Method Article
The Use of 3D Echocardiography in Surgical Planning of the Mitral Valve in Pediatric Cardiology
In This Article
Summary
3D echocardiography of the mitral valve in pediatric cardiology produces full anatomic reconstructions that contribute to improved surgical management. Here, we outline a protocol for 3D acquisition and post-processing of the mitral valve in pediatric cardiology.
Abstract
Mitral valve disease in pediatric cardiology is complex and can involve a combination of annular, leaflet, chordae tendineae and papillary muscle abnormalities. Transthoracic two-dimensional echocardiography (2DE) remains the primary diagnostic imaging technique utilized in pediatric surgical planning. However, given that the mitral valve is a three-dimensional (3D) structure, the addition of 3D echocardiography (3DE) to better define the mechanisms of stenosis and/or regurgitation is advantageous. Transthoracic 3DE technology has improved with advances in probe technology and ultrasound scanners, producing images with good spatial resolution and adequate temporal resolution. Specifically, the addition of pediatric 3D transducers with higher frequencies and a smaller footprint provides better 3DE imaging in children. Improved efficiency of 3DE acquisition and analysis allow 3D assessment of the mitral valve to be more easily integrated by the sonographer, the cardiologist and the surgeon in mitral valve assessment. This improvement was also made possible by the postprocessing software optimization.
In this method paper, we aim to describe the transthoracic 3DE assessment of the mitral valve in children and its use in the surgical planning of pediatric mitral valve disease. Firstly, the 3DE assessment begins by selecting the correct probe and by obtaining a view of the mitral valve. Then, the appropriate data acquisition method should be selected based on the individual patient. Next, optimization of the data set is critical in order to properly balance spatial and temporal resolution. During live scanning or following acquisition, the data set can be cropped using innovative tools that allow the user to quickly obtain an infinite number of cut planes or volumetric reconstructions. The cardiologist and surgeon can view the mitral valve en face; thus, accurately reconstructing its morphology in order to support medical or surgical planning. Finally, a review of some clinical applications is proposed, showing examples in pediatric mitral valve managements.
Introduction
The mitral valve apparatus is a complex structure consisting of the mitral valve annulus, leaflets, chordae tendineae and left ventricular papillary muscles1,2. Pediatric mitral valve disease consists of an extensive range of morphologic abnormalities associated with congenital and acquired heart anomalies3. The description of the morphology of mitral valve disease and its underlying mechanisms are key parameters for the surgical planning4. This requires the use of accurate diagnostic imaging modalities. Echocardiography is established as one of the primary diagnostic techniques used in pediatric mitral valve disease5. Specifically, two-dimensional (2D) echocardiography in pediatric mitral valve disease remains the most widely used diagnostic method. However, due to the nature of 2D imaging, the sonographer, the cardiologist and the surgeon must mentally reconstruct this complex 3D structure to determine the pathological mechanisms.
With the ability to produce anatomically correct views and an infinite number of cut planes, three-dimensional (3D) echocardiography has the ability to enhance mitral valve imaging. The value of 3D echocardiography is shown in its ability to provide specific information about annular shape and dynamics, leaflet scallop prolapse and the zone of leaflet coaptation6,7. While 3D transesophageal echocardiography (TEE) has been shown to be the most accurate ultrasound modality in identifying adult mitral valve pathology8, 3D transthoracic echocardiography (TTE) is more feasible in children due to a better acoustic window. 3D TTE has been proven to be highly accurate in discerning simple vs. complex mitral valve lesions and the need for surgical intervention9. Additionally, acquiring a 3D volumetric dataset allows surgeons and cardiologists to collaborate in post-processing, further enhancing surgical planning.
3D TTE technology has continued to improve with advancement in probe technology, ultrasound processing power, and post-processing efficiencies. The current 3D matrix probes can now acquire a full volume single-beat data set at a volume rate of approximately 25 volumes per second10. It is possible to further increase the volume rate of a single-beat data set above 25 volumes per second depending on the ultrasound vendor, probe technology and volume optimization. However, if the ECG gated (sub volumes) full volume method is used, this number can more than double, providing volumes rates that are needed in children. The higher heart rates in children compared to adults require higher temporal 3D resolution for diagnostic accuracy. Additionally, the development of specific pediatric 3D probe technology allowed for a higher scanning frequency, providing better spatial resolution that is crucial regarding the small size of the mitral valve and its apparatus11. Despite all these technological improvements, the vendors have managed to produce probes with footprints adapted to the anatomy of small children to maintain an optimal acoustic window. Lastly, new post-processing features, such as a quick cropping tools, allow for efficient post-processing.
In this paper, we describe the technique for 3D TTE assessment of the mitral valve in children, which can be applied to any ultrasound system with 3D TTE application. Additionally, post-processing of the 3D data will be reviewed and its benefit in the surgical planning. Finally, we will discuss some clinical applications of 3D imaging in children and include some examples.
Protocol
This protocol follows the guidelines of our institution's human research ethics committee.
NOTE: For the implementation of this protocol, a General Electric (GE) Vivid E95 or Philips Epiq 7C ultrasound system is used. On the GE Vivid E95 system, the user has a choice between the 4Vc-D (adult probe) or 6Vc-D (pediatric probe). On the Philips Epiq 7C, the user has a choice between the X5-1 (adult probe) or X7-2 (pediatric probe). See Figure 1.
1. Patient setup and probe selection
- Position the patient in a left lateral decubitus position when possible. See Figure 1, step A.
- Select the appropriate 3D matrix probe, pediatric or adult, based on the patient size and imaging window quality. In the majority of pediatric patients under the age of ten, a high frequency (pediatric) probe can be used when imaging from a parasternal imaging window due to the close proximity of the mitral valve. Over the age of ten, use of a pediatric probe can be attempted, however with excellent image quality on older children, the adult probe is more ideal. See Figure 1, step B.
NOTE: If the user only has access to an adult 3D matrix probe, for smaller pediatric patients, increase the scanning frequency for optimal spatial resolution.
2. Probe positioning and 2D image optimization
- Apply a generous amount of gel to the selected 3D matrix probe.
NOTE: The optimal imaging window for 3D mitral assessment is a modified low parasternal long axis view. From this view, the mitral valve apparatus is in close proximity to the probe and the mitral valve leaflets to be relatively perpendicular to the ultrasound beam. In addition, a low parasternal long axis view provides full visualization of the entire mitral valve apparatus. See Figure 1, step C. - To obtain a modified low parasternal long axis view, position the probe on the chest in a standard parasternal long axis echocardiography view.
- Slide the probe laterally on the chest until the mitral valve leaflets are more perpendicular to the ultrasound beam and the 2D imaging window is optimal (this position will be between the standard parasternal window and standard apical window).
NOTE: If the patient does not have an optimal modified low parasternal view, a standard parasternal window and apical window in combination will allow full visualization of the mitral valve anatomy. - Center the mitral valve in the ultrasound sector by rocking the probe. Rocking the probe involves motion in the long axis of the probe along a fixed point while changing the angle of insonation away from 90 degrees. In 3D imaging, center the area of interest in the ultrasound sector to allow for a narrower volume and therefore better temporal resolution.
- Slide the probe laterally on the chest until the mitral valve leaflets are more perpendicular to the ultrasound beam and the 2D imaging window is optimal (this position will be between the standard parasternal window and standard apical window).
3. 3D Volume acquisition method
- Begin by activating the 3D button on the ultrasound console (may also be labelled 4D by some vendors) to enter a full volume display. The full volume display should begin as a real-time full volume.
NOTE: 3D Zoom can also be used to obtain a 3D data set of the mitral valve, however with its limited region of interest, would not be recommended because including surrounding structures can be important for surgical management. - If the patient is cooperative and able to hold their breath, use ECG gated full volume acquisition (see Figure 1, step E). Choose the number of sub volumes (heart beats) to use for the acquisition; on most ultrasound systems the number of sub volumes can be set between 2-6 (see Figure 1, step H). The higher number of sub volumes used during acquisition will result in a higher volume rate (increased temporal resolution) but can result in stitch artifacts related to breathing or motion as the sub volumes are put together.
- If the patient is uncooperative or unable to hold their breath, real-time 3D full volume acquisition will eliminate the potential for "stitch" artifacts (see Figure 1, step F). However, the reduced temporal resolution is not ideal in children and will require the user to either sacrifice volume size (region of interest) or spatial resolution to compensate (both discussed in the next step).
4. 3D volume optimization (see Figure 1, step G)
- Optimize the full volume size to include all the mitral valve annulus, chordae, papillary muscles and aortic valve where possible.
NOTE: With ECG gated acquisition, a larger volume of data can be acquired because of an increase in volume rate achieved via sub volumes.- A smaller volume of data will be required for real-time acquisition, in order to maintain a reasonable frame rate. Do this by narrowing the elevation plane and imaging in a parasternal short axis to allow for full visualization of the mitral valve leaflets and annulus (see Figure 2).
- Optimize 3D signal-to-noise ratio (quality of the images) by increasing the ultrasound line density when possible. An increase in ultrasound line density will result in a decrease in volume rate. Different vendors have variable terminology for this function. On the GE Vivid E95 ultrasound system, optimize the line density using the Frame Rate knob. On the Philips Epiq 7C ultrasound system, optimize the line density using the Image Quality touch screen button.
- With ECG gated acquisition, increase the 3D volume line density because the use of sub volumes will maintain a good volume rate.
- With real time acquisition, balance the 3D volume line density with an acceptable volume rate for the patient's heart rate.
- Set the 3D gain settings higher than 2D gain settings to minimize drop-out in the mitral valve leaflets. Gain can be decreased during post-processing to further optimize the cropped image if needed.
5. Storing the 3D full volume acquisition (see Figure 1, step I)
- If using ECG gated acquisition, ask the patient to hold their breath and remain still. Then activate the number of sub volumes (heart beats) selected. Wait at least the number of beats selected before pressing Store (the more sub volumes that are selected will result in a longer storing process)
- Ensure there are no "stitch" artifacts present and the entire mitral valve is visible in the 3D volume before storing the final volume.
- If using real time acquisition, store the final volume once all optimization is complete.
6. 3D color Doppler acquisition
- Separately obtain a color Doppler 3D volume acquisition by adding color Doppler and following steps 3-5 of the protocol. Optimize the color Doppler box size as narrow as possible while including the entire mitral valve annulus. Set the color Doppler velocity scale between 60-80 cm/s.
- Use ECG gated acquisition to maintain an adequate volume rate. Follow step 5.1 to store the 3D color Doppler volume.
NOTE: The addition of color Doppler to a 3D volume significantly reduces temporal resolution, making its feasibility in children difficult.
7. Post processing and cropping of the mitral valve
NOTE: Post processing and cropping of the mitral valve can be performed directly on the ultrasound system for immediate results. However, there is also dedicated GE software (EchoPAC) and Philips software (QLAB) that provide the same functions from a reviewing station. In addition, TomTec provides a universal software for post processing and cropping 3D datasets from both vendors.
- Load the stored 3D volume of the mitral valve in a 3-panel multi-planar display (2D lateral plane, 2D elevation plane, and 3D reconstruction) and activate the quick cropping tool. The quick cropping tool requires two clicks and allows the user to crop in any plane.
NOTE: Different vendors will have variable terminology for the quick cropping tool. On the GE Vivid E95 ultrasound system, this cropping tool is labelled "2 Click Crop". On the Philips Epiq 7C ultrasound system, this cropping tool is labelled "Quick Vue". - To obtain an en face view of the mitral valve viewing from the left atrium (Surgeon's view) follow the below steps (see Figure 3, step E).
- Working from the 2D lateral plane (low parasternal long axis in this protocol), position the first curser within the left atrium, just above the mitral annulus. After the first position is set, drag the curser across the mitral valve towards the ventricular side and align the crop line parallel to the mitral valve annulus. Position the second curser within the left ventricle, ensuring the mitral valve leaflets are captured within your crop lines, and set this point (see Figure 3 step B).
- The recommended display orientation for the mitral valve en face is anterior up12. Using the trackball, rotate the 3D mitral valve to position the aortic valve at the top of the screen.
- To obtain an en face view of the mitral valve viewing from the left ventricle, simply flip the previous step cropped image 180 degrees (on some vendor systems there is a flip crop function that accomplishes this quickly) (see Figure 3, step F).
- Crop the color Doppler 3D volume of the mitral valve in the same orientation as step 7.3.
- Obtain a view of the mitral valve sub-valvar apparatus including the chordae tendineae and papillary muscles.
- Working from the 2D lateral plane (low parasternal long axis in this protocol), position the first curser in the middle of the left ventricle. After the first position is set, drag the curser towards the posterior wall of the left ventricle and align the crop lines parallel with the long axis of the left ventricle. Position the second curser below the posterior wall and set this point (see Figure 4).
- Optimize the 3D gain and compression settings.
- Optimize the 3D gain settings to its lowest setting while maintaining minimal to no mitral valve leaflet drop-out.
- Optimize 3D compression settings to include a wider or narrower range of color shades. 3D compression can improve 3D depth perception. On the Philips Epiq 7 system, adjusting the 3D compression is performed by rotating the Compression knob. On the GE Vivid E95 system, adjusting the 3D compression is performed by rotating the Active Mode gain knob.
- Store the optimized, cropped 3D views of the mitral valve as separate cine loop clips.
النتائج
A good quality 3D data set of the mitral valve in pediatric echocardiography will have an optimal volume rate that is appropriate for assessing leaflet motion and excellent spatial resolution that utilizes superior axial resolution. To assess the success of the protocols 3D ECG gated acquisition, first determine whether any significant "stitch" artifact is present. In the presence of no artifact and if the acquisition was made using an excellent quality 2D low parasternal long-axis view, this 3D data set will provide diagnostic information about the entire mitral valve complex.
If ECG gated full volume cannot be used due to a significant "stitch" artifact caused by patient breathing and/or movement, real time 3D should be used. While this method will likely not provide one single volume of the entire mitral valve complex, utilizing a real time 3D acquisition with a narrow volume (in the elevation plane) will allow for better temporal resolution. With real time 3D acquisition, imaging of the mitral valve annulus and leaflets will be best visualized from a parasternal short axis window. While imaging of the mitral valve chordae tendineae and papillary muscles will be best visualized from an apical two chamber view (see Figure 5, case 1C).
Color Doppler, when added to a 3D full volume acquisition of the mitral valve, can enhance the assessment of mitral regurgitation. When viewed from the left ventricle, 3D color Doppler provides diagnostic information about mitral regurgitation location and vena contracta area (see Figure 3). However, it is worth noting that the addition of color Doppler significantly reduces temporal resolution, making its feasibility in children difficult.
Post-processing of 3D echocardiography data sets with the ability to obtain an infinite number of cut planes and anatomically accurate reconstructions provides one of the greatest benefits this method has in comparison to 2D echocardiography13. By producing en face 3D views of the mitral valve, the assessment of mitral regurgitation can be enhanced with true anatomic visualization of prolapsing/flail scallops, isolated clefts and zones of non-coaptation (see Figure 3). Additionally, there is available post-processing software that can quantify leaflet diameter, leaflet area, coaptation length and leaflet tenting height14, all of which is not provided with standard 2D imaging. In the assessment of mitral stenosis, 3D echocardiography can provide direct planimetry of the mitral valve orifice area13. This method is more accurate than 2D echocardiography by allowing the user to obtain cut planes that identify the smallest orifice area. In addition, direct planimetry of a 3D data set is possible without the use of dedicated 3D software. Also, post-processing of the 3D data set in mitral valve stenosis, allows visualization of the sub-valvar apparatus for accurate morphology and measurements of chordae tendineae length. With the continued evolution of efficient post-processing techniques, the ability to obtain accurate 3D reconstructions now requires a minimal time commitment.
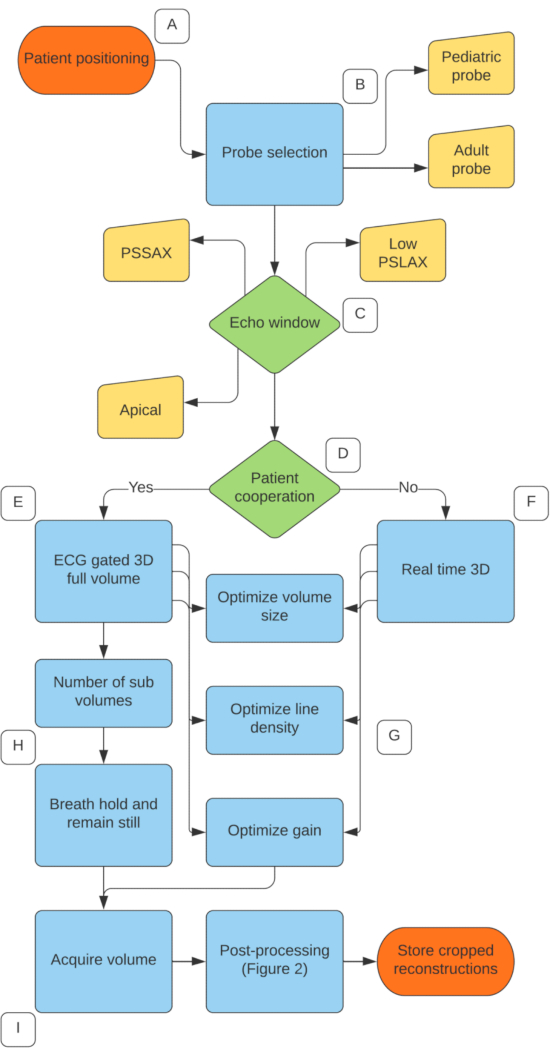
Figure 1. 3D Mitral Valve acquisition protocol. (A) Patient positioned in left lateral decubitus position. (B) Select the correct probe and scan at the highest frequency possible. (C) Choose the best 2D echocardiography window, with the low parasternal long axis considered most ideal. (D) Determine whether the patient can hold their breath and remain still. (E) If yes, choose the ECG gated full volume acquisition. (F) If no, choose the real time 3D acquisition. (G) For both methods, adjust volume size, line density and gain to optimize temporal and spatial resolution. (H) For ECG gated acquisition, choose the number of sub volumes and ask patient to breath hold and remain still. (I) Acquire volume. Please click here to view a larger version of this figure.
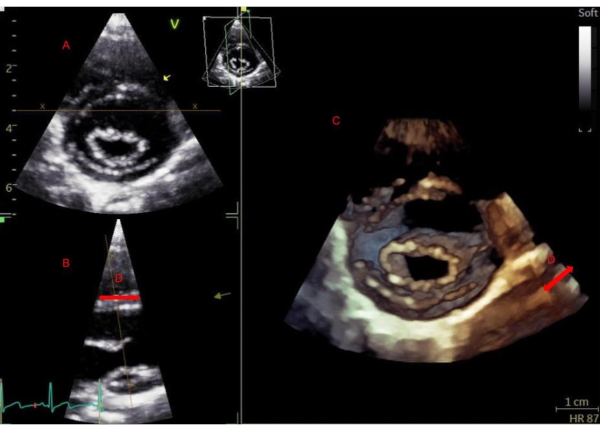
Figure 2. Real-time 3D acquisition parasternal short axis. Real-time 3D acquisition parasternal short axis. Beginning in a parasternal short-axis view of the mitral valve (A), activating real-time 3D will display the elevation plane (B) and the 3D rendered view (C). Narrowing the elevation plane (D) is important to achieve optimal resolution for a real-time acquisition. Please click here to view a larger version of this figure.
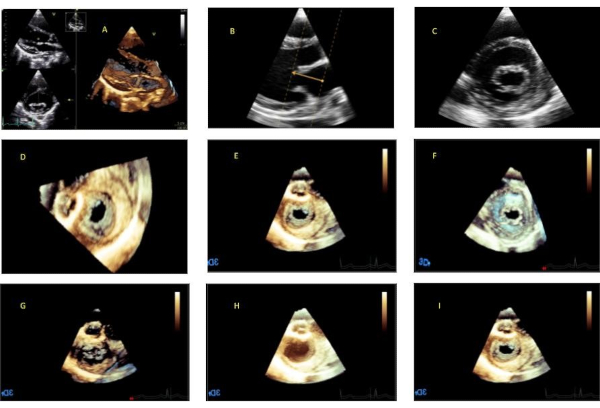
Figure 3. Post-processing workflow. (A) Begin with a multiplanar display. (B) Activate quick crop function and position first point within the left atrium above the mitral annulus. Drag cursor across the mitral valve and align crop lines perpendicular to mitral annulus. Position second point within the left ventricle to set depth of reconstruction. (C) Orthogonal 2D plane showing mitral valve in short axis. (D) 3D reconstruction showing en face view of the mitral valve from the left atrial perspective. (E) Rotate image around the z axis to position anterior up (aorta at top of image). (F) Flip image 180 degrees around the y axis to visualize the mitral valve en face from the ventricular perspective. (G) Gain settings too low. (H) Gain settings too high. (I) Gain settings optimal. Please click here to view a larger version of this figure.
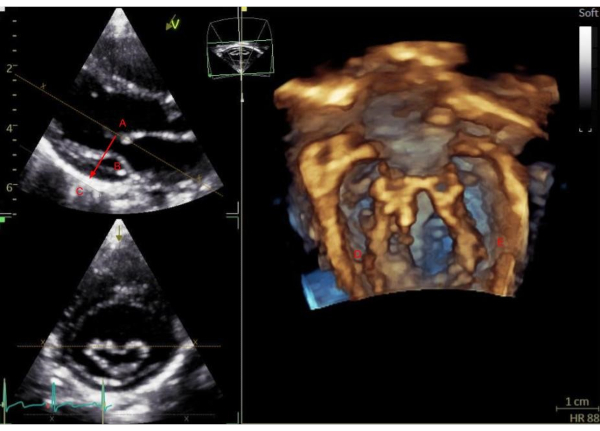
Figure 4. Mitral valve sub-valvar apparatus workflow. Mitral valve sub-valvar apparatus workflow. Using a 3D volume acquisition of the mitral valve from a low parasternal imaging window, activate the quick cropping tool. Position the first curser in the center of the left ventricle (A), drag the cropping line towards the posterior wall (B) and set the second curser posterior to the left ventricle (C). The 3D rendered view on the right shows the postero-medial mitral attachments (D) and antero-lateral mitral attachments (E). Please click here to view a larger version of this figure.
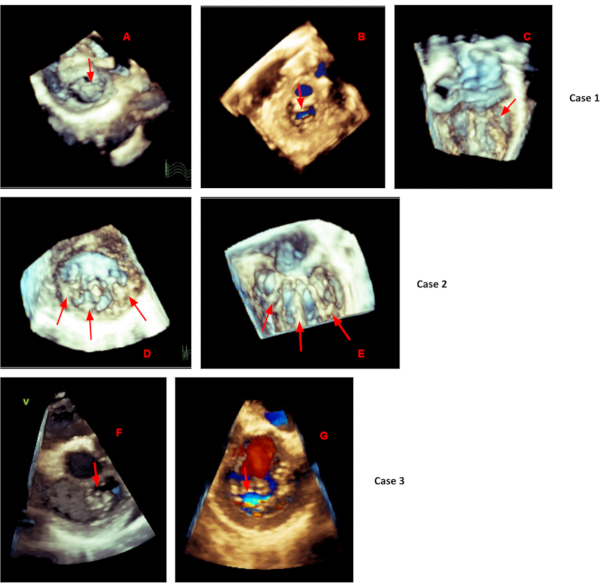
Figure 5. Mitral valve 3D cases. Mitral valve 3D Cases. Case 1 showing mitral valve anterior leaflet prolapse (A) with central mitral regurgitation (B) cropped from an ECG gated dataset acquired from a low parasternal long axis imaging window. (C) A real time 3D acquisition from an apical 2 chamber view showing short posterior leaflet chordae restricting motion. Case 2 showing three left ventricular papillary muscles (D) with the mitral valve chordae attachments (E) cropped from an ECG gated dataset acquired from a low parasternal long axis imaging window. Case 3 showing prolapse of the A3 and P3 scallops (F) and corresponding mitral regurgitation (G) cropped from an ECG gated dataset acquired from a low parasternal long axis imaging window. Please click here to view a larger version of this figure.
Discussion
For the operator/sonographer, 3D echocardiography is often met with several challenges. First, by nature there is significant variation in patient size, heart rate and cooperation during a pediatric echocardiography exam. These parameters make it difficult to have 3D specific protocols and therefore make the 3D acquisition operator dependent. Often training for sonographers is focused primarily on 2D imaging, leaving a gap in knowledge with regards to 3D image acquisition and interpretation. In addition, 3D temporal resolution is reduced when compared to 2D imaging, and the inability in some children to use ECG gated (sub volumes) acquisition, to increase volume rate, make this modality a challenge. In children with high heart rates, maintaining a high temporal resolution is critical to appreciate real time movement of the mitral valve leaflets. Another challenge associated with 3D imaging in children is access to a high frequency pediatric probe (for an optimal spatial resolution). While present adult 3D probes have a wide frequency bandwidth, their footprint and lower frequency is often not well suited for small children.
The use of one ultrasound probe for both the 2D and 3D assessment can add significant efficiencies to the 3D assessment process in children. New 3D ultrasound probes offer excellent 2D images, color Doppler and 3D volume quality. The ability to quickly capture a 3D volume at any point during the exam is particularly important in children. Secondly, for the operator, it is important to understand, through continuous training, what constitutes a good quality 3D acquisition. Additionally, to optimize the 3D assessment in children, before the acquisition the operator should determine what 3D information is important for the patient in order to guide the assessment process. For the reviewing cardiologist and surgeon, having direct access to post processing software will allow them to produce 3D reconstructions that will aid in surgical planning.
The quality of a 3D data set depends greatly on the quality of the 2D image. Thus, careful consideration should be paid to optimizing the 2D image of the mitral valve. Beginning in the most optimal 2D window available for each individual patient, parasternal or apical, will result in the best 3D image14. However, when possible, 3D TTE of the mitral valve from a parasternal long axis window will demonstrate most anomalies best15. As stated in the protocol, a small movement of the probe to a low parasternal long axis window will result in less leaflet drop out and consequently a better 3D image of the mitral valve. It is worth noting that when 2D image quality is technically difficult, the 3D acquisition will not produce clinically useful data.
Inherently, mitral valve assessment by 2D echocardiography requires multiple images and geometric conventions. Instead, 3D echocardiography provides any number of 2D slices and anatomically accurate 3D reconstructions of the mitral valve in one volumetric acquisition. Generally, the mitral valve by echocardiography is images with good acoustic quality, making this valve well suited for 3D assessment. Additionally, 3D imaging is no longer time consuming with improvements in acquisition methods and post-processing techniques. As mentioned in the protocol, an en face view of the mitral valve from the perspective of the left atrium (surgeon's view) can be easily interpreted by surgeons. The addition of 3D echocardiography preoperatively allows for immediate review in the operating room with the surgeon. This provides an opportunity for the cardiologist and surgeon to agree on a clear mechanism and therefore improve surgical planning. Specifically in congenital heart disease, the ability to visualize the function of the mitral valve relative to its surrounding structures is a strong advantage of 3D echocardiography16. The need for 3D assessment of the mitral valve extends beyond the valve itself. This diagnostic modality has been shown to provide more accurate and reproducible left ventricular (LV) volumes17 and left atrial (LA) volumes18 that correlate better with cardiac magnetic resonance (CMR). Assessing LV and LA volumes accurately are important for mitral valve surgical planning as they are used in determining hemodynamic significance.
There remain many current limitations to 3D echocardiography, particularly 3D echocardiography in pediatric congenital heart disease. To apply 3D imaging in congenital mitral valve disease requires both a high level of anatomical understanding and 3D imaging proficiency. Furthermore with current technology, small children pose a significant challenge to 3D echocardiography with regards to their inability to cooperate with breath holding and their size. Also, there remain temporal resolution challenges with real time 3D imaging and children due to their higher heart rates. Continued improvements in real time 3D echocardiography technology will further emphasize its importance in pediatric cardiology.
Disclosures
No conflict of interest
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| 4Vc-D probe | General Electric | Ultraspound probe (GE) | |
| 6Vc-D probe | General Electric | Ultraspound probe (GE) | |
| Epiq 7C | Philips | Ultrasound system | |
| Vivid E95 | General Electric | Ultrasound system | |
| X5-1 | Philips | Ultraspound probe (Philips) | |
| X7-2 | Philips | Ultraspound probe (Philips) |
References
- Perloff, J. K., Roberts, W. C. The mitral valve apparatus. Functional anatomy of mitral regurgitation. Circulation. 46, 227-239 (1972).
- Ho, S. Y. Anatomy of the mitral valve. Heart. , 5-10 (2002).
- Sousa Uva, M., et al. Surgery for congenital mitral valve disease in the first year of life. The Journal of Thoracic and Cardiovascular Surgery. 109 (1), 164-176 (1995).
- Honjo, O., Mertens, L., Van Arsdell, G. S. Atrioventricular Valve Repair in Patients With Single-ventricle Physiology: Mechanisms, Techniques of Repair, and Clinical Outcomes. Pediatric Cardiac Surgery Annual. 14, 75-84 (2011).
- Banerjee, A., Kohl, T., Silverman, N. H. Echocardiographic evaluation of congenital mitral valve anomalies in children. American Journal of Cardiology. 76, 1284-1291 (1995).
- Lang, R. M., Tsang, W., Weinert, L., Mor-Avi, V., Chandra, S. Valvular Heart Disease: The Value of 3-Dimensional Echocardiography. Journal of the American College of Cardiology. 58 (19), 1933-1944 (2011).
- Gripari, P., et al. Transthoracic echocardiography in patients undergoing mitral valve repair: comparison of new transthoracic 3D techniques to 2D transoesophageal echocardiography in the localization of mitral valve prolapse. The International Journal of Cardiovascular imaging. 34, 1099-1107 (2018).
- Pepi, M., et al. Head-to-Head Comparison of Two- and Three-Dimensional Transthoracic and Transesophageal Echocardiography in the Localization of Mitral Valve Prolapse. Journal of the American College of Cardiology. 48 (12), 2524-2530 (2006).
- Tamborini, G., et al. Pre-operative transthoracic real-time three-dimensional echocardiography in patients undergoing mitral valve repair: accuracy in cases with simple vs. complex prolapse lesions. European Journal of Echocardiography. 11, 778-785 (2010).
- Lang, R. M., Addetia, K., Narang, A., Mor-Avi, V. 3-Dimensional Echocardiography: Latest Developments and Future Directions. JACC: Cardiovascular Imaging. 11 (12), 1854-1878 (2018).
- Simpson, J. M. Real-time three-dimensional echocardiography of congenital heart disease using a high frequency paediatric matrix transducer. European Journal of Echocardiography. 9, 222-224 (2008).
- Lang, R. M., et al. EAE/ASE Recommendations for Image Acquisition and Display Using Three-Dimensional Echocardiography. Journal of the American Society of Echocardiography. 25, 3-46 (2012).
- Surkova, E., et al. Current Clinical Applications of Three-Dimensional Echocardiography: When the Technique Makes the Difference. Current Cardiology Reports. 18, 109 (2016).
- Kutty, S., Colen, T., Smallhorn, M., J, F. Three-dimensional echocardiography in the assessment of congenital mitral valve disease. Journal of the American Society of Echocardiography. 27, 142-154 (2014).
- Simpson, J., et al. Three-dimensional echocardiography in congenital heart disease: an expert consensus document from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. European Heart Journal - Cardiovascular Imaging. 17, 1071-1097 (2016).
- Sugeng, L., et al. Use of real-time 3-dimensional transthoracic echocardiography in the evaluation of mitral valve disease. Journal of the American Society of Echocardiography. 19, 413-421 (2006).
- Badano, L. P., et al. Current clinical applications of transthoracic three-dimensional echocardiography. Journal of Cardiovascular Ultrasound. 20 (1), 1-22 (2012).
- Mor-Avi, V., et al. Real-time 3D echocardiographic quantification of left atrial volume: multicenter study for validation with CMR. JACC Cardiovascular Imaging. 5, 769-777 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved