Method Article
Flux-Based Assay for the Identification of Autophagy Modulators for Osteoarthritis
In This Article
Summary
This paper details monitoring autophagy flux to identify new molecules by cell-based imaging screening.
Abstract
Autophagy is a central mechanism to regulate homeostasis. Alterations of autophagy contribute to aging-related diseases. Phenotypic methods to identify regulators of autophagy could be used for the identification of novel therapeutics. This article describes a cell-based imaging screening workflow developed to monitor autophagic flux using LC3 as a reporter of autophagic flux (mCherry-EGFP-LC3B) in human chondrocytes. Data acquisition is performed using an automated High Content Imaging Screening System microscope. An algorithm-based automated image analysis protocol was developed and validated to identify molecules activating autophagic flux. Critical steps, explanatory notes, and improvements over current autophagy monitoring protocols are reported. Physiologically relevant phenotypic screening approaches to target hallmarks of aging can facilitate more effective drug discovery strategies for age-related musculoskeletal diseases.
Introduction
Many chronic diseases are associated with the hallmarks of aging, including defective autophagy1. Osteoarthritis (OA) is the most prevalent joint disease and has a major impact in restricting everyday activities in the aging population, but neither preventive measures nor disease-modifying treatments are yet available2.
Joint aging and OA are associated with hallmarks that define the progression of cartilage degeneration, including defective autophagy and senescence3,4. Targeting autophagy in musculoskeletal tissues can help find therapeutic innovations for rheumatologic diseases5,6. Pharmacological modulation of autophagy is a promising, relevant mechanism for intervention in preclinical disease models7. In OA, autophagy activation has been used to prevent joint dysfunction8. Methods to monitor autophagy based on robust, reproducible protocols that allow quantitative analysis can be used to identify novel agents and facilitate the pharmacological targeting of disease-relevant hallmarks of aging.
Autophagy flux reflects degradation activity and is a relevant measurement to identify new molecules activating autophagy9. This study describes a method developed to determine autophagic flux by measuring autophagic degradation activity using an autophagy reporter cell line in human chondrocytes (TC28a2). mCherry-EGFP-LC3- transient expression allows simultaneous monitoring of autolysosome formation and degradation events by quantifying the differences in the pH sensitivity between GFP and mCherry LC3 signals in the lysosomes of live cells10.
Adapting this flow cytometry reported method to a stable expression imaging monitoring system in live chondrocytes may allow the identification of molecules activating autophagic flux in the context of cartilage biology.
Protocol
1. Generation of autophagy reporter cell line of immortalized human chondrocytes
NOTE: The generation of an autophagy reporter cell line by stable transfection of pBABE-mCherry-GFP-LC3 by establishing a flow cytometry quantitative readout was described previously11. Two cell lines were used in the retroviral transfection: HEK 293-T17 during the cotransfection process and T/C28a2 in the infection step.
- Cotransfection process
- Prepare the growth medium for HEK 293-T17 cells: 500 mL of Eagle’s minimum essential medium (EMEM), 10% fetal bovine serum (FBS), and 1% penicillin-streptomycin (P/S). Store the growth medium at 4 °C and warm to 37 °C before each use.
- Seed 1 x 106 HEK 293-T17 cells per well into 100 mm cell culture plates, incubate at 37 °C and 5% CO2, and make sure that they are at ~75% confluence before starting the cotransfection process.
- Remove the culture medium, rinse with Hank's balanced salt solution (HBSS), and add 8 mL of EMEM with 2% FBS and 1% P/S.
- In a conical tube, add 10 μg, 3 μg, and 7 μg of pBABE-puro mCherry-EGFP-LC3B, VSV.G, and pCL-Eco plasmids, respectively. Then add 200 μL of 1x reduced serum medium (Table of Materials). Add transfection reagent (Table of Materials) as a nonliposomal mixture of lipids to have a 1:3 ratio (1 μg DNA : 3 μg transfection reagent; final volume = 60 μL).
NOTE: A total of 20 μg of mix is prepared for each 10 cm cell plate to be transfected. - Incubate the mixture for 20 min at room temperature. Carefully transfer the transfection mix dropwise to HEK 293-T17 packaging cells. Incubate the cells at 37 °C and 5% CO2 at least 48 h before checking for green and red protein expression by fluorescent microscopy.
NOTE: Upon these conditions, more than 75% of the cells should be positive for fluorescence. Ensure that transfection efficiency is high (e.g., more than 75% of the cells are positive). Use high-quality plasmid DNA (e.g., predominantly supercoiled and free of genomic DNA, RNA, and protein; highly concentrated; free of endotoxins and salts) as well as high-quality cell cultures (e.g., homogeneous, low passage, monolayer, transfected in the exponential growth phase, free of mycoplasma).
- On the same day of cotransfection, prepare T/C28a2 chondrocytes.
- Prepare T/C28a2 cell growth medium: 500 mL of Dulbecco's modified Eagle medium (DMEM), 10% fetal calf serum (FCS), and 1% P/S. Store growth medium at 4 °C and warm to 37 °C before each use.
- Seed 2 x 105 T/C28a2 chondrocytes in two 6 well multiplates and incubate for 48 h at 37 °C and 5% CO2.
- Retroviral infection process
- Aspirate culture medium from HEK 293-T17 retroviral packaging cells obtained from step 1.1.5. Rinse 1x with 4 mL of HBSS and aspirate HBSS.
- Add 2 mL of trypsin and wait 2 min at 37 °C. Neutralize trypsin by adding 6 mL of HEK 293-T17 culture medium and tranfer cells and medium into 15 mL conical tubes.
- Centrifuge cells at 400 x g for 5 min. Tranfer supernatant to a sterile filter through a 0.45 μm membrane.
- Aspirate the T/C28a2 chondrocyte culture medium from the 6 well multiplates. Infect T/C28a2 cells by adding 2 mL of viral suspension (step 1.3.3) per well into 6 well multiplates. Use one well for control (i.e., untransfected cells), adding 2 mL of T/C28a2 cell growth medium instead of viral suspension.
- Incubate for 48 h at 37 °C and 5% CO2.
- To perform the selection process, use an antibiotic selection on transfected cells to eliminate untransfected cells and to obtain a homogenous cell population.
NOTE: The pBABE-puro mCherry-EGFP-LC3B retroviral vector provides mammalian antibiotic resistance to puromycin, which enables selection of a stable cell culture after viral transfection. Kill curves for optimal puromycin concentration of T/C28a2 chondrocytes should be set up in advance to establish the appropriate conditions to select the cell clones carrying the transgene with high expression levels.- Prepare T/C28a2-pBABE-puro mCherry-EGFP-LC3B stable cell line growth medium: 500 mL of DMEM, 10% FCS, 1% P/S, and 2.5 µL/mL puromycin. Store medium at 4 °C and warm to 37 °C before each use.
- Change the medium. For each 6 well multiplate, aspirate culture medium, rinse 1x with 2 mL of HBSS and aspirate it. Add 2 mL of medium and incubate at 37 °C and 5% CO2. This is the beginning of the selection process.
- Replace the medium every 2 days and observe dead cells and puromycin resistant cell populations. Compare results to the untransfected control cells, which are not resistant to puromycin and die.
- To start clonal selection, ensure that the monoclonal populations grew. For each 6 well multiplate:
- Add 200 µL of medium per well into a 96 well multiplate.
NOTE: Each single cell selected from the 6 well multiplate will be plated in individual wells of the 96 well multiplate. - Use a microscope to identify each monoclonal population in the 6 well multiplates. Colonies formed from single cells can be observed.
- Aspirate culture medium from 6 well multiplates, rinse 1x with 2 mL of HBSS, and aspirate HBSS.
- Add 10 µL of trypsin to each monoclonal population to tranfer single cells into each well of the 96 well multiplates. Incubate for 48 h at 37 °C and 5% CO2.
- Add 200 µL of medium per well into a 96 well multiplate.
- After 48 h, replace the medium with 100 μL of fresh medium per well. Repeat this procedure until colonies form due to expansion of single cells.
- Once a single cell has expanded well, plate each monoclonal population in individual wells of 24 well, 6 well, and 100 mm cell culture plates.
- Generate a stable monoclonal cell line using a single cell sorting method.
- Aspirate culture medium from 100 mm cell culture plates. Rinse 1x with 8 mL of HBSS and aspirate it.
- Add 2 mL of trypsin on each plate and incubate for 2 min at 37 °C. Neutralize trypsin by adding 8 mL of culture medium.
- Centrifuge cells at 252 x g for 5 min and discard the supernatant. Add 4 mL of HBSS to each cell pellet, resuspend it carefully, and transfer it to cytometry tubes.
- Inject each cell sample into a flow cytometer (Table of Materials) with cell sorting technology and separate cells with high green and red fluorescence.
- Tranfer selected cells to 15 mL conical tubes and centrifuge cells at 252 x g for 5 min. Add cell pellet to a T-25 flask with 9 mL of stable cell line growth medium. Incubate at 37 °C and 5% CO2.
NOTE: After cell sorting, three monoclonal populations (Table 1) should be obtained.
- After 24 h, remove the medium and add 8 mL of fresh medium. Repeat this procedure every 2 days until the cells reach confluence.
NOTE: To study autophagic flux, two controls can be used: rapamycin as a high autophagic flux signal reference and chloroquine as a low autophagic flux signal reference. Prepare a 10 mM rapamycin stock solution in dimethyl sulfoxide (DMSO) and a 30 mM chloroquine stock solution in H2O and store at -20 °C. To select the best monoclonal population by testing the expression mCherry and GFP, two methods can be used: flow cytometry (step 1.10) or high content fluorescent image analysis (step 1.11). - Flow cytometry
- Seed 2.5 x 105 cells per well of each monoclonal population in 12 well plates at least 24 h prior to treatment in 1 mL of stable cell line growth medium and incubate at 37 °C and 5% CO2.
- On the day of the treatment, remove the medium, add 1 mL of stable cell line growth medium supplemented with 2% FCS per well. Add 10 μM rapamycin and 30 μM chloroquine to the stable cell line growth medium with 2% FCS and incubate for 16 h at 37 °C and 5% CO2.
- Remove media, rinse with 1 mL of HBSS per well, and aspirate it.
- Add 500 µL of trypsin in each well, incubate 1 min at 37 °C and neutralize the reaction by adding 500 µL of culture medium. Scrape cells and transfer them to conical tubes.
- Centrifuge the cells at 252 x g for 5 min and aspirate the supernatant. Rinse the cell pellets with 500 µL of phosphate buffered saline (PBS). Centrifuge at 252 x g for 5 min and discard the supernatant.
- Add 500 µL of PBS, tranfer cells to cytometry tubes, and inject each sample into the cytometry equipment. For each condition, collect 10,000 events.
- To select the monoclonal population by flow cytometry, use a 488 nm laser to excite EGFP and a 633 nm laser for mCherry. Then, establish a ratio of mCherry and EGFP fluorescence to select the cell population with high and low autophagic flux.
- High content fluorescent image analysis
- Seed 4 x 103 cells of each monoclonal population per well in 384 well plates (black wall/clear bottom) in growth medium supplemented with 10% FCS, 1% P/S, and 2.5 µL/mL puromycin. Incubate at 37 °C and 5% CO2 for 24 h prior to treatment.
- Remove the medium and add 10 μM rapamycin and 30 μM chloroquine in 50 µL of stable cell line growth medium supplemented with 2% FCS per well. Incubate for 16 h at 37 °C and 5% CO2.
- Remove media, rinse with 50 µL of HBSS per well, and aspirate it. Fix cells with 4% paraformaldehyde (PFA) for 10 min at 37 °C. Remove PFA and rinse with 50 µL of HBSS per well.
- To identify the cell nuclei, stain with Hoechst 33342 (2.5 µg/mL) for 10 min at 37 °C. Aspirate Hoechst 33342 dye, rinse with 50 µL of HBSS per well, and add 50 µL of PBS per well.
- Perform data acquisition and analysis of autophagic flux obtained by HTS platform as described in section 3.
NOTE: The criteria to select the monoclonal population is based on whether autophagic flux increased or decreased with rapamycin or chloroquine, respectively (Table 1). Clones with high levels of mCherry and GFP expression are more appropiate for accurate high content fluorescent image analysis quantitation of autophagy flux.
2. Image-based autophagic flux assay in live chondrocytes
NOTE: After selecting a clone, start the assay to quantify autophagic flux.
- Using a handheld electronic 384 channel pipette, seed 4 x 103 cells of the selected clone (step 1.11) per well into 384 well plates (black wall/clear bottom) in 50 µL of stable cell line growth medium supplemented with 10% FCS, 1% P/S, and 2.5 µL/mL puromycin per well. Incubate at 37 °C and 5% CO2 for 24 h prior to treatment.
- On the day of the experiment, prepare an interval plate for compound addition.
- Add a volume of each compound/controls and stable cell line growth medium to the interval plate using acoustic liquid handling technology and an electronic 384 channel pipette, respectively, to get an interval concentration.
NOTE: The controls in this assay included stable cell line growth medium supplemented with 2% FCS, 30 μM chloroquine, and 10 μM rapamycin. Interval concentrations will depend on the desired final concentration. For example, in this assay, chloroquine and rapamycin were added in 50 µL of stable cell line growth medium per well to obtain an interval concentration of 300 µM and 100 µM, respectively. - Using a microplate washer robot, aspirate medium from cell plate in an automated fashion and add 45 µL of stable cell line growth medium supplemented with 2% FCS per well.
- Then transfer 5 µL per well from the interval plate to the assay plate to get a final concentration using aother automated liquid handler workstation.
- Add a volume of each compound/controls and stable cell line growth medium to the interval plate using acoustic liquid handling technology and an electronic 384 channel pipette, respectively, to get an interval concentration.
- Incubate at 37 °C and 5% CO2 for 16 h. Using a microplate washer robot, aspirate the medium.
- Fix cells with 50 µL of 4% PFA per well for 10 min at 37 °C. Wash with 50 µL of HBSS per well.
- Stain the nuclei with Hoechst 33342 (2.5 µg/mL) for 10 min at 37 °C. Aspirate Hoechst 33342 and rinse with 50 µL of HBSS per well.
- Add 50 µL of PBS per well. The plate is now ready for reading, data collection, and subsequent analysis.
NOTE: This protocol is described for high throughput screening (HTS). However, if few compounds are used, they can be added manually, without automatic instruments. Minimal manipulation of cells and supernatant using calibrated liquid handling instruments and/or electronic pipettes facilitate successful preparation of cell culture monolayers for quantitation of autophagy flux by high content fluorescent image analysis.
3. Data acquisition and analysis of autophagic flux
- For data acquisition, use a high content screening system (HCS) and an automated microscope, which allows automated image acquisition and analysis by image processing algorithm.
- To develop an acquisition protocol, select several channels and exposure times (Table 2) to identify the nuclei and the formation of autophagosomes and autolysosomes during autophagic flux events.
- For each well, collect images from four fields covering the well surface and in four stacks starting from 8 µm separated by a working distance of 0.2 µm. Capture the images using a 60x objective for detailed cell imaging.
- Analyze the data using the image analysis software provided by the HCS microscope. To develop a robust analysis protocol algorithm for autophagy flux monitoring, select the most appropriate method for automated cell image segmentation and for specifically detecting the speckles corresponding to LC3 activation. Perform the final analysis sequence by adding methods, cell segmentation steps, and quantification instructions as described below:
- Click the Find Nuclei building block and choose the nuclear dye channel Hoechst 33342. Select Method and choose the output population Nuclei.
- Click the Find Cytoplasm building block and choose the cytoplasmic dye channel Fluorescein. Select Method | Individual Threshold.
- Click the Find Cytoplasm (2) building block and choose the cytoplasmic dye channel RFP. Select Method | Individual Threshold.
- Click the Population building block and select the study population Nuclei. Select the method Common filters (Remove border objects) and choose the output population Nuclei Inside Borders.
- Click the Find Spots building block and choose the specific marker channel Fluorescein. Select the study population Nuclei Inside borders and select the study region Cytoplasm. Select Method and choose the output population Spots Inside Borders.
- Click the Find Spots (2) building block and choose the specific marker channel RFP. Select the study population Nuclei Inside borders and select the study region Cytoplasm (2). Select Method and choose the output population Spots (2) Inside Borders.
- Click the Calculate Intensity Properties building block and choose the specific marker channel Fluorescein. Select the study population Nuclei Inside Borders and select the study region Spot. Select Method and choose the output properties Intensity Spot Fluorescein Inside Borders.
- Click the Calculate Intensity Properties building block and choose the specific marker channel RFP. Select the study population Nuclei Inside Borders and select the study region Spot (2). Select Method and choose the output properties Intensity Spot (2) RFP Inside Borders.
- Click the Define Results building block, select population Nuclei Inside Borders, select Number of Objects of Nuclei (Mean), select Relative Intensity Spots Fluorescein – mean per well, and select Relative Intensity Spots (2) – RFP – mean per well.
- To quantify autophagic flux, use the same method described previously for flow cytometry analysis11. Establish the mCherry/EGFP ratio of relative intensity spots.
NOTE: Cells with higher flux are less green, which increases the mCherry/EGFP ratio in the cell. Identifying and accurately quantifying a meaningful number of the relevant signals coming from the autophagy flux events in the cells is very important to establish statistically significant differences.
Results
Autophagic flux can be monitorized in cells by pharmacological modulation or by cell stress response. Inmortalized Human Chondrocytes (T/C28a2) were employed to develop an autophagy reporter cell line using LC3 as a fluorescent reporter (mCherry-EGFP-LC3B) for autophagy. Figure 1 shows the schematic workflow of the screening assay starting with the development of the autophagy reporter cell line in human chondrocytes (mCherry-EGFP-LC3-T/C28a2), to the induction or inhibition of autophagic flux by pharmacological modulation, and then the data analysis performed with a High Content Imaging Microscope. Figure 2 shows the schematic representation of the cell-based imaging assay to identify autophagy modulators by monitoring autophagic flux. This basis of the system lies in the higher sensitivity of EGFP fluorescence to the acidic environment of the autolysosome relative to mCherry. Figure 3 shows the sequence of analysis to quantify autophagic flux from cell-based images. Figure 4 shows representative images of low and high autophagic flux represented by the ratio of mCherry/GFP of chloroquine and rapamycin, respectively.

Figure 1: Schematic overview of the workflow used to determine autophagic flux by cell-based imaging assay. To create an autophagy reporter cell line, two cell lines were used in the retroviral transfection, the HEK 293-T17 cell line during the cotransfection process and the T/C28a2 immortalized human chondrocytes in the infection step. The pBABE-puro-mCherry-EGFP-LC3B plasmid was introduced into the cells as described previously10 and puromycin was used to select a stable cell culture after viral transfection. Cells were seeded into 384 well plates (4 x 103 cells per well) in growth medium and incubated at 37 °C and 5% CO2 for 24 h prior to treatment. Then, medium was removed and growth medium supplemented with 2% FCS and 1% P/S, and 2.5 μL/mL puromycin. Cells were treated with 30 μM chloroquine and 10 μM rapamycin. A high content screening system was used for data acquisition. Several channels and exposure times were selected to develop a reading protocol. Data were analysed using image analysis software and an analysis protocol was selected. Finally, the mCherry/EGFP ratio of relative intesity spots was confirmed. Please click here to view a larger version of this figure.
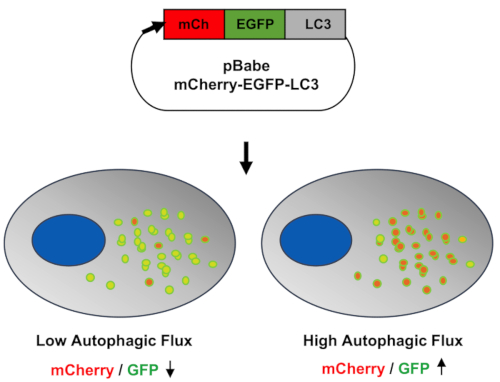
Figure 2: Representation of cell-based imaging assay to identify autophagy modulators. The pBABE-puro-mCherry-EGFP-LC3B plasmid was introduced into the cells. When autophagy was activated, the cargo was engulfed by the phagophores and autophagosomes were created. LC3 was lipidated and incorporated into autophagosomal membranes where LC3 could interact with cargo receptors. Due to the basic environment in the autophagosomes and EGFP pKa ≥ 6.0, green fluorescence was emited. When autophagy flux continued, autophagosomes fused with lysosomes, autolysosomes were created, the pH decreased, and red-orange fluorescence was emited. Please click here to view a larger version of this figure.
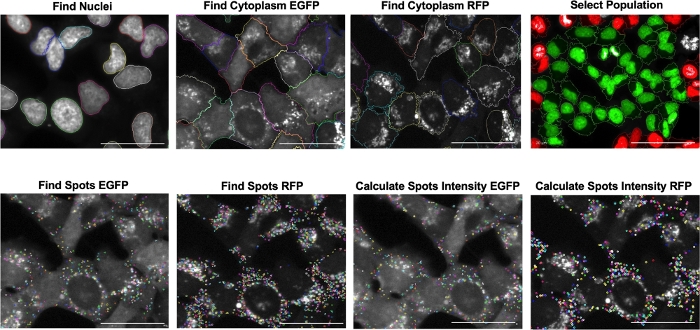
Figure 3: Sequence of analysis to quantify autophagic flux from cellular images using different building blocks to create a protocol. Nuclei were identified using the Hoechst channel. Then, the cytoplasm was identified using two channels, fluorescein (EGFP) and RFP. Next, border objects were removed to select the cell population. The software identifies whole cells in green and incomplete cells in red. In each cytoplasm identified, accumulation of speckels (called spots) was seen. Finally, relative intensity spots from each cytoplasm were calculated and autophagy flux was quantified based on the ratio of mCherry/EGFP associated to LC3 activation. Scale bar = 50 μm. Please click here to view a larger version of this figure.

Figure 4: Quantification of mCherry-EGFP-LC3 T/C28a2 chondrocytes treated with chloroquine and rapamycin by imaging. Culture medium supplemented with 2% FCS was used as a control. Chloroquine was used as autophagic flux inhibitor. When the autophagic flux was activated, autophagosomes were created, but the fusion between autophagosomes and lysosomes did not occur. Therefore, autophagosomes accumulated, green fluorescence was emitted, and the ratio of mCherry/EGFP decreased. Additionally, rapamycin was used as an autophagic flux activator. Autophagosomes fused with lysosomes, pH decreased, and red-orange fluorescence was emitted, resulting in an increase in the ratio of mCherry/EGFP. Scale bar = 50 μm. Please click here to view a larger version of this figure.
| Monoclonal population | Conditions | Ratio mCherry/EGFP flow cytometry | Ratio mCherry/EGFP HTS |
| Clon B | DMEM 2% FCS | 1 | 1 |
| Rapamycin 5 μM | 1.5 | 1.13 | |
| Chloroquine 30 μM | 0.96 | 0.76 | |
| Clon D | DMEM 2% FCS | 1 | 1 |
| Rapamycin 5 μM | 1.32 | 1.22 | |
| Chloroquine 30 μM | 1.05 | 0.76 | |
| Clon H | DMEM 2% FCS | 1 | 1 |
| Rapamycin 5 μM | 1.64 | 1.12 | |
| Chloroquine 30 μM | 0.88 | 0.87 |
Table 1: Data obtained by flow cytometry and HTS to quantify autophagic flux and select the best monoclonal population.
| Channels | Emission (nm) | Excitation (nm) | Exposure (ms) |
| Brightfield | 652−760 | Transmission | 20 |
| Hoechst | 410−480 | 360−400 | 100 |
| Fluorescein | 500−550 | 460−490 | 700 |
| RFP | 560−630 | 520−550 | 1000 |
Table 2: Image acquisition settings for high content screening analysis of the autophagic flux process. Emission and excitation wavelength and exposure time of each channel.
Discussion
Defective autophagy is an important hallmark of aging-related joint degeneration, but neither preventive nor disease-modifying treatments targeting autophagy are yet available for cartilage degeneration2. Given its relevance and clinical implications, autophagy has become a target of interest for drug discovery and development, although methods to directly monitor changes in this key homeostasis mechanism has proved challenging.
Here, a cell-based phenotypic assay to determine the autophagic flux in live human chondrocytes is described. A plasmid containing a dual reporter of autophagy activation, mCherry-EGFP-LC3, is used to simultaneously monitor autolysosome formation and degradation events due to differences in the pH sensitivity of GFP and mCherry signals in live cells by flow cytometry11. Human chondrocytes with stable, high expression of mCherry-EGFP-LC3B can be used to accurately monitor the dynamics of autophagosome flux. A cell-based imaging assay using chondrocytes can be used to identify autophagy modulators, because the dynamic pH changes due to autolysosome formation can be observed in real-time. When autophagy is activated, phagophore and autophagosomes are created and catch disposable cargo. LC3 lipidation, inclusion into autophagosomal membranes, and interaction with cargo receptors provide a basic environment of pKa ≥ 6.0, corresponding to green fluorescence emission. If autophagy flux is sustained, autophagosomes fuse with lysosomes to generate autolysosomes, decreasing pH and emitting red-orange fluorescence.
Critical steps for setting up the protocol, include getting high transfection efficiency by using high-quality cell cultures and DNA to generate viral particles, the eficient selection of the cell clones carrying high levels of the transgene by optimal antibiotic treatment and flow cytrometry sorting, the gentle manipulation of cell monolayers to make sure that high-quality images allow quantitative analysis of autophagy flux, as well as the accurate identification of a meaningful number of relevant signals coming from the autophagy flux events.
Current methods rely heavily on flow cytometry, which requires cells to be in suspension, making information on cell–cell communication and intracellular events difficult to obtain. Although accurate and suitable to purify small or complex subpopulations, sorting can be too slow and provide the data only in terms of the average of surface receptor densities. Therefore, cell-based imaging presents an advantage in monitoring and measuring autophagy by detection of markers by static analyses, which cannot distinguish between upregulation and degradation inhibition, for example12. Also, high sensitivity for image acquisition and automated methods allowing the capture and the analysis of the images are only possible by using High Content Screening Systems. Automatic quantitative analyses show consistent low autophagy signals (green) obtained by exposing chondrocytes to chloroquine, while high autophagy activation signals are obtained by exposing chondrocytes to rapamycin (red-orange). These opposite effects allow the systematic identification of agents (e.g., libraries of small molecules, genomic loss-of-function or gain-of-function screening libraries) that modulate autophagy in large scale experiments. Indeed, by using this cell-based assay in a drug repurposing approach, the PPARα fibrate used as a lipid lowering drug was identified as a candidate disease-modifying therapeutic for osteoarthritis13.
This method adapts flow cytometry to a stable expression imaging monitoring system in live chondrocytes. This protocol may allow the identification of molecules activating autophagy flux in the context of cartilage biology, as well as a method to screen agents affecting these mechanisms.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This study was supported by Instituto de Salud Carlos III- Ministerio de Ciencia, Innovación y Universidades, Spain, Plan Estatal 2013-2016 and Fondo Europeo de Desarrollo Regional (FEDER), “Una manera de hacer Europa”, PI14/01324 and PI17/02059, by Innopharma Pharmacogenomics platform applied to the validation of targets and discovery of drugs candidates to preclinical phases, Ministerio de Economía y Competitividad. We also thank the Foundation for Research in Rheumatology (FOREUM) for their support.
Materials
| Name | Company | Catalog Number | Comments |
| 100 mm cell culture plate | Corning | 430167 | Cell culture plate |
| 12-well multiplate | Corning | 353043 | Cell culture plate |
| 15 mL Centrifuge conical tube | Falcon-Corning | 352095 | Centrifuge conical tube |
| 24-well multiplate | Corning | 351147 | Cell culture plate |
| 25 cm2 Cell Culture Flask | Falcon-Corning | 353014 | Cell culture flask |
| 384-well multiplate Cell Carrier | Perkin Elmer | 6007550 | Cell culture plate |
| 6-well multiplate | Corning | 351146 | Cell culture plate |
| 96-well multiplate | Corning | 353077 | Cell culture plate |
| Acoustic liquid handling technology | Labcyte | − | https://www.labcyte.com |
| Chloroquine | Sigma-Aldrich | C6628 | autophagic flux inhibitor |
| Dimethyl Sulfoxide (DMSO) | Sigma-Aldrich, St. Louis, MO | D2650 | Disolvent |
| Dulbecco's Modified Eagle's Medium (DMEM) | Lonza, Basel, Switzerland | BE-604F | T/C28a2 growth medium |
| Eagle's Minimum Essential Medium (EMEM) | ATCC | 30–2003 | HEK 293-T17 growth medium |
| FACScalibur cytometer | Becton Dickinson, CA | − | https://www.bdbiosciences.com/en-eu |
| Fetal Bovine Serum (FBS) | Sigma-Aldrich, St. Louis, MO | F9665 | HEK 293-T17 serum |
| Fetal Calf Serum (FCS) | Gibco by Life Technologies, CA | 26010–074 | Serum |
| FuGene | Promega | E2691 | A nonliposomal mixture of lipids as a plasmid delivery method to create autophagy reported cell line |
| Handheld electronic 384 channel pipette | Integra | − | https://www.integra-biosciences.com/united-states/en/electronic-pipettes/viaflo-96384#downloads |
| Hank's Balanced Salt Solution (HBSS) | Sigma-Aldrich | H6648 | Buffer |
| HEK 293-T17 | ATCC | CRL-11268 | Kidney cell line. Cells were used to facilitate retroviral packaging |
| High Content Screening System | Perkin Elmer | − | https://www.perkinelmer.com/es/product/operetta-cls-system-hh16000000 |
| Hoechst 33342 | Thermo Fisher Scientific | 62249 | DNA staining |
| Image Analysis Software | Perkin Elmer | − | https://www.perkinelmer.com/es/product/harmony-4-9-office-license-hh17000010 |
| Liquid Handler workstation | Perkin Elmer | − | https://www.perkinelmer.com/es/category/janus-liquid-handler-workstations |
| Microplate washer robot | Biotek | − | https://go.biotek.com/405tradein |
| Opti-MEM® (1x) | Thermo Fisher Scientific | 11058 | Transfection medium |
| Paraformaldehyde (PFA) | Sigma-Aldrich | 158127 | Fixer |
| pBABE-puro mCherry-EGFP-LC3B | Addgene, Cambridge, MA | 22418 | Plasmid |
| pCL-Eco | Addgene, Cambridge, MA | 12371 | Plasmid |
| Penicillin-Streptomycin (P/S) | Sigma-Aldrich | P0781 | Antibiotic |
| Phosphate-buffered saline (PBS) | MP Biomedicals | 2810305 | Buffer |
| Puromycin | Sigma-Aldrich, St. Louis, MO | P8833 | Antibiotic |
| Rapamycin | Calbiochem, Germany | 5053210 | autophagic flux activator |
| Software CellQuestPro | Becton Dickinson | − | https://www.bdbiosciences.com/en-eu |
| Syringe filters 0.45 μm | Corning | CLS431220 | Sterile filter |
| T/C28a2 | − | − | human chondrocytes cell line |
| Trypsin | Gibco by Life Technologies, CA | 15400054 | Trypsin used with T/C28a2 cells |
| Trypsin | Sigma-Aldrich, St. Louis, MO | SM-2002-C | Trypsin used with HEK 293-T17 cells |
| VSV.G | Addgene, Cambridge, MA | 14888 | Plasmid |
References
- Lopez-Otin, C., Blasco, M. A., Partridge, L., Serrano, M., Kroemer, G. The hallmarks of aging. Cell. 153 (6), 1194-1217 (2013).
- Lotz, M. K., Carames, B. Autophagy and cartilage homeostasis mechanisms in joint health, aging and OA. Nature Reviews Rheumatology. 7 (10), 579-587 (2011).
- Carames, B., Taniguchi, N., Otsuki, S., Blanco, F. J., Lotz, M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis & Rheumatology. 62 (3), 791-801 (2010).
- Carames, B., Olmer, M., Kiosses, W. B., Lotz, M. K. The relationship of autophagy defects to cartilage damage during joint aging in a mouse model. Arthritis & Rheumatology. 67 (6), 1568-1576 (2015).
- Kroemer, G. Autophagy: a druggable process that is deregulated in aging and human disease. The Journal of Clinical Investigation. 125 (1), 1-4 (2015).
- Leidal, A. M., Levine, B., Debnath, J. Autophagy and the cell biology of age-related disease. Nature Cell Biology. 20 (12), 1338-1348 (2018).
- Maiuri, M. C., Kroemer, G. Therapeutic modulation of autophagy: which disease comes first. Cell Death & Differentiation. 26 (4), 680-689 (2019).
- Vinatier, C., Dominguez, E., Guicheux, J., Carames, B. Role of the inflammation- autophagy-senescence integrative network in osteoarthritis. Frontiers in Physiology. 9, 706 (2018).
- du Toit, A., Hofmeyr, J. S., Gniadek, T. J., Loos, B. Measuring autophagosome flux. Autophagy. 14 (6), 1060-1071 (2018).
- N'Diaye, E. N., et al. PLIC proteins or ubiquilins regulate autophagy-dependent cell survival during nutrient starvation. EMBO Reports. 10 (2), 173-179 (2009).
- Gump, J. M., Thorburn, A. Sorting cells for basal and induced autophagic flux by quantitative ratiometric flow cytometry. Autophagy. 10 (7), 1327-1334 (2014).
- Yoshii, S. R., Mizushima, N. Monitoring and Measuring Autophagy. International Journal of Molecular Sciences. 18 (9), 1865 (2017).
- Nogueira-Recalde, U., et al. Fibrates as drugs with senolytic and autophagic activity for osteoarthritis therapy. EBioMedicine. 45, 588-605 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved