11 as a guide.
NOTE: If the operator chooses not to use a surgical drape, disinfect a larger area and place a drape below the biopsy site to ensure that any minor skin bleeding from the biopsy site is contained. - Draw up an appropriate volume of local anesthetic (e.g., 20 mL of 1% lidocaine, see Table of Materials) in a syringe. Ensure that the amount of local anesthetic administered to the patient does not exceed the maximum recommended dose for their weight. Attach a 21 G hypodermic needle to the syringe.
NOTE: The optimal length of the 21 G needle will depend on the length of the needle pathway from the skin to the biopsy area. In most patients, a length of 10 cm is optimal. - Open the sterile US transducer biopsy kit (see Table of Materials) and place its contents on the sterile mobile table.
- With assistance, position the sterile transducer cover over the transducer, ensuring that the previously applied US gel is evenly distributed between the "footprint" of the transducer and the sterile cover.
- Place the two elastic bands from the sterile biopsy kit at the base of the transducer to ensure a snug fit between the transducer and the transducer cover, without any significant air trapped below the cover.
- Attach the plastic handle from the sterile US transducer biopsy kit to both the US transducer and the US biopsy guidance system.
- Insert the 21 G needle adapter into the plastic handle of the US transducer biopsy guide system.
- Apply sterile gel from the sterile biopsy kit to the transducer.
8. Injection of local anesthetics
- Position the transducer at the previously chosen optimal area for performing the biopsy and ensure the selected needle angle is optimally placed with respect to the chosen biopsy area. Secure the transducer with the hand holding it to ensure complete stability during the injection of the local anesthetic.
- Take the syringe with the local anesthetic and place it in the biopsy guidance system without advancing it into the patient's skin. Ensure that the syringe is positioned in a way that aligns the tip of the needle parallel to the transducer's footprint.
NOTE: This maximizes the surface area of the needle tip visible on the ultrasound screen, allowing for optimal visualization. - Inform the patient that local anesthetic will be applied and emphasize the importance of remaining still even if the application causes discomfort.
- Slowly introduce the syringe with the local anesthetic into the skin and subcutaneous tissue (Figure 4).
NOTE: While doing so, ensure the following: (1) continuous visualization of the needle tip on the ultrasound screen, (2) aspiration before injecting to confirm that the local anesthetic is not being injected into a blood vessel, (3) focus on applying the majority of the local anesthetic in the subcutaneous area just below the skin and in the area just superficial to the parietal pleura, as these areas are the most sensitive (Figure 5). - After applying the local anesthetic, slowly withdraw and remove the syringe.
- Remove the transducer from the patient's skin and wait at least 3 min to ensure the full effect of the local anesthetic before proceeding.
9. Biopsy procedure
- Remove the 21 G needle adapter from the plastic handle of the US transducer biopsy guide system and insert the 18 G needle adapter instead.
- Prepare the core biopsy needle by ensuring that the spring of the biopsy needle can be "loaded," "fired" without excessive force, and inform the patient that the sound generated when the biopsy needle is "fired" is normal.
- "Load" the core biopsy needle for the predetermined biopsy length (usually 1 or 2 cm) as determined in step 5.9.
- Using a scalpel, make a small incision of a few millimeters in the skin corresponding to the hole left by the needle from applying the local anesthetic. If the incision is painful, wait an additional 2 min and then retest with the scalpel blade. If there is still pain, return to step 8.2 and apply additional local anesthetic, being careful not to exceed the maximum recommended dose.
- Take the core biopsy needle and place it in the biopsy guidance system without advancing it into the patient's skin.
- Position the transducer with the mounted core biopsy needle on the patient's skin at the biopsy site.
- Insert the tip of the core biopsy needle a few millimeters into the previously made hole in the skin using a scalpel.
- Carefully adjust the angle and rotation of the transducer while maintaining good skin contact to ensure: (1) optimal image quality along the entire length of the needle/biopsy pathway and (2) optimal placement of the biopsy pathway with respect to the biopsy area and avoidance of vital structures and ribs. Once the optimal position is found, secure the transducer with the hand.
- Gradually advance the core biopsy needle with the other hand while closely monitoring the progression of the needle tip on the US screen (Figure 6).
NOTE: Once the needle tip is placed just superficially to the parietal pleura, the next steps will depend on whether the lung lesion moves in synchrony with the patient's breathing or if it is fixed to the chest wall. - Ask the patient to hold their breath when the target lesion is optimally positioned in relation to the biopsy pathway. Once the patient is holding their breath, continue to step 9.11. Note that the time for steps 9.11 to 9.13 should be minimized to avoid accidental respiratory movement of the lesion while the biopsy needle is inserted.
- Advance the biopsy needle tip through the pleura until the tip is positioned just next to the "biopsy target area" in the needle pathway.
- Advance the inner stylet of the core biopsy needle corresponding to the predetermined biopsy length when "loading" the needle (step 9.3). While advancing the inner stylet, ensure that the specimen notch is optimally placed within the target biopsy area (Figure 7).
- Inform the patient that the biopsy needle will now be "fired."
- "Fire" the core biopsy needle and subsequently slowly retract and remove the core biopsy needle from the patient. Once the needle has been removed, also remove the transducer.
- Reload the core biopsy needle by pulling back on the plunger. Advance the inner stylet and remove the tissue specimen, placing it into the relevant biopsy container (Figure 8). This may require assistance using a small needle.
- Once the biopsy has been removed from the specimen notch, retract the inner stylet, and once again place the core biopsy needle in the US transducer biopsy guidance system.
- Repeat steps 9.5 to 9.14 until a sufficient number of biopsies have been obtained.
NOTE: When repeating step 9.8, ensure: (1) there are no immediate US signs of complications (e.g., loss of lung sliding, suggesting pneumothorax), and (2) adjust the biopsy angle slightly (if possible) to obtain biopsies from different areas within the target lesion. - Once the last biopsy has been placed in the biopsy specimen container, close the container and add any relevant fixation material/liquid to the container in accordance with local policy. Attach any necessary patient and sample identifier labels, double-checking that they are correct.
10. Postprocedural thoracic ultrasound
- After the last biopsy, conduct TUS and evaluate for any immediate complications (e.g., pneumothorax, hemothorax) at the biopsy site. If no signs are present, apply a sterile dressing to cover the small biopsy incision.
- With the patient in the supine position, examine the anterior surface of the hemithorax for signs of pneumothorax, and with the patient in the sitting position, assess the posterolateral surface for signs of developing hemothorax.
11. Postprocedural patient observation and information
- If the patient exhibits no symptoms, has stable vital signs, and shows no ultrasound signs of immediate complications, remove patient monitoring. Leave the peripheral venous catheter in place.
- Allow the patient to dress in their own clothes and instruct them to wait in the waiting room for 1 h.
- If, within 1 h after the procedure, the patient has not displayed any signs or symptoms and is considered clinically stable, remove the peripheral venous catheter and discharge the patient with the necessary paperwork. If the patient does show signs or symptoms or is deemed clinically unstable by the staff, conduct further clinical and radiological assessments to determine the next steps.
- Prior to discharge, ensure that the patient and any relatives have comprehended the following: (1) the oral and written information regarding how to respond in case signs of complications develop after discharge and (2) the plan for when and how the patient will receive the results of the obtained biopsy.
Results
The overall diagnostic yield of US-TTNB in patients with lung lesions has been reported as 88.7% in a meta-analysis by DiBardiono et al.18. However, it should be noted that other studies have reported lower diagnostic yields of US-TTNB17,23,25. Several patient factors have been shown to affect the diagnostic yield of US-TTNB, including: (1) whether there is a malignant or non-malignant condition; (2) the size of the target lesion; (3) the presence of invasive growth; (4) the length of pleural contact17,25,28,29.
The US-TTNB protocol described above is for obtaining a US-TTNB core needle biopsy (Figure 8) for histological assessment, but it is also possible to use the same principles for obtaining an ultrasound-guided transthoracic needle aspiration (US-TTNA) for cytology assessment. A single study has indicated that the diagnostic yield of US-TTNA and US-TTNB might vary between different types of lung cancer, suggesting that the choice of sampling technique might not always be a matter of obtaining the "largest possible biopsy"30. Some studies have described a combined procedure approach in which US-TTNA is initially performed, followed by US-TTNB in the same procedure, possibly increasing the diagnostic yield24,30.
US-TTNB can also be combined with contrast-enhanced ultrasound (CEUS) as an extension of the preprocedural thoracic ultrasound assessment31. The use of preprocedural CEUS has been shown to increase the diagnostic yield of US-TTNB32. As with other invasive chest procedures, US-TTNB can also be combined with rapid on-site evaluation (ROSE) of the obtained tissue samples to improve diagnostic efficiency33.
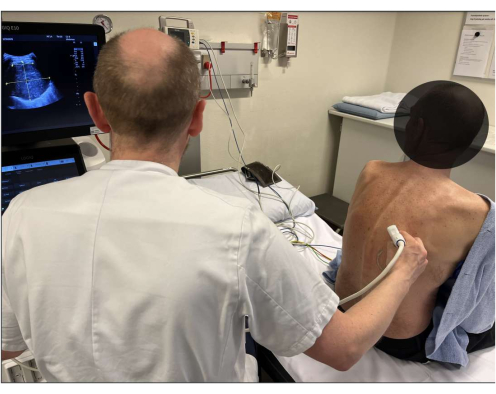
Figure 1: Patient positioning. Baseline 2D thoracic ultrasound assessment of the target lesion is performed before the procedure, simultaneously determining the optimal patient positioning for the intervention. Please click here to view a larger version of this figure.
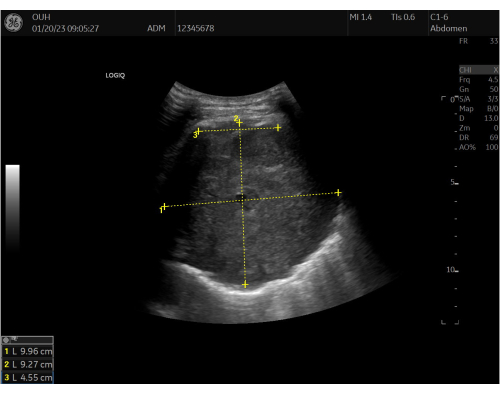
Figure 2: Baseline ultrasound measurements. Baseline 2D thoracic ultrasound assessment and inserted measurements of the target lesion before the procedure. Measurements for the width (1), depth (2), and length of pleural contact (3) are marked in the ultrasound image. The lesion is visualized as a well-defined, rounded, hyperechoic (grey) structure. Please click here to view a larger version of this figure.

Figure 3: Prepared procedure trolley. The equipment required for the US-TTNB (Ultrasound-Guided Transthoracic Needle Biopsy) procedure is organized and ready on a sterile trolley. Please click here to view a larger version of this figure.
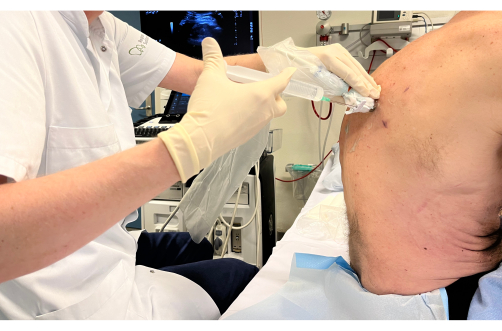
Figure 4: Injection of local anesthetic. The syringe containing the local anesthetic is positioned in the biopsy guidance system and injected into the skin and subcutaneous tissue with continuous visualization of the needle tip on the ultrasound screen. Please click here to view a larger version of this figure.

Figure 5: Assessment of local anesthetic needle tip placement. The tip of the local anesthetic needle is visible (A) at the border zone between the pleural line and the underlying lung tumor. Please click here to view a larger version of this figure.

Figure 6: Core biopsy needle placement. The core biopsy needle is gradually introduced while carefully monitoring the advancement of the needle tip on the ultrasound screen. Please click here to view a larger version of this figure.

Figure 7: Core biopsy needle prior to biopsy. The core needle biopsy is visible just before it is "fired." The needle tip (A) is positioned approximately 2 cm within the tumor, allowing for a full 2 cm core biopsy. Please click here to view a larger version of this figure.
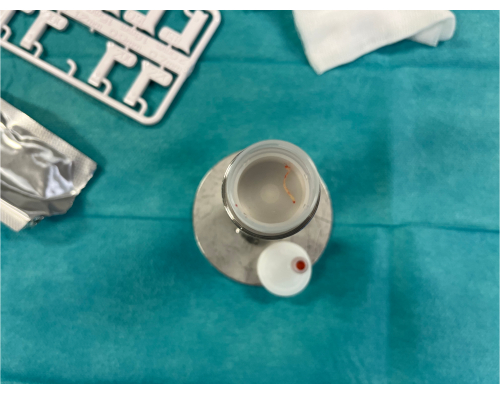
Figure 8: Obtained core biopsy specimen. A 2 cm core biopsy specimen from the lung tumor has been placed in the specimen container. Please click here to view a larger version of this figure.
Discussion
Appropriate patient selection and a careful initial TUS assessment are crucial steps before performing US-TTNB. If the lung lesion cannot be visualized, US-TTNB is not a viable option. Ideally, TUS should be conducted before scheduling the procedure during the clinic visit to prevent last-minute cancellations in the procedural room17. This approach also allows for more comprehensive planning and a potential shift to an alternative invasive procedure if the lesion cannot be visualized17.
Three studies directly comparing US-TTNB with computed tomography transthoracic needle biopsy (CT-TTNB) have found comparable diagnostic yields19,20,34. These findings are consistent with the comparison between US-TTNB and CT-TTNB as part of the previously mentioned meta-analysis by DiBardiono et al.18.
The complication rate for US-TTNB is generally very low17. The pooled proportion of patients experiencing pneumothorax following US-TTNB has been noted to be 4.4%18, significantly lower than when directly compared to CT-TTNB18,19,20,21.
While studies assessing US-TTNB procedure time are limited, when compared with CT-TTNB, US-TTNB has a shorter median procedure time20,21. From cost, radiation, and environmental perspectives, US-TTNB is significantly less burdensome than CT-TTNB, which exposes both patients and staff to radiation and has a greater carbon footprint than ultrasound20,35.
Despite numerous studies on education, training, and competency assessment in the field of thoracic ultrasound, research specifically evaluating these aspects in relation to US-TTNB is rare and is not included in the European Respiratory Society's certified training program in thoracic ultrasound15,16,36,37. A single study has assessed US-TTNB learning curves and found that several operators with proficiency in thoracic ultrasound did not achieve competency in US-TTNB despite performing multiple procedures38. Therefore, further research on education, training, and competency assessment in US-TTNB is still needed.
The described US-TTNB approach can be modified by incorporating contrast-enhanced ultrasound (CEUS) as part of the initial baseline thoracic ultrasound assessment before the biopsy is performed. This approach offers improved visualization of the specific biopsy site within the lesion, more accurate differentiation between benign and malignant lung tissue, and easier identification of necrotic areas31. CEUS-guided US-TTNB can be seamlessly integrated into the protocol described above, and the literature reports a significant increase in diagnostic yield compared to conventional US-TTNB32,39.
While the focus of our protocol has been on its use in patients with lung lesions suspected of malignancy in the lungs, the protocol's principles remain generally applicable when sampling other structures in the chest, such as the parietal pleura or the anterior/superior mediastinum9,14,17,40,41,42,43,44. The clinical utility of US-TTB and its utilization by pulmonologists are expected to expand in the coming years.
In summary, US-TTNB is a safe, cost-effective procedure with a high diagnostic yield in patients with subpleural lung lesions suspected of malignancy. When technically feasible, US-TTNB should be considered the transthoracic biopsy technique of choice.
Disclosures
The authors have nothing to disclose.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| Ambu BlueSensor R | Ambu | R-00-S/25 | ECG patches |
| BD Nexiva Closed IV Catheter System (20 GA x 1.25 in)(1.1 x 32 mm) | BD | 383667 | Intravenous accesskit |
| BD PosiFlush SP Syringe 5 mL | BD | 306574 | Syringe with 5 mL 0.9% NaCl |
| Blunt Fill Needle with 5 Micron Filter (18 G x 11/2") | Sol-Millennium Medical Inc. | BN1815F | 18 G needle |
| C1-6VN ultrasound transducer | GE Healthcare | 5476279 | Ultrasound transducer |
| C2-9D ultrasound transducer | GE Healthcare | 5405253 | Ultrasound transducer |
| CARBON STEEL SURGICAL BLADES | Swann-Morton | 203 | Scalpel blade |
| Disinfection wipe (82% ehanol + 0.5% chlorhexidine) | Vitrex Medical A/S | 527297 | Disinfection wipe |
| Disposable needle single use (0.80 mm x 80 mm) | Misawa Medical Industry Co., Ltd. | K070001 | 80 mm 21 G hypodermic needle |
| EKO GEL | Ekkomed A/S | 29060008-29 | Ultrasound gel |
| Formalin system, buffered formalin solution 450 mL | Sarstedt | 5,11,703 | Biopsy specimen contained and relevant fixation liquid (e.g. formaldehyde) |
| GAMMEX Latex | Ansell | 330048075 | Sterile gloves |
| KD-JECT 20 mL | KD Medcial GmbH | 820209 | 20 mL syringe |
| Klorhexidin sprit 0.5% 500 mL | Fuckborg Pharma | 212045 | Disinfectant |
| Lidokain Mylan 10 mg/mL, 20 mL | Mylan | NO-6042A1 | Local anesthetic (20 mL, 2% lidocaine) |
| LOGIQ E10 | GE Healthcare | NA | High-end ultrasound machine |
| Mölnlycke BARRIER Adhesive Aperture Drape (50 x 60 cm / 6 x 8 cm) | Mölnlycke Healthcare AB | 906693 | Adhesive surgical drape with a central hole |
| Mölnlycke Gauze 10 x 10 cm | Mölnlycke Healthcare AB | 158440 | Swaps for applying disinfectant |
| Philips IntelliVue X2 | Philips | NA | Patient monitoring system |
| Raucodrape PRO 75 x 90 cm | Lohmann & Rauscher International GmbH & Co | 33 005 | Sterile drape for procedure table |
| SEMICUT 18 G x 100 mm | MDL | PD01810 | 18 G x 100 mm core biopsy needle |
| SEMICUT 18 G x 160 mm | MDL | PD01816 | 18 G x 160 mm core biopsy needle |
| S-Monovette, 25 mL, for Formalin system, (LxØ): 97 x 25 mm, with paper label | Sarstedt | 9,17,05,001 | Biopsy specimen container |
| Sterican (0.80 x 120 mm BL/LB) | Braun | 4665643 | 120 mm 21 G hypodermic needle |
| Tegaderm I.V. | 3M | 1633 | I.V. Transparent film dressing with border |
| Ultra-Pro II Disposable Replacement Kits | CIVCO | 610-608 | For use with GE Healthcare C2-9 transducer |
| Ultra-Pro II In-Plane Ultrasound Needle Guides-Multi-Angle | CIVCO | H4913BA | For use with GE Healthcare C2-9 transducer |
| Verza Needle Guidance System for VerzaLink™ Transducers | CIVCO | 610-1500-24 | For use with GE Healthcare C1-6 transducer |
| Verza Ultrasound Needle Guidance System | CIVCO | H4917VB | For use with GE Healthcare C1-6 transducer |
References
- Maconachie, R., Mercer, T., Navani, N., McVeigh, G., Guideline, C. Lung cancer: diagnosis and management: summary of updated NICE guidance. BMJ. 364, 1049 (2019).
- Rasmussen, T. R., et al. Lungecancer - Visitation, Diagnose, Stadie. , (2020).
- Herth, F. J. F., Shah, P. L., Gompelmann, D. Interventional Pulmonology. , (2017).
- Juul, A. D., et al. Does the addition of radial endobronchial ultrasound improve the diagnostic yield of electromagnetic navigation bronchoscopy? a systematic review. Respiration. 101 (9), 869-877 (2022).
- Kuijvenhoven, J. C., et al. Endobronchial ultrasound for the diagnosis of centrally located lung tumors: a systematic review and meta-analysis. Respiration. 99 (5), 441-450 (2020).
- Li, Y., Yang, F., Huang, Y. Y., Cao, W. Comparison between computed tomography-guided core and fine needle lung biopsy: A meta-analysis. Medicine (Baltimore). 101 (9), e29016 (2022).
- Fu, Y. F., Zhang, J. H., Wang, T., Shi, Y. B. Endobronchial ultrasound-guided versus computed tomography-guided biopsy for peripheral pulmonary lesions: A meta-analysis. Clin Respir J. 15 (1), 3-10 (2021).
- Chandrasekhar, A. J., Reynes, C. J., Churchill, R. J. Ultrasonically guided percutaneous biopsy of peripheral pulmonary masses. Chest. 70 (5), 627-630 (1976).
- Laursen, C. B., et al. European respiratory society statement on thoracic ultrasound. Eur Respir J. 57, 2001519 (2020).
- Laursen, C. B., Graumann, O., Rahman, N. M. Thoracic Ultrasound - new challenges, new horizons. Ultraschall Med. 42, 226-227 (2021).
- Hallifax, R. J., et al. Physician-based ultrasound-guided biopsy for diagnosing pleural disease. Chest. 146 (4), 1001-1006 (2014).
- Corcoran, J. P., et al. Ultrasound-guided pneumothorax induction prior to local anaesthetic thoracoscopy. Thorax. 70 (9), 906-908 (2015).
- Bedawi, E. O., et al. Intercostal vessel screening prior to pleural interventions by the respiratory physician: a prospective study of real world practice. Eur Respir J. 55 (4), 1902245 (2020).
- Mei, F., et al. Diagnostic yield and safety of image-guided pleural biopsy: a systematic review and meta-analysis. Respiration. 100 (1), 77-87 (2021).
- Pietersen, P. I., et al. Lung ultrasound training: a systematic review of published literature in clinical lung ultrasound training. Crit Ultrasound J. 10 (1), 23 (2018).
- Pietersen, P. I., Laursen, C. B., Petersen, R. H., Konge, L. Structured and evidence-based training of technical skills in respiratory medicine and thoracic surgery. J Thorac Dis. 13 (3), 2058-2067 (2021).
- Laursen, C. B., et al. Ultrasound-guided lung biopsy in the hands of respiratory physicians: diagnostic yield and complications in 215 consecutive patients in 3 centers. J Bronchology Interv Pulmonol. 23 (3), 220-228 (2016).
- DiBardino, D. M., Yarmus, L. B., Semaan, R. W. Transthoracic needle biopsy of the lung. J Thorac Dis. 7 (Suppl 4), S304-S316 (2015).
- Yamamoto, N., et al. Efficacy and safety of ultrasound (US) guided percutaneous needle biopsy for peripheral lung or pleural lesion: comparison with computed tomography (CT) guided needle biopsy. J Thorac Dis. 11 (3), 936-943 (2019).
- Sconfienza, L. M., et al. Pleural and peripheral lung lesions: comparison of US- and CT-guided biopsy. Radiology. 266 (3), 12112077 (2012).
- Lee, M. H., Lubner, M. G., Hinshaw, J. L., Pickhardt, P. J. Ultrasound guidance versus CT guidance for peripheral lung biopsy: performance according to lesion size and pleural contact. AJR Am J Roentgenol. 210 (3), W110-W117 (2018).
- Chen, C. C., Hsu, W. H., Huang, C. M., Hsu, J. Y., Chiang, C. D. Ultrasound-guided fine needle aspiration biopsy of small pulmonary nodules abutting to the chest wall. Zhonghua Yi Xue Za Zhi (Taipei). 57 (2), 106-111 (1996).
- Diacon, A. H., et al. Safety and yield of ultrasound-assisted transthoracic biopsy performed by pulmonologists. Respiration. 71 (5), 519-522 (2004).
- Koegelenberg, C. F., et al. Diagnostic yield and safety of ultrasound-assisted biopsies in superior vena cava syndrome. Eur Respir J. 33 (6), 1389-1395 (2009).
- Meena, N., Bartter, T. Ultrasound-guided percutaneous needle aspiration by pulmonologists: a study of factors with impact on procedural yield and complications. J Bronchology Interv Pulmonol. 22 (3), 204-208 (2015).
- Laursen, C. B., Rahman, N. M., Volpicelli, G. . Thoracic Ultrasound. Vol 79, (2018).
- Roberts, M. E., et al. British Thoracic society guideline for pleural disease. Thorax. , (2023).
- Dallari, R., Gollini, C., Barozzi, G., Gilioli, F. Ultrasound-guided percutaneous needle aspiration biopsy of peripheral pulmonary lesions. Monaldi Arch Chest Dis. 54 (1), 7-10 (1999).
- Liao, W. Y., et al. US-guided transthoracic cutting biopsy for peripheral thoracic lesions less than 3 cm in diameter. Radiology. 217 (3), 685-691 (2000).
- Diacon, A. H., et al. Ultrasound-assisted transthoracic biopsy: fine-needle aspiration or cutting-needle biopsy. Eur Respir J. 29 (2), 357-362 (2007).
- Laursen, C. B., Graumann, O., Moller, T. V., Davidsen, J. R. Contrast-enhanced Ultrasound-guided Transthoracic Lung Biopsy. Am J Respir Crit Care Med. 194 (5), e5-e6 (2016).
- Jacobsen, N., et al. Clinical applications of contrast-enhanced thoracic ultrasound (CETUS) compared to standard reference tests: a systematic review. Ultraschall Med. 43 (1), 72-81 (2020).
- Koegelenberg, C. F., et al. The diagnostic yield and safety of ultrasound-assisted transthoracic fine-needle aspiration of drowned lung. Respiration. 81 (1), 26-31 (2011).
- Adams, R. F., Gleeson, F. V. Percutaneous image-guided cutting-needle biopsy of the pleura in the presence of a suspected malignant effusion. Radiology. 219 (2), 510-514 (2001).
- McAlister, S., et al. The carbon footprint of hospital diagnostic imaging in Australia. Lancet Reg Health West Pac. 24, 100459 (2022).
- Pietersen, P. I., et al. Development of and gathering validity evidence for a theoretical test in thoracic ultrasound. Respiration. 98 (3), 221-229 (2019).
- Pietersen, P. I., et al. Objective structured clinical examination in basic thoracic ultrasound: a European study of validity evidence. BMC Pulm Med. 23 (1), 15 (2023).
- Laursen, C., et al. Learning curves for ultrasound guided lung biopsy in the hands of respiratory physicians. European Respiratory Journal. 48 (suppl 60), PA3850 (2016).
- Safai Zadeh, E., et al. WFUMB technological review: how to perform contrast-enhanced ultrasound of the lung. Ultrasound Med Biol. 48 (4), 598-616 (2022).
- Ahmed, M., Daneshvar, C., Breen, D. Neck Ultrasound for the detection of cervical lymphadenopathy in sarcoidosis: an alternative to endobronchial ultrasound. J Bronchology Interv Pulmonol. 26 (3), 225-227 (2019).
- van Overhagen, H., et al. Metastases in supraclavicular lymph nodes in lung cancer: assessment with palpation, US, and CT. Radiology. 232 (1), 75-80 (2004).
- Koegelenberg, C. F., et al. The diagnostic yield and safety of ultrasound-assisted transthoracic biopsy of mediastinal masses. Respiration. 81 (2), 134-141 (2011).
- Psallidas, I., et al. A Pilot feasibility study in establishing the role of ultrasound-guided pleural biopsies in pleural infection (The AUDIO study). Chest. 154 (4), 766-772 (2018).
- Koegelenberg, C. F., et al. Direct comparison of the diagnostic yield of ultrasound-assisted Abrams and Tru-Cut needle biopsies for pleural tuberculosis. Thorax. 65 (10), 857-862 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved