Method Article
Development of a Murine Model for Femoral Artery Anastomotic Stenosis
In This Article
Summary
Here we present a murine model for femoral artery anastomosis, offering researchers a valuable animal model to study and simulate vascular anastomotic stenosis. This development is crucial for advancing our understanding of the pathophysiology underlying this condition and facilitating more accurate and effective research into vascular diseases.
Abstract
In vascular surgery, vascular anastomosis is a common reconstructive technique used to restore blood flow. However, anastomotic restenosis is a frequent postoperative complication, primarily caused by surgery-induced vascular injury, intimal hyperplasia, and inflammatory responses. The mouse femoral artery anastomosis model is widely used to investigate the mechanisms of anastomotic restenosis and vascular repair. Microscopically guided end-to-end femoral artery anastomosis allows precise simulation of vascular injury and repair processes following surgery, providing a reliable experimental tool for studying the pathological mechanisms related to restenosis. This study focuses on refining the surgical technique for femoral artery anastomosis in mice. Through refinements in surgical techniques and optimization of technical details, we have achieved a marked increase in the success rate and reproducibility of the model. Specific improvements include refined vascular handling techniques during surgery, the selection of suture materials, and the optimization of suturing methods to minimize anastomotic leakage and postoperative occlusion. The study also emphasizes the observation of intimal hyperplasia, vascular remodeling at the anastomotic site, and long-term vessel patency. Through this research, we provide a concise and efficient operational guide for performing mouse femoral artery anastomosis, offering reliable technical support for experimental studies in vascular surgery. This work lays a solid foundation for subsequent investigations into related mechanisms and evaluations of therapeutic intervention.
Introduction
Vascular anastomosis is a fundamental technique in revascularization procedures, playing a pivotal role in restoring blood flow and promoting tissue repair. However, the occurrence of intimal hyperplasia (IH) at the anastomotic site often leads to restenosis, which significantly compromises long-term vascular patency and negatively impacts clinical outcomes and patient prognosis1,2. IH is closely associated with intraoperative vascular injury, characterized by abnormal proliferation and migration of smooth muscle cells (SMCs) and excessive deposition of extracellular matrix1. These complex and interrelated pathological processes underline the critical need to elucidate the precise mechanisms of IH to inform preventive and interventional strategies against restenosis.
Due to their reproducibility and precise control, murine models of femoral artery anastomosis have been widely adopted in research on vascular repair and associated pathological mechanisms3,4,5. End-to-end anastomosis in mice allows accurate simulation of post-surgical anastomotic injury, enabling dynamic observation of IH and vascular remodeling. These models provide an ideal platform to study interactions between endothelial cells and SMCs post-surgery and to evaluate the role of inflammatory responses in IH development6. By combining histological analysis and molecular biomarker detection, researchers can comprehensively identify key drivers of IH, offering critical insights into its underlying mechanisms and potential therapeutic targets.
The development of IH is driven by multiple factors, with hemodynamic changes being a critical contributor1,7,8. At the anastomotic site, regions of low shear stress and abnormal oscillatory shear index (OSI) are primary stimuli for SMCs proliferation and migration1,7. Furthermore, compliance mismatches and turbulent blood flow around the anastomosis exacerbate endothelial injury, accelerating IH progression8. These findings underscore the necessity of optimizing surgical techniques and selecting appropriate materials to mitigate pathological changes at the anastomotic site.
In recent years, drug-coated balloons (DCBs) have demonstrated efficacy in reducing IH. Anti-proliferative agents, such as paclitaxel, effectively inhibit SMCs proliferation and migration, significantly reducing the incidence of restenosis9. However, challenges persist in high-flow systems like arteriovenous grafts, where rapid fluctuations in shear stress and high blood flow rates may diminish the efficacy of DCBs1. Future studies should focus on improving the applicability of DCBs in varied hemodynamic environments while leveraging advancements in biomaterials science to develop more personalized and effective solutions for post-surgical restenosis. In addition to localized interventions, systemic factors such as diabetes, atherosclerosis, and endothelial dysfunction significantly influence IH development10. Therefore, clinical strategies should prioritize comprehensive management of these systemic conditions to enhance overall vascular health. Concurrently, the identification and monitoring of novel biomarkers for IH progression could provide opportunities for early intervention. The integration of artificial intelligence into surgical planning offers another promising avenue, allowing for the computational design of optimized anastomotic configurations, thereby improving surgical success rates and prolonging vascular patency.
In the study of post-surgical IH and associated pathological mechanisms, the femoral artery anastomosis model stands out for its precision and reproducibility11. This model, employing microsurgical techniques to create end-to-end anastomosis of the femoral artery in mice, accurately mimics localized surgical trauma at the anastomotic site. The advantages of this model become particularly evident when compared to models such as wire-induced injury or other alternatives. A major technical advantage of the femoral artery anastomosis model is its ability to induce highly localized and controlled vascular injury12. The surgical trauma allows for a focused impact on the anastomotic region, closely mimicking the injury patterns encountered in clinical vascular surgery. In contrast, wire-induced injury models, while simpler in technique, often result in extensive endothelial denudation, making it difficult to replicate localized trauma observed in real-life anastomotic surgeries13. Furthermore, the variability in the depth and extent of wire-induced damage across different trials potentially diminishes the reproducibility of results. The extensive and diffuse nature of the damage in wire injury models makes it less relevant for investigating the localized IH that is specifically associated with anastomotic regions.
In this study, utilizing a murine model of femoral artery anastomosis, we systematically refined surgical techniques to enhance model success rates and ensure long-term patency of the anastomotic site. Leveraging this established foundation, our study delved into the molecular and cellular mechanisms underlying IH, including regulatory pathways that govern SMCs migration and proliferation, as well as the role of inflammatory mediators in the progression of IH. Through this research, we aim to contribute novel theoretical insights into the mechanisms of post-anastomotic restenosis and establish an experimental foundation for the development of therapeutic strategies specifically targeting IH.
Protocol
This study was approved, and the animals were handled in accordance with the Guidelines for the Management and Use of Laboratory Animals in China. The research strictly adhered to the ethical requirements of animal experiments, with approval from the Animal Ethics Committee (Approval Number: SWMU20221109-019). Here, 8-week-old healthy C57BL/6 mice of either gender, weighing between 20-22 g, were utilized for the present study. The animals were housed at the Laboratory Animal Center of Southwest Medical University (SWMU).
1. Preoperative procedures
- Anesthetize mice with 3% isoflurane inhalation in accordance with institutionally approved protocols. After the onset of anesthesia, verify the complete loss of reflexes, such as the lack of response to toe-pinch, to ensure deep anesthesia before proceeding with the surgery. After inducing anesthesia, reduce the concentration of isoflurane to 1%-1.5% to maintain the anesthetic state, ensuring that the animals remain in deep anesthesia throughout the surgical procedure.
- Position the mouse supine on a surgical platform, extending the hind limbs without overstretching.
- Apply a depilatory cream to the thigh area for approximately 1 min to remove hair, then clean the area thoroughly to remove any residual cream and stray hairs.
- Disinfect the surgical site with iodine solution 3x to prepare for the subsequent surgical steps.
2. Vascular anastomosis of the femoral artery
- Under a stereomicroscope, make an incision approximately 1.5 cm long along the axis of the femur in the mid-thigh region using a microscalpel. After incising the skin, perform blunt dissection to separate the subcutaneous tissue until the femoral artery and femoral vein are exposed. The femoral artery is located laterally to the femoral vein, and the nerve is positioned superiorly and laterally to the artery.
- Carefully dissect the tissue to fully expose a 1 cm section of the femoral artery. Using hemostatic forceps, gently separate the muscles and deep fascia to expose the nerves, artery, and vein. During this process, the nerve is located on the outermost layer, the artery is in the middle, and the vein is on the inside.
- Gently separate the femoral artery and femoral vein using blunt dissection. Be cautious to use fine forceps to separate the connective tissue to fully expose the femoral artery. Place a sterile pad beneath the femoral artery to prevent accidental needle injury during the procedure. Subsequently, gently clamp the femoral artery with forceps to induce a hematoma.
- .Using small hemostatic forceps, clamp the proximal and distal ends of the femoral artery. Use fine scissors to neatly and symmetrically transect the femoral artery.
- Draw up 1% heparinized saline (1% heparin in normal saline) in a 1 mL syringe for injection at the anastomosis of the femoral artery. Add saline as 3-4 drops at a time, with several repeated rinses to remove any blood clots from the artery.
- To provide support for the vessel during subsequent suturing and simulate the potential damage caused by a guidewire intervention, insert a 1 cm long 6-0 surgical suture into the femoral artery. Ensure proper alignment and smoothness of the femoral artery to facilitate the suturing process.
- Use a 12-0 surgical suture for anastomosis and adjust the needle angle under the microscope to ensure that the needle exits from the inner side to the outer side of the vessel.
- Create eight puncture sites, four on the proximal and four on the distal ends of the femoral artery.
- Ensure that the puncture sites are located at the anterior, posterior, left, and right points of the vessel, with corresponding sites at the distal and proximal ends. When selecting these points, ensure the vessel is accessible, avoid critical anatomical structures, and maintain good blood flow during anastomosis.
- Make the puncture diameter small enough to minimize damage to the vessel but large enough for necessary procedures. A 12-0 suture needle is typically used for puncture.
- Cut four lengths of 12-0 suture, each 3-4 cm long, and thread each through the corresponding puncture hole. Begin with tying a loose knot to avoid tangling the sutures.
- Remove the 6-0 suture used for vessel support and securely tie the knots. Release the hemostatic forceps after the anastomosis is completed.
- Gently scrape the femoral artery with curved forceps from the proximal to the distal end to check for patency, ensuring that blood flows freely through the vessel. Additionally, carefully inspect the anastomosis site for any signs of obvious leakage.
3. Postoperative suture
- Suture the skin of the lower limb by using a 6-0 surgical suture, employing an interrupted suture to ensure precise alignment and secure approximation of the tissue. After suturing the wound, apply iodine tincture to the sutured area to disinfect the wound and further reduce the risk of infection.
- Maintain sterile conditions post-surgery. Do not leave the animal unattended until it has regained sufficient consciousness to maintain sternal recumbency. Do not return the animals that have undergone surgery to the company of other animals until they are fully recovered.
4. Postoperative observation and sampling
- Regularly inspect the surgical site for any signs of inflammation, excessive swelling, or discharge. Document the animal's condition and provide appropriate care as needed.
- At 4 weeks post-surgery, humanely euthanize the mice in accordance with approved ethical guidelines. For euthanasia, administer an overdose of pentobarbital sodium (150 mg/kg, intraperitoneal injection), ensuring a painless and humane endpoint. Once unconsciousness and cessation of reflexes are confirmed, initiate tissue collection.
NOTE: In the preliminary studies, we found that harvesting the samples during the 3rd week or earlier was not conducive to the formation of intimal hyperplasia. Conversely, collecting samples at the 5th week led to excessive intimal hyperplasia. This excessive growth not only impeded the observation of subsequent experiments but also posed potential health risks to the mice. So the 4th week was selected here. - Collect femoral artery samples, centering on the anastomosis site, extending approximately 1 cm in both directions from the anastomosis. Using fine scissors, excise the femoral artery along with all surrounding muscle tissue.
- After excision, rinse the sample in PBS to remove residual blood, then fix it in 10% neutral formalin for 1-2 days for further preservation and analysis.
NOTE: A 10% neutral formalin is a classic choice for tissue fixation, effectively cross-linking proteins and preserving the integrity of tissue structure, making it particularly suitable for the long-term storage of tissues. In contrast, 4% paraformaldehyde (PFA) is a milder fixative that is better suited for the finer preservation of intracellular structures (such as nucleic acids and proteins) and is commonly used for immunohistochemistry or immunofluorescence analysis. The primary aim of this study is to observe the histological changes in blood vessels (such as intimal hyperplasia and vascular remodeling) rather than the precise localization of intracellular molecules or proteins. Therefore, the fixation effect of formalin is sufficient to meet the experimental needs. If the research requires higher molecular fidelity (such as the preservation of RNA or proteins), PFA may be a better choice.
5. Dehydration and embedding of the femoral artery
- After fixation, place the samples in embedding boxes. Rinse the femoral artery samples with running water for 6-8 h before being processed for tissue dehydration using an automated tissue processor.
- Pour melted paraffin into embedding molds, carefully pick the femoral artery up using heated forceps, and vertically embed it into the mold containing the molten paraffin. Separate the lid and bottom of the embedding box, with the bottom placed on top of the embedding mold. Add a small amount of melted paraffin to fix it in place, serving as the base for the paraffin block. Air bubbles should be meticulously avoided.
- When the wax block has cooled to the point where a transparent wax film forms on the surface, place it on a freezing table to rapidly cool.
- Remove the embedded wax block from the embedding mold and trim the excess paraffin encircling the tissue block carefully using a sharp blade.
6. Preparation of paraffin sections of the femoral artery
- Mount the blade on the microtome, ensuring the knife is sharp for sectioning.
- Fix the paraffin block onto the holder and adjust the block relative to the blade to the appropriate position for sectioning.
- Trim the block to ensure the embedded tissue can be completely sectioned. Set the thickness of the initial sections to 15-20 µm.
- Adjust the section thickness to approximately 4 µm and proceed with sectioning.
- Lift the sections gently using a brush and transfer them with specialized fine tweezers to a slide box filled with warm water (approximately 45 °C) to facilitate floating.
- Transfer the spread sections onto microscope slides. Position the slides at a 45° angle to drain excess water. Afterward, place the slides in an oven to dry, typically at 37 °C for 2 h, followed by baking at 60 °C for 1 h.
7. Hematoxylin-Eosin staining
- Hydrate the sections using a series of graded ethanol concentrations, including absolute ethanol, 95%, 80%, and 70% ethanol, with each step taking approximately 5 min. Subsequently, rinse the sections with distilled water to remove any residual ethanol.
- Stain the sections with hematoxylin for approximately 8-10 min and thoroughly wash 3x with running water to remove excess stain.
- To differentiate the stained sections, apply 1% hydrochloric acid alcohol solution for a brief period of 5-7 s.
- To intensify the blue color, treat the sections with a 1:400 ammonia solution for 1 min.
- After soaking in 75% ethanol, stain the sections with eosin for 36 s. Following staining, sequentially dehydrate the sections with an ascending series of ethanol concentrations (80%, 90%, 95%, and 100%), with each step lasting for 3-5 s.
- Finally, bake the sections at 37 °C in an oven for 10 min, apply a couple of drops of sealing agent on a glass slide, cover the specimen with a coverslip to secure them in place, and allow it to air dry naturally.
Results
In vascular anastomosis surgery, mechanical injury to the vessel wall can activate intimal cells and trigger proliferation. Changes in blood flow velocity and direction after the anastomosis can also stimulate the proliferation of intimal cells. The vascular remodeling process and the long-term instability of blood flow can also persistently stimulate intimal cells, ultimately leading to thickening.
To confirm the success of the femoral artery anastomosis model, hematoxylin and eosin staining was performed on the harvested femoral artery sections. The presence of significant intimal hyperplasia was observed, indicating successful vascular remodeling and confirming the success of the anastomosis procedure (Figure 1A).
To evaluate intimal hyperplasia, the boundaries of key vascular structures, including the lumen, the internal elastic lamina (IEL), and the external elastic lamina (EEL), were marked and identified14. Based on these boundaries, area measurements were performed using image analysis software. The intimal area was calculated by measuring the total area enclosed by the IEL and subtracting the lumen area to obtain the actual intimal region area. The medial area was determined by measuring the total area enclosed by the EEL and subtracting the area of the IEL, yielding the actual medial region area. To assess the extent of intimal hyperplasia, the ratio of the intimal area to the medial area (intimal/medial ratio) was calculated, providing a clear and quantitative representation of the degree of intimal thickening (Figure 1B).
Specific detection of the vascular endothelial marker CD31 and the smooth muscle marker α-SMA was performed using the immunohistochemical staining method in both the normal control group and the experimental group after femoral artery anastomosis surgery (Figure 2).

Figure 1: Hematoxylin and eosin staining of femoral artery anastomotic tissue section. (A) After 4-weeks of surgery, femoral artery samples were harvested from the left hind limb of the mouse. The arterial tissue was embedded in paraffin and sectioned to a thickness of 4 µm. Hematoxylin and eosin staining were performed on the sections, revealing significant intimal hyperplasia in the femoral artery. (B) The statistical histogram showed that the ratio of neointima to media increased significantly in the anastomotic vessels. The data are presented as mean ± standard error of the mean (SEM). The statistical significance was assessed using the independent t-test. The y-axis represents the ratio of the intimal area to the medial area, expressed as a percentage. Please click here to view a larger version of this figure.
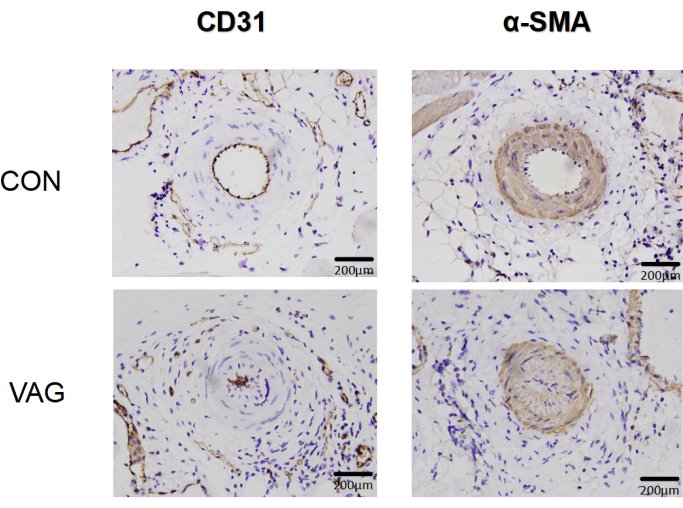
Figure 2: Immunohistochemical analysis of vascular endothelial and smooth muscle markers after femoral artery anastomosis surgery. Immunohistochemical analysis revealed alterations in the morphology and distribution of CD31-labeled vascular endothelial cells, as well as the postoperative proliferation of α-SMA-labeled smooth muscle cells, in both the normal and surgical groups. Please click here to view a larger version of this figure.
Discussion
Vascular anastomosis is a crucial technique in vascular reconstruction surgery, with its animal model playing a key role in studying the mechanisms of postoperative restenosis. This model offers a controlled approach to investigating vascular pathological changes, particularly in understanding the origin of over-proliferating cells in the neointima during restenosis. The source of proliferating smooth muscle cells (SMCs) becomes a crucial issue when severe arterial injury occurs after vascular anastomoses, resulting in the apoptosis of SMCs in the media. SMCs derived from the media of blood vessels are not the only ones involved; stem cells in the adventitia may also differentiate into new SMCs, potentially contributing to neointimal hyperplasia15. Questions have been raised about the origin of these cells, whether they come from existing SMCs or stem cells, or if other adventitial cells, such as stem cells, play a role through proliferation, migration, and differentiation. The experimental arterial anastomosis model is a valuable tool to study the migration of adventitial cells into the neointima and their transformation into SMCs following arterial injury. By surgically disrupting the vessels, the elastic layer is also disrupted, allowing adventitial cells to migrate towards the inner layer. This model offers important insights into the cellular mechanisms involved in the response to arterial injury. Each precise maneuver during the vascular anastomosis procedure critically impacts vascular integrity and the overall success of the surgical model. When extending the hind limb of the mouse, avoid over-straightening, as excessive tension may damage the artery's elasticity, complicating subsequent suturing and potentially leading to microtears in the vessel wall, which could compromise blood flow and the success rate of the anastomosis. During dissection and isolation, it is essential to carefully distinguish the artery, vein, and nerve, particularly avoiding any damage to the vein. Venous injury may cause excessive bleeding, obstructing the surgical field and increasing the complexity of the procedure. After isolating the femoral artery, clamp the proximal and distal ends with fine hemostatic forceps, taking care to avoid stretching the vessel. This precaution minimizes vessel wall damage and, upon releasing the clamps, allows blood flow to resume gradually, reducing endothelial cell stress and potential damage.
Choosing the appropriate suture is critical for preserving vessel integrity and ensuring smooth anastomosis. The suture size and type should align with the specific requirements of the vessel being handled. Selecting an appropriate length of 6-0 suture to support the artery is a critical step. Excessive length of the suture may rub against the small hemostatic clips and cause endothelial injury. Furthermore, during vascular suturing, ensure the needle passes from the inner to the outer vessel wall in a single motion to minimize endothelial trauma. This step is critical, as endothelial damage increases the risk of postoperative thrombosis and impacts subsequent functional assessments of the vessel16. Each puncture point should be precisely aligned, with symmetrical holes on both the proximal and distal ends. Adjust the number of stitches according to the vessel diameter; for example, smaller vessels may require fewer stitches (e.g., three instead of four) to reduce suture density, prevent blood flow obstruction, and ensure model patency for further analyses. Choosing a 12-0 suture is essential for reducing trauma to the vessel wall, as its thin profile minimizes the physical impact on vascular structure and preserves endothelial cell integrity, which is critical for postoperative healing and blood flow patency. Using a thinner suture helps maintain structural integrity within the model vessel, which supports sustained blood flow and provides a reliable basis for subsequent analysis. Throughout the suturing process, maintain consistent tension in the suture to avoid loosening or excessive tightening, which could restrict local blood flow.
After removing the hemostatic forceps, minor blood leakage may occur, which is expected; however, maintaining continuous blood flow is crucial to the long-term stability of the model. Postoperative observation is essential to ensuring model success. During the 4 weeks following surgery, the mouse's overall health should be closely monitored, including checking for bleeding, infection, or thrombosis at the surgical site, to ensure survival and the absence of major complications. Consistently observing the blood flow at the surgical site not only verifies anastomosis patency but also provides high-quality support for further analysis, establishing a strong foundation for the model's application in long-term studies.
In this study, we aim to evaluate anastomotic stenosis caused by vascular injury and intimal hyperplasia following revascularization. While the mouse model provides valuable insights into the initial biological response to vascular injury, there are inherent limitations to its applicability in mimicking human vascular conditions. Firstly, the progression of intimal hyperplasia in mice, typically observed over a few weeks, may not fully replicate the chronic development observed in humans over months or years, which constrains the assessment of long-term vascular changes17. Furthermore, the physiological differences between mouse and human vascular systems, including variations in vessel size, healing rates, and cellular responses, present challenges for direct translational application of findings. Precise control of injury severity in the mouse femoral artery is also challenging, as minor deviations in surgical technique can significantly impact the vessel's response, introducing variability to outcomes. Finally, the microsurgical demands of working on small vessels increase the technical complexity and risk of inconsistent injuries, which may affect the reproducibility of results. These factors collectively emphasize the need for careful interpretation of this model's findings when considering their relevance to human vascular pathophysiology.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
We would like to extend our sincere thanks to Prof. Qingbo Xu and Yanhua Hu from Zhejiang University for their valuable technical assistance. This work was supported by the National Natural Science Foundations of China (grant numbers 82070502 and 32171099), the Sichuan Science and Technology Program (grant numbers 2025HJRC0035, 2024NSFSC0709), and Luzhou-Southwest Medical University Joint Project (2024LZXNYDJ021, 2024LZXNYDJ014)
Materials
| Name | Company | Catalog Number | Comments |
| 6-0 Nylon Suture with Needle | Ningbo Chenghe | 240102 | |
| 12-0 Nylon Suture with Needle | Ningbo Lingqiao | 22064 | |
| Electro-heating standing-temperature incubator | Shanghai Boxun | HPX-9272MBE | |
| Eosin Staining Solution | Servicebio | G1005-2 | |
| Formaldehyde Solution | KESHI | 50-00-0 | |
| Hematoxylin Staining Solution | Servicebio | G1005-1 | |
| Heparin Sodium | Solarbio | H8060 | |
| MAGSCANNER KF-PRO-002 | KFBIO | KFPBL00200107003 | |
| Mounting medium | Wuxi Jiangyuan | 220810 | |
| OLYMPUS SZ2-ILST | OLYMPUS CORPORATION | SN 9B40828 | |
| Paraffin embedding machine | YAGUANG | YB-7LF | |
| Phosphate-Buffered Saline | Solarbio | P1010 |
References
- Haruguchi, H., Teraoka, S. Intimal hyperplasia and hemodynamic factors in arterial bypass and arteriovenous grafts: A review. J Artificial Organs. 6 (4), 227-235 (2003).
- Huang, C., et al. Outcome and risk factors of restenosis post percutaneous transluminal angioplasty at juxta-anastomotic of wrist autogenous radial-cephalic arteriovenous fistulas: A retrospective cohort study. Ann Vas Surg. 93, 234-242 (2023).
- Pruthi, N., Tyagi, G., Gohil, D. End-to-side microvascular anastomosis on rat femoral vessels using only 2-throw knot interrupted sutures - evaluation of feasibility and patency rates on rat femoral vessels model. World Neurosurg. 148, e145-e150 (2021).
- Yücel, H. C., et al. Effectiveness of 1α-25-dihydroxyvitamin d3 active substance on anastomosis safety in the rat femoral artery end-to-end anastomosis experimental model: Macroscopic and histological analyses. J Plastic Reconstruct Aesthetic Surg. 97, 310-319 (2024).
- Godden, D. R. P., Little, R., Weston, A., Greenstein, A., Woodwards, R. T. M. Catecholamine sensitivity in the rat femoral artery after microvascular anastomosis. Microsurgery. 20 (5), 217-220 (2000).
- Lu, Y., et al. Endothelial ripk1 protects artery bypass graft against arteriosclerosis by regulating smc growth. Sci Adv. 9 (35), e8939 (2023).
- Ghista, D. N., Kabinejadian, F. Coronary artery bypass grafting hemodynamics and anastomosis design: A biomedical engineering review. Biomed Eng Online. 12 (1), 129 (2013).
- Surovtsova, I. Effects of compliance mismatch on blood flow in an artery with endovascular prosthesis. J Biomech. 38 (10), 2078-2086 (2005).
- Matsuura, S., et al. Effect of drug-coated balloons in treatment of stenosis of the femoral artery and vein bypass graft not responding to plain old balloon angioplasty: A case report. Surg Case Rep. 5 (1), 204 (2019).
- Funk, S. D., Yurdagul, A., Orr, A. W. Hyperglycemia and endothelial dysfunction in atherosclerosis: Lessons from type 1 diabetes. Int J Vas Medicine. 2012, 1-19 (2012).
- Akelina, Y. Ballestín Aeds. Microsurgery 101. , (2024).
- Curaj, A., Zhoujun, W., Staudt, M., Liehn, E. A. Induction of accelerated atherosclerosis in mice: The "wire-injury" model. J Vis Exp. (162), e54571 (2020).
- Oh, J. G., Ishikawa, K. Experimental models of cardiovascular diseases: Overview. Methods Mol Biol. 1816, 3-14 (2018).
- Lipke, E. A., West, J. L. Localized delivery of nitric oxide from hydrogels inhibits neointima formation in a rat carotid balloon injury model. Acta Biomater. 1 (6), 597-606 (2005).
- Dong, Z. F., et al. Role of smooth muscle progenitor cells in vascular mechanical injury and repair. Medicine Novel Technol Devices. 16, 100178 (2022).
- Yau, J. W., Teoh, H., Verma, S. Endothelial cell control of thrombosis. BMC Cardiovasc Disord. 15, 130 (2015).
- Jia, G., Aroor, A. R., Jia, C., Sowers, J. R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim Biophys Acta. 1865 (7), 1802-1809 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved