Method Article
A Mouse Model of Hemorrhagic Transformation Induced by Acute Hyperglycemia Combined with Transient Focal Ischemia
In This Article
Summary
This study presents a protocol for establishing a highly reproducible animal model of hemorrhagic transformation (HT) using middle cerebral artery occlusion/reperfusion (MCAO/R) in C57BL/6 mice with acute hyperglycemia.
Abstract
Hemorrhagic transformation (HT) is a serious complication that can occur as a result of thrombolytic therapy following ischemic stroke (IS), and it poses significant limitations on the clinical application of recombinant tissue plasminogen activator (rt-PA). Unfortunately, there are currently no effective interventions available for HT in clinical practice. Therefore, there is an urgent need for stable and reliable experimental animal models to elucidate the pathogenesis of HT and develop effective intervention strategies. This study presented a protocol for establishing a mouse model of HT induced by acute hyperglycemia combined with transient focal ischemia (tMCAO). Male C57BL/6J mice were injected with 30% glucose to induce hyperglycemia and then subjected to 60 min of tMCAO with reperfusion. The infarct volume, integrity of the blood-brain barrier (BBB), and degree of intracranial hemorrhage were assessed at 24 h after MCAO. The results showed that glucose injection led to transient hyperglycemia (14.3-20.3 mmol/L), which significantly increased both the infarct volume and the incidence of HT. Hematoxylin-eosin (H&E) staining indicated significant hemorrhagic lesions within the infarction zone in hyperglycemic mice. Additionally, hyperglycemic mice exhibited aggravated BBB disruption, as shown by more severe leakage of Evans blue (EB) and FITC-Dextran. In conclusion, acute hyperglycemia reliably and consistently resulted in macroscopic HT in a mouse model of tMCAO. This reproducible model offers a valuable tool for investigating the pathological mechanisms of HT and developing corresponding therapeutic interventions.
Introduction
Cerebral infarction is the primary cause of disability and the second leading cause of death in adults worldwide1. The acute phase plays a crucial role in the progression of cerebral infarction, serving as a pivotal time point for disease treatment. Early and timely restoration of blood flow in the penumbra area is essential to prevent further brain cell death, with thrombolysis and interventional therapy representing the mainstays for acute cerebral infarction (ACI) treatment. However, hemorrhagic transformation (HT) poses a significant complication following thrombolysis and interventional therapy, occurring in 15%-30% of patients with ischemic stroke, thereby limiting their application to some extent2,3. The occurrence of HT significantly increases the risk of mortality and disability, affecting the prognosis of ACI. Therefore, it is of great clinical significance to investigate the pathological mechanisms of HT and to identify effective therapeutic targets.
Currently, thread embolism-induced middle cerebral artery occlusion (MCAO) is frequently utilized as a model of HT in rodents4. Prolonged obstruction can result in massive cerebral infarction involving the cortex and striatum, potentially leading to secondary HT. Thread MCAO does not require craniotomy, is highly reproducible, and produces focal brain damage and HT similar to a human stroke. However, this mechanical model has some distinct disadvantages, including high early mortality rates and low long-term survival rates5. Another frequently used HT model is the thrombolysis model, in which blood clot formation is induced firstly in the target vessel, followed by the use of thrombolytic drugs (e.g., rt-PA, warfarin) to dissolve the clot, mimicking the clinical process of HT in ischemic stroke6,7. Despite replicating the pathological process of clinical thrombolytic therapy to a large extent, HT animal models induced by rt-PA or warfarin are intricate to implement and associated with high animal mortality as well as variable incidence and location of bleeding. In order to advance basic and clinical translational research on HT after cerebral infarction, it is essential to establish a reproducible animal model of HT that is easy to operate and offers high stability.
Hyperglycemia is a significant contributor to HT following cerebral ischemia/reperfusion (I/R)8. Several retrospective studies have analyzed the clinical data of patients undergoing mechanical thrombectomy, revealing that elevated blood glucose levels upon admission are linked to a higher incidence of spontaneous HT3. In diabetic stroke patients, hyperglycemia significantly increases the risk of HT and leads to more severe neurological deficits9,10. Researchers have developed HT models by inducing cerebral I/R in diabetic animal models through MCAO. However, the diabetes-MCAO model has a long experimental duration, complex procedure, and high costs11,12. A reliable model of HT can be established by inducing acute hyperglycemia through intraperitoneal injection of glucose and integrating it with a cerebral I/R model generated by the suture technique. This method is easily performed with consistent bleeding position and effectively imitates the clinical features of post-stroke hyperglycemia. However, there are significant differences in crucial conditions such as ischemic time and glucose concentration; additionally, the stability of the model and the incidence of HT are inconsistent in different literature.
Our research group extensively utilized the acute hyperglycemia-MCAO method to establish an HT model. Furthermore, we conducted a comprehensive series of experiments to explore the relationship between ischemic time, blood glucose concentration, HT incidence rate, and animal mortality. These experiments ultimately led to identifying optimal conditions for creating a post-cerebral infarction HT model. This study presents a detailed protocol for establishing an acute hyperglycemia-induced HT model using intraperitoneal injection of 30% glucose combined with embolic MCAO.
Protocol
The experimental protocol was approved by the Institutional Animal Care and Use Committee of Jianghan University (JHDXLL2024-080) and conducted in accordance with the Experimental Animal Ethical Guidelines issued by the Center for Disease Control of China. Adult male C57BL/6J mice weighing 21–26 g were used in this study. The details of the reagents and equipment used are listed in the Table of Materials.
1. Animal grouping and acute hyperglycemia inducing
- House the mice in the laboratory animal center of Jianghan University in a 12-h light/dark cycle controlled environment (20–22 °C, 50%–55% humidity) with food and water ad libitum.
- Randomly divide the mice into four groups: Sham + saline (n = 12), Sham + glucose (n = 12), MCAO + saline (n = 12), and MCAO + glucose (n = 20).
2. Preoperative preparation
NOTE: All experimental mice fasted for 12 h before surgery.
- Administer systemic analgesia (meloxicam, 5 mg/kg subcutaneous) and local anesthesia to the incision site (bupivacaine, 2 mg/kg subcutaneous) peri-operatively.
- Sterilize all surgical instruments, cotton swabs, and surgical sutures by autoclaving at the conditions of 121 °C and 15 psi for 30 min.
- Wipe the surgical platform and surrounding work area with 75% ethanol, and turn on the heating switch with the temperature set to 37 °C.
- Anesthetize the mouse with a 3% isoflurane-20% oxygen gas mixture in the anesthesia induction chamber. Then, evaluate the toe pinch reflex to test the depth of anesthesia.
- Move the mouse on the surgical platform in the prone position, and quickly insert the mouse's nose in the nose cone. Open the gas flow to the nose cone, closing off flow to the anesthesia induction chamber. Adjust the proportion of isoflurane to 2% and set the flow of the gas mixture to 0.4 mL/min for anesthesia maintenance.
- Apply eye gel to the mouse's eyes to maintain moisture during surgery.
- Shave the hair of the neck region by using the animal clipper. Clean and disinfect the skin by alternating applying with iodine and 70% ethanol using a disinfectant cotton stick.
- Cover a sterile gauze on the neck and cut an opening to expose the operative region.
3. Baseline cerebral blood flow measurement
- Shave the hair of scalp region by using the animal clipper. Clean and disinfect the scalp by alternating applying with iodine and 70% ethanol using a disinfectant cotton stick.
- Make a 1.0 cm long midline incision in the skin over the frontal region to expose the cranial fontanelle. Keep the skull moist with normal saline.
- Prepare the Laser Doppler Flowmetry (LDF) instrument and hold the LDF probe tip perpendicular to surface of the left parietal skull (1 mm posterior and 5 mm lateral to the bregma); once the FLUX is stable and record the baseline value.
NOTE: The unit of measurement for FLUX is Perfusion Units (PU), with a measurement range of 0-1000 PU. Ideal baseline CBF on a Laser Doppler Flowmetry are >600 PU. A continuous period of 5 seconds with a fluctuation range of less than 10% is considered as valid FLUX reading. - Suture the muscle and the skin separately using 5.0 PGA absorbable suture. Apply diclofenac sodium gel and mupirocin ointment to the wound.
4. MCAO surgical procedure
NOTE: MCAO is performed using a modified thread-occlusion method, as previously described by Chiang et al.13.
- Gently turn the mouse over to a supine position and administer intraperitoneally 30% glucose (7.2 mL/kg) or normal saline to the mice 15 min before the surgical procedure.
NOTE: Mice from Sham + glucose and MCAO + glucose groups received intraperitoneal injections of glucose. Mice from Sham + saline and MCAO + saline groups received intraperitoneal injections of normal saline. - Shave the hair of neck region by using the animal clipper. Clean and disinfect the skin by alternating applying with iodine and 70% ethanol using a disinfectant cotton stick. Cover a sterile gauze on the neck and cut an opening to expose the operative region.
NOTE: Ensure the incision is kept straight to achieve optimal visualization of the carotid artery. - Make a 1.5 cm midline incision on the ventral aspect of the neck using a scalpel. Pull apart the subcutaneous tissue and superficial fascia by using surgical tweezers.
NOTE: Make sure that the incision is kept straight to achieve optimal visualization of the carotid artery. - Beneath the superficial fascia, locate the submandibular gland and an inverted triangle formed by three muscles: the sternohyoid, positioned at the midline over the trachea; the posterior belly of the digastric muscle, recognizable by its shiny white tendinous part; and finally, the sternomastoid muscle.
- Perform blunt dissection within the inverted triangle to identify and separate the left common carotid artery (CCA).
- Dissect the vagus nerve adjacent to the CCA and mucosal tissue surrounding the blood vessels using ophthalmic forceps. Make a ringer on CCA using a 5.0 polyglycolic acid (PGA) absorbable suture, but keep the suture untensioned.
- Separate upward along the CCA, a "Y" bifurcation can be observed to divide the CCA into the external carotid artery (ECA) and internal carotid artery (ICA).
- Separate the bifurcation to fully expose the ECA and ICA. Loop and tightly tie a 5.0 PGA absorbable suture around the ECA distally from the bifurcation.
NOTE: Ensure a sufficient distance between the permanent ligation site and vascular bifurcation to prevent the thread from dislodging during the rotation of the ECA. - Temporarily clamp both the CCA and ICA using two 8 mm x 2 mm light micro serrafine arterial artery clamps to block the blood flow from the CCA to the ICA.
- Stretch the suture tie on the ECA distal and CCA to straighten the ECA segment.
- Use microscissors to make a small incision between the two suture ties on the ECA.
- Insert a silicon-coated monofilament suture (30 mm long, 3–4 mm coated silicon) into the ECA. Loop and slightly tie a second 5.0 PGA absorbable suture on the ECA near the bifurcation to prevent the monofilament suture from backing out.
- Completely cut the ECA distal to the permanent ligation and remove the artery forceps clamp from the ICA.
- Withdraw the suture to the bifurcation of the CCA, then carefully retract and rotate the ECA stump. Adjust the insertion direction of the suture and slowly insert it, approximately 9.0–10.0 mm from the CCA bifurcation into the ICA, to occlude the MCA.
- Tighten the second PGA absorbable suture around the ECA and remove the artery clamp from the CCA.
- Suture the muscle and the skin separately using 5.0 PGA absorbable suture. Apply diclofenac sodium gel and mupirocin ointment to the wound.
- Gently turn the mouse over to the prone position and repeat step 3.4. to record the blood flow following MCA occlusion.
NOTE: Mice exhibiting a blood flow reduction of less than 40% of the baseline value following MCAO were excluded from the study14. - Turn on the heating switch of recovery cage with the temperature set to 37°C. Place the mouse in the recovery cage during the post-occlusion period (60 min).
5. Monofilament removal and reperfusion
- Re-anesthetize the mouse as previously described just before the occlusion period should end.
- Clamp the CCA using a microclip artery clamp.
- Partially retract the monofilament from the ICA until the silicon-coated tip becomes visible through the ICA.
- Place another microclip artery clamp on the ICA above the silicon-coated tip.
- Completely withdraw the monofilament and tightly ligate the ECA stump.
- Remove the microclip artery clamp from ICA and CCA, respectively.
- Suture the muscle and the skin layer by layer using 5.0 PGA absorbable suture.
- Place the mouse in the recovery chamber and monitor all mice until they are fully awake. Administer meloxicam (5 mg/kg, subcutaneously) for pain relief every 12 h for up to 24 h.
6. Blood glucose measurement
NOTE: Blood glucose levels were measured at the following time points: (1) just before MCAO surgery (baseline), (2) immediately after the insertion of the monofilament (15 min after glucose injection), (3) immediately after the withdrawal of the monofilament immediately after the insertion of the monofilament (75 min after glucose injection).
- Wipe the mouse' tail with an alcoholic cotton ball to make the tail vein fully hyperaemic.
- Cut off the tail tip by 1–2 mm using surgical scissors.
- Gently squeeze along the root of the tail to the tip of the tail, facilitating the blood to flow out of the incision.
- Position the sample absorption tank of the test paper at the edge of the blood droplet.
NOTE: The blood will be drawn into the test paper due to the siphoning effect. - Read the blood glucose meter reading and record the result.
NOTE: Exclude the mice whose blood glucose levels did not rise above 10 mmol/L after 15 min of glucose injection. - Remove any surplus blood using a cotton ball and apply pressure to halt the bleeding.
7. 2,3,5-Triphenyltetrazolium Chloride (TTC) staining
- Euthanize the mouse by intraperitoneal injection of an overdose of pentobarbital (300 mg/kg).
- Once the mouse stops breathing, promptly immobilize it with a supine position.
- Open the thorax using scissors, and cut out the diaphragm to expose the heart.
- Insert a 23 G injection needle into the left ventricle of the mouse and incise the auricular appendix with scissors.
- Perfuse 15 mL of normal saline until the fluid draining from the auricular appendix appears clear and transparent.
- Decapitate the mouse and cut open the scalp to fully expose the skull using scissors. Insert the scissors tip 2 mm slightly in front of the coronal suture of the skull to pry it open.
- Use tweezers to remove the mouse's brain tissue intact.
- Place the brain tissue in a rodent brain matrice and cut it into 2 mm coronal slices.
- Transfer the slices to a 24-well plate and incubate them with 2% TTC solution at 37 °C for 15 min.
- Start the scanning software and set the parameter settings to a resolution of 1200 dpi and a JPG format for image analysis.
- Clip the tissue sections out from the 24-well plate with curved forceps and arrange the sections on the glass scanning plate.
- Scan the tissue sections and then export the images for measurement analysis.
- Measure the infarct area by correcting for tissue swelling. Subtract the non-infarct area of the ipsilateral side from the area of the contralateral side using ImageJ software15. Assess the infarct size as a percentage of the contralateral hemisphere. For additional details on infarct area analysis, refer to Friedländer et al.16.
8. Gross observation
- Repeat steps 7.1–7.8.
- Clip the tissue sections out from the brain matrice with curved forceps and arrange the sections on the glass scanning plate.
- Scan the brain coronal sections as described in steps 7.10 and 7.12.
9. Hematoxylin and eosin (H&E) staining
- Repeat steps 7.1–7.4. Slowly perfuse 15 mL of normal saline, followed by perfusion of approximately 15 mL of paraformaldehyde (PFA).
- Repeat steps 7.6–7.7. Fix the brain tissue with 4% PFA for 24 h at room temperature.
- Place the fixed brain tissue into the automated tissue dehydrator for dehydration, vitrification, and wax immersion.
- Embed the tissue in paraffin wax and cut the brain tissue into 5 μm thick coronal slices by microtome.
- Dewax the slice by immersing it in xylene three times, with each immersion lasting 8 min. Then, gradually immerse the slice in ethanol solutions of decreasing concentrations (100%, 95%, 90%, 80%, 70%), followed by distilled water, with each step lasting 5 min.
- Stain the slice with hematoxylin solution for 3 min. Then, differentiate it by immersing it in 5% hydrochloric acid alcohol for 5 s.
- Incubate the slice with eosin solution for 1 min, then dehydrate and hyalinize it with gradient ethanol solution (90%, 95%, 100%) and xylene.
- Mount the section with neutral resins.
- Capture the images of H&E stained brain slices under a bright field using fluorescence microscopy.
10. Determination of Evans Blue (EB) lekage
NOTE: For details on this procedure, please refer to Wang et al.17.
- Inject 2% (w/v) Evans Blue (EB) solution (2 mL/kg) via the tail vein 23 h post-MCAO.
- Repeat steps 7.1–7.7 one hour after EB injection, and capture a photo of the entire brain.
- Place the brain in a rodent brain matrice and cut it coronally into 2 mm slices. Scan the brain coronal sections as described in steps 7.10–7.12 to observe EB leakage.
- Divide the slices into right and left portions, and put each one into a centrifuge tube.
- Homogenize every slice of brain tissue in 50% trichloroacetic acid, with a ratio of 100 mg of brain tissue to 0.4 mL of trichloroacetic acid.
- After being incubated at 4 °C overnight, centrifuge the homogenate for 30 min at 10,000 x g (at 4 °C).
- Transfer the supernatant liquid into another centrifuge tube and dilute it four-fold with ethanol.
- Measure the absorbance of the supernatant liquid at 620 nm using a spectrophotometer.
- Convert absorbance values to the concentration of EB using a standard curve of EB in ethanol (e.g., at 31.25, 62.5, 125, and 250 ug/mL).
NOTE: The result is presented as a microgram of EB per gram of brain tissue.
11. Determination of FITC-Dextran leakage
- Inject FITC-Dextran (10 KDa, 6 mg /mL, diluted in 0.01M PBS; 4 mL/kg) into the tail vein 24 h post-MCAO, allowing it to circulate in the blood for 10 min.
- Repeat steps 7.1–7.7 to perform perfusion and remove the intact brain tissue.
- Fix the brain tissue in 4% PFA for 24 h at room temperature in darkness.
- Transfer the fixed brain tissue into the sucrose solution with gradient concentrations of 10%, 20%, and 30% (diluted in 0.01M PBS) for dehydration.
- Embed the brain tissue using optimal cutting temperature compound (OCT) and cut into 30 μm thick brain tissue sections. Transfer the sections onto a microscope slide using an inoculating loop and ensure they adhere to the surface of the slide.
- Mount the brain tissue sections using a mounting medium containing 4',6-diamidino-2-phenylindole (DAPI).
- Start the Laser confocal microscopy and its control software.
- Fix the microscope slide on the object stage and locate the infarct area under 200x eyepiece.
- Set the resolution to 1024 x 1024 and adjust the gain value and exposure time to obtain the clearest image possible.
- Acquire the images of the FITC-Dextran stained region of cerebral infarction under an excitation wavelength of 488 nm.
النتائج
The experimental procedure of this study is illustrated in Figure 1. Briefly, the mice underwent thread occlusion-induced MCAO for 60 min, followed by reperfusion. Glucose (30% in normal saline, 7.2 mL/kg body weight) was intraperitoneally administered 15 min before MCAO. Blood glucose levels were measured at baseline (before glucose injection), immediately after MCAO, and at the time of reperfusion. After 24 h of reperfusion, the mice were euthanized, and brain tissues were collected for analysis of infarct volume, blood-brain barrier leakage, and histopathology. As depicted in Figure 2, all four groups exhibited similar baseline blood glucose levels. However, the mice that received glucose injections showed significantly elevated blood glucose levels compared to those in the saline groups.
As depicted in Figure 3A, no visible infarction was observed on brain slices in the Sham + saline or Sham + glucose group, while marked cerebral infarction was clearly revealed by TTC staining on brain slices from both MCAO groups. Furthermore, mice injected with glucose exhibited a greater infarct volume compared to those injected with normal saline in the different MCAO groups. According to the statistical analysis, the infarct volume was 20.8% ± 0.8% and 45.1% ± 1.6% in the MCAO + saline group and MCAO + glucose group, respectively (Figure 3B).
Macroscopic observations revealed no hemorrhages in the left brain hemisphere of either the MCAO + saline group or the Sham + saline/glucose group. In contrast, multiple punctate hemorrhages were observed in the ischemic brain hemisphere of the MCAO + glucose group (Figure 4A). Scanning images of consecutive coronal brain sections showed that the hemorrhages in the MCAO + glucose group were distributed within the infarction regions of the cortex and striatum (Figure 4A). Additionally, histological analysis using H&E staining demonstrated significant blood cell infiltration into the infarction regions of the cortex and striatum in the MCAO+glucose group (Figure 4B). Conversely, no blood cell infiltration was observed in either the MCAO+saline group or the Sham + saline/glucose group.
As illustrated in Figure 5A, no leakage of EB was observed in the brain slices of the Sham + glucose group. However, both MCAO groups showed distinct blue staining due to EB leakage. Additionally, compared to mice injected with saline, those injected with glucose exhibited increased EB permeability at 24 h post-MCAO. In comparison to the Sham + saline group, the MCAO + saline group demonstrated a significant increase in EB content (4.2 µg/g ± 1.8 µg/g vs. 28 µg/g ± 0.7 µg/g, p < 0.01), which was further elevated in the MCAO + glucose group (28 µg/g ± 0.7 µg/g vs. 65.5 µg/g ± 6.0 µg/g, p < 0.01) (Figure 5B).As illustrated in Figure 5C, the leakage of FITC-Dextran was more intense in the ischemic cerebral cortex and striatum of the MCAO + glucose group compared with MCAO + saline group.
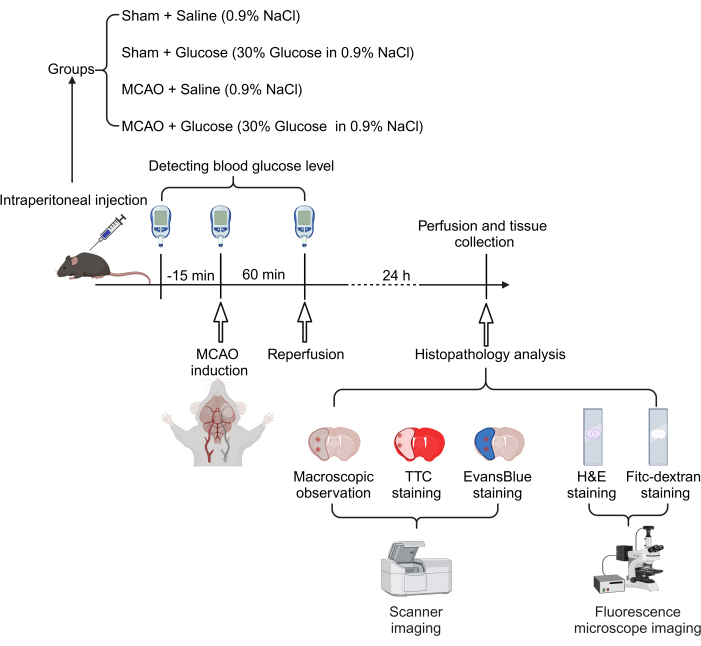
Figure 1: Schematic diagram of the experimental procedure. Mice were intraperitoneally injected with 30% glucose or saline before MCAO surgery, with blood glucose monitored during the procedure. 24 h after reperfusion, mice were euthanized for pathological and histological analysis. Please click here to view a larger version of this figure.

Figure 2: Changes in blood glucose levels at various time points following the injection of 30% glucose. Data are presented as mean ± SD. N = 10-20. ***p < 0.001 compared to the Sham + saline group. Please click here to view a larger version of this figure.
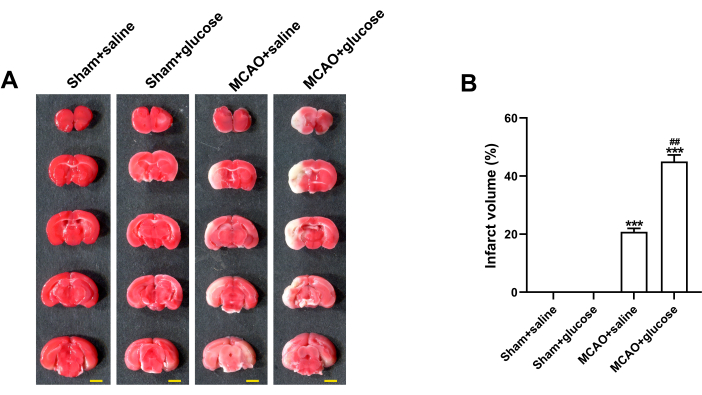
Figure 3: Hyperglycemia worsened ischemic brain damage 24 h after reperfusion. (A) Representative images of TTC-stained coronal slices, with normal brain tissue appearing pinkish-red and ischemic infarct areas appearing grayish-white. Scale bar = 5 mm. (B) Statistical analysis of infarct volume. Data are presented as mean ± SD. N = 3. ***p < 0.001 compared to the Sham + saline group, ##p < 0.01 compared to the MCAO + saline group. Please click here to view a larger version of this figure.
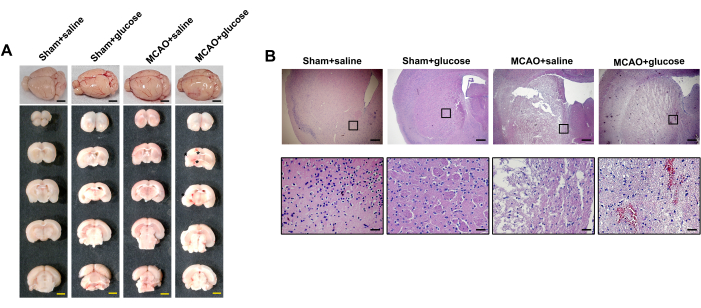
Figure 4: Hyperglycemia increased the risk of HT at 24 h after reperfusion. (A) Representative images of gross observation and coronal slices. The asterisk indicated the visible hemorrhagic foci. Scale bar = 5 mm. (B) Representative H&E-stained half-brain images (top) and enlarged images (bottom). Scale bar = 200 µm for half-brain images; Scale bar = 20 µm for enlarged images. Please click here to view a larger version of this figure.

Figure 5: Hyperglycemia aggravated BBB disruption at 24 h after reperfusion. (A) Representative images of Evans blue leakage. Scale bar = 5 mm. (B) Statistical analysis of Evans blue leakage in the ischemic hemispheres. Data are presented as mean ± SD, N = 3, **p < 0.01, ***p < 0.001 compared with Sham+saline group, ##p < 0.01 compared with MCAO + saline group. (C) Representative images of FITC-Dextran stained ischemic cerebral cortex and striatum. Scale bar = 10 µm. Please click here to view a larger version of this figure.
Discussion
The current protocol is designed to create a reliable animal model of hemorrhagic transformation following ischemic stroke, which can replicate the harmful effects of vessel revascularization under hyperglycemic conditions. Among the various risk factors for ischemic stroke, the blood glucose level within 24 h after the onset of stroke is positively correlated with the exacerbation of cerebral injury and increased mortality3,18. Numerous clinical investigations have also demonstrated that poststroke hyperglycemia is one of the most critical risk factors for HT and poorer neurological outcomes19. In the present protocol, 19 out of 20 mice were successfully induced to HT under acute hyperglycemia, resulting in a successful modeling ratio of approximately 95%. Unfortunately, four mice died from severe cerebral hematoma within 24 h after the MCAO surgery and were therefore excluded from further analysis.
Hyperglycemia-induced HT animal models have been utilized in recent preclinical research. Won et al. demonstrated that hyperglycemia promoted HT in a rat model of tPA stroke treatment20. However, the pathogenic factors in Won's HT model were more complex, as tPA itself may increase the risk of HT. In contrast, the current protocol utilized acute hyperglycemia combined with MCAO and achieved a significant success rate of HT in mice. Notably, variability exists among the hyperglycemia-induced HT models regarding glucose concentration, duration of ischemia, model reliability, and occurrence of HT21,22,23,24. Some studies utilized 50% glucose injection to induce significantly higher blood glucose levels (20-28 mmol/L), surpassing the typical range observed in stroke patients. The rate of HT in these experiments ranges from 70% to 73.3%, with a mortality rate recorded at 37.5% in one experimental model. In contrast, the current experimental procedures involved using a 30% glucose injection to induce acute hyperglycemia maintained between 14.3 mmol/L and 20.3 mmol/L, better aligning with the range of blood glucose values found in patients. Additionally, these procedures required a fasting period for experimental mice prior to surgery to regulate their preoperative baseline blood glucose levels to approximately around 5 mmol/L; failure to do so may result in an elevated baseline blood level of around 10 mmol/L, leading subsequently to excessively high blood glucose levels, and ultimately, higher mortality rates among the mice.
In a recent study, MacDougall et al. discovered that hyperglycemia led to an increase in the size of the infarct, particularly in a type 1 diabetes model25. The current findings indicate that the group administered with glucose exhibited a larger infarct size after 60 min of ischemia compared to the group under saline conditions. This result is consistent with a previous experiment that demonstrated the detrimental impact of high glucose on infarct progression in the penumbra area18. In this protocol, preoperative 30% glucose injection resulted in cerebral punctate hemorrhage after 24 h post-MCAO. Consistent with macroscopic gradation findings, histological analysis using H&E staining revealed significant blood cell infiltration into the ischemic striatum and cortex in acute hyperglycemia mice. Naturally, there are other methods available for evaluating HT, such as hemoglobin measurement through ELISA or magnetic resonance imaging (MRI). Although the pathophysiological mechanism underlying HT remains elusive, BBB disruption is considered to be its leading cause after cerebral ischemia26. Therefore, BBB disruption can indirectly reflect the severity of HT. This protocol used EB staining to test BBB permeability. EB-stained brain slices visually demonstrate changes in BBB permeability and allow for quantitative measurement of EB dye content that has permeated into brain tissue. In recent years, other tracers such as Fluorescein-sodium and FITC-Dextran have been extensively utilized to assess BBB permeability in animal models of neurological diseases27,28. So, we also performed a Dextran staining experiment to delineate the location and extent of vascular endothelial injury in more detail by observing the leakage degree of FITC-Dextran.
The current protocol has several limitations that cannot be overlooked. Firstly, the MCAO surgery requires a high level of proficiency from the operator. It is crucial to ensure timely delivery of the embolic thread into the MCA and accurate placement and depth of the occlusion. Meeting these requirements necessitates extensive training for the experiment operator. Secondly, due to the potential for HT to worsen cerebral injury, postoperative care is essential in improving the survival rates of experimental animals. Lastly, it is important to acknowledge that clinical stroke patients often have multiple risk factors for HT, such as diabetes, high blood pressure, atrial fibrillation, and age29. Therefore, the present animal model cannot fully replicate the complex mechanisms involved in these risk factors.
In conclusion, this protocol effectively replicated the HT triggered by acute hyperglycemia in a cerebral ischemia-reperfusion mouse model. This model demonstrates good repeatability, high stability, and a high animal survival rate, making it suitable for widespread use in preclinical research of HT.
Disclosures
The authors have no conflicting interests to disclose.
Acknowledgements
Figure 1 was created with BioRender software (https://www.biorender.com/). This study was supported by grants from the guiding project of the Natural Science Foundation of Hubei Province (No. 2022CFC057).
Materials
| Name | Company | Catalog Number | Comments |
| 2,3,5-Triphenyltetrazolium Chloride (TTC) | Sigma-Aldrich | 108380 | The dye for TTC staining |
| 24-well culture plate | Corning Incorporated | CLS3527 | The vessel for TTC staining |
| 30% glucose injection | Kelun Pharmaceutical | H42021188 | Acute hyperglycemia induction |
| 4% paraformaldehyde | Wuhan Servicebio Technology Co., Ltd. | G1101 | Tissue fixation |
| 5.0 Polyglycolic acid absorbable suture | Jinhuan Medical Co., Ltd | KCR531 | Equipment for surgery |
| 96-well culture plate | Corning Incorporated | CLS3596 | EB content measuring |
| Anesthesia machine | Midmark Corporation | VMR | Anesthesia for animal |
| Antifade Mounting Medium with DAPI | Beyotime Biotech | P0131 | Mount for tissue sections |
| Automation-tissue-dehydrating machine | Leica Biosystems | TP1020 | Dehydrate tissue |
| Confocal microscopy | Leica Biosystems | STELLARIS 5 | Image acquisition |
| Diclofenac sodium gel | MaYinglong Pharmaceutical | H10950214 | Analgesia for animal |
| Eosin staining solution | Servicebio Technology | G1001 | The dye for H&E staining |
| Evans Blue | Aladdin | E104208 | EB staining |
| Eye gel | Guangzhou Pharmaceutical | H44023098 | Material for surgery |
| Fitc-dextran | Sigma-Aldrich | 60842-46-8 | BBB permeability assessing |
| Fluorescence microscope | Olympus | BX51 | Image acquisition |
| Frozen microtome | Leica Biosystems | CM1900 | Use for frozen sections |
| Glucometer | YuWell | 580 | Blood glucose measurement |
| Hematoxylin staining Solution | Servicebio | G1004 | The dye for H&E staining |
| Iodine | Lircon | 20020059 | Material for surgery |
| Isoflurane | Rwd Life Science | R510-22-10 | Anesthesia for animal |
| Laser doppler blood flow meter | Moor Instruments | moorVMS | Blood flow monitoring |
| MCAO Sutures | Rwd Life Science | 907-00023-01 | Material for surgery |
| Meloxicam | Boehringer-Ingelheim | J20160020 | Analgesia for animal |
| Microsurgical instrument kit | Rwd Life Science | SP0003-M | Equipment for surgery |
| Microtome | Thermo Fisher Scientific | HM325 | Tissue section production |
| Microtome blade | Leica Biosystems | 819 | Tissue section production |
| Mupirocin ointment | GlaxoSmithKline | H10930064 | Anti-infection for animal |
| Neutral balsam | Absin Bioscience | abs9177 | Seal for H&E staining |
| Paraffin embedding center | Thermo Fisher Scientific | EC 350 | Produce paraffin blocks |
| Pentobarbital sodium | Sigma-Aldrich | P3761 | Euthanasia for animal |
| Phosphate buffered saline | Beyotime Biotech | C0221A | Rinse for tissue section |
| Scanner | EPSON | V330 | Tissue scanning |
| Shaver | Shenzhen Codos Electrical Appliances Co.,Ltd. | CP-9200 | Equipment for surgery |
| Spectrophotometer | Thermo Fisher Scientific | 1510-02362 | EB content measuring |
| Sucrose solution | Shanghai Macklin Biochemical | 57-50-1 | Dehydration for tissue |
| Tissue-Tek O.C.T. Compound | Sakura | 4583 | Tissue embedding medium |
| Trichloroacetic acid | Sigma-Aldrich | T6399 | EB content measuring |
References
- Patel, P., Yavagal, D., Khandelwal, P. Hyperacute management of ischemic strokes: JACC focus seminar. J Am Coll Cardiol. 75 (15), 1844-1856 (2020).
- Goncalves, A., et al. Thrombolytic tPA-induced hemorrhagic transformation of ischemic stroke is mediated by PKCβ phosphorylation of occludin. Blood. 140 (4), 388-400 (2022).
- Adebayo, O. D., Culpan, G. Diagnostic accuracy of computed tomography perfusion in the prediction of hemorrhagic transformation and patient outcome in acute ischaemic stroke: A systematic review and meta-analysis. Eur Stroke J. 5 (1), 4-16 (2020).
- Fluri, F., Schuhmann, M. K., Kleinschnitz, C. Animal models of ischemic stroke and their application in clinical research. Drug Des Devel Ther. 9, 3445-3454 (2015).
- Knight, R. A., et al. Prediction of impending hemorrhagic transformation in ischemic stroke using magnetic resonance imaging in rats. Stroke. 29 (1), 144-151 (1998).
- Orset, C., et al. Combination treatment with U0126 and rt-PA prevents adverse effects of the delayed rt-PA treatment after acute ischemic stroke. Sci Rep. 11 (1), 11993 (2021).
- Kwon, I., et al. Hemorrhagic transformation after large cerebral infarction in rats pretreated with dabigatran or warfarin. Stroke. 48 (10), 2865-2871 (2017).
- Mi, D., et al. Correlation of hyperglycemia with mortality after acute ischemic stroke. Ther Adv Neurol Disord. 11, 1756285617731686 (2018).
- Seners, P., Turc, G., Oppenheim, C., Baron, J. C. Incidence, causes and predictors of neurological deterioration occurring within 24 following acute ischaemic stroke: A systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. 86 (1), 87-94 (2015).
- Reshi, R., et al. Hyperglycemia in acute ischemic stroke: Is it time to re-evaluate our understanding. Med Hypotheses. 107, 78-80 (2017).
- Graham, M. L., Schuurman, H. J. Validity of animal models of type 1 diabetes, and strategies to enhance their utility in translational research. Eur J Pharmacol. 759, 221-230 (2015).
- Rehni, A. K., Liu, A., Perez-Pinzon, M. A., Dave, K. R. Diabetic aggravation of stroke and animal models. Exp Neurol. 292, 63-79 (2017).
- Chiang, T., Messing, R. O., Chou, W. H. Mouse model of middle cerebral artery occlusion. J Vis Exp. 48, e2761 (2011).
- Ma, F., Sun, P., Zhang, X., Hamblin, M. H., Yin, K. J. Endothelium-targeted deletion of the miR-15a/16-1 cluster ameliorates blood-brain barrier dysfunction in ischemic stroke. Sci Signal. 13 (621), eaay5686 (2020).
- Wanson, R. A., et al. A semiautomated method for measuring brain infarct volume. J Cereb Blood Flow Metab. 10 (2), 290-293 (1990).
- Friedländer, F., et al. Reliability of infarct volumetry: Its relevance and the improvement by a software-assisted approach. J Cereb Blood Flow Metab. 37 (8), 3015-3026 (2017).
- Wang, H. L., Lai, T. W. Optimization of Evans blue quantitation in limited rat tissue samples. Sci Rep. 4, 6588 (2014).
- Wang, C., et al. Association between red blood cell distribution width and hemorrhagic transformation in acute ischemic stroke patients. Cerebrovasc Dis. 48 (3-6), 193-199 (2019).
- Zhu, B., et al. Stress hyperglycemia and outcome of non-diabetic patients after acute ischemic stroke. Front Neurol. 10, 1003 (2019).
- Won, S. J., Tang, X. N., Suh, S. W., Yenari, M. A., Swanson, R. A. Hyperglycemia promotes tissue plasminogen activator-induced hemorrhage by increasing superoxide production. Ann Neurol. 70 (4), 583-590 (2011).
- Zhang, F. H., et al. Rosiglitazone attenuates hyperglycemia-enhanced hemorrhagic transformation after transient focal ischemia in rats. Neuroscience. 250, 651-657 (2013).
- Soejima, Y., et al. Hyperbaric oxygen preconditioning attenuates hyperglycemia-enhanced hemorrhagic transformation after transient MCAO in rats. Med Gas Res. 2 (1), 9 (2012).
- Chen, C., Ostrowski, R. P., Zhou, C., Tang, J., Zhang, J. H. Suppression of hypoxia-inducible factor-1alpha and its downstream genes reduces acute hyperglycemia-enhanced hemorrhagic transformation in a rat model of cerebral ischemia. J Neurosci Res. 88 (9), 2046-2055 (2010).
- Lin, Y., et al. Docosahexaenoic acid attenuates hyperglycemia-enhanced hemorrhagic transformation after transient focal cerebral ischemia in rats. Neuroscience. 301, 471-479 (2015).
- MacDougal, E. L., Herman, W. H., Wing, J. J., Morgenstern, L. B., Lisabeth, L. D. Diabetes and ischaemic stroke outcome. Diabet Med. 35 (9), 1249-1257 (2018).
- Yang, C., Hawkins, K. E., Doré, S., Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am J Physiol Cell Physiol. 316 (2), C135-C153 (2019).
- Natarajan, R., Northrop, N., Yamamoto, B. Fluorescein isothiocyanate (FITC)-dextran extravasation as a measure of blood-brain barrier permeability. Curr Protoc Neurosci. 79, 9.58.1-9.58.15 (2017).
- Ahishali, B., Kaya, M. Evaluation of blood-brain barrier integrity using vascular permeability markers: Evans Blue, Sodium Fluorescein, Albumin-Alexa Fluor conjugates, and Horseradish Peroxidase. Methods Mol Biol. 2367, 87-103 (2021).
- Kovács, K. B., Bencs, V., Hudák, L., Oláh, L., Csiba, L. Hemorrhagic transformation of ischemic strokes. Int J Mol Sci. 24 (18), 14067 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved