Method Article
Kidney Procurement in a Preclinical Large Animal Model
* These authors contributed equally
In This Article
Summary
Here, we present a protocol that details a surgical model for kidney procurement in a preclinical swine model for subsequent machine perfusion or transplantation.
Abstract
Machine perfusion has evolved as a viable strategy for ex vivo organ assessment, monitoring, treatment, optimization, as well as to prolong preservation times. Large animal models have been paramount for the development and optimization of these technologies. However, in order to ensure graft quality and data reproducibility, standardized and clinically translatable surgical techniques for organ and tissue procurement should be followed. Thus, here, we describe an optimized protocol for kidney procurement in a preclinical swine model. Kidney recovery is performed using mixed breed (Yorkshire cross/mix) pigs. Briefly, following sterile disinfection and draping of the surgical field, a complete midline incision is performed to gain optimal access to both kidneys. The ureter, renal vein, and artery are dissected until their origin from the inferior vena cava and the aorta, respectively. After complete renal dissection, the ureter is tied and cut distally. The donor animal is then fully heparinized with 100 IU per kg/body weight. Next, the renal artery is clamped close to the aorta, and the renal vein is clamped close to the vena cava using a Satinsky vascular clamp. The kidney graft is then resected, and the renal artery is immediately cannulated back table. The kidney will then be flushed with an ice-cold preservation solution and stored on ice until either machine perfusion or transplantation. Finally, the renal artery stump is ligated with a 2-0 silk ligature, and the vena cava is closed with a 6-0 polypropylene suture. This technique recovers kidneys and simulates either a living (single kidney) or deceased (dual kidney) donor setting. The single kidney recovery offers the advantage to perform a subsequent autotransplantation. In the deceased donor model, blood can be collected prior to euthanasia by inserting blood bag needles directly into the aorta, thereby exsanguinating the animal and providing blood for ex vivo machine perfusion.
Introduction
Kidney transplantation is the optimal treatment for end-stage renal disease (ESRD), providing improved quality of life and long-term outcomes as compared to dialysis1. Despite advances in organ preservation, dozens of people die each day from ESRD while on the waitlist for a kidney transplant2. Machine perfusion is a growing field that increases preservation times, enabling extended donor networks and more efficient organ allocation. This technology also allows for ex vivo organ monitoring and optimization, thereby minimizing the effects of ischemia-reperfusion injury (IRI). When compared to static cold storage (SCS), machine perfusion has been shown to significantly reduce the incidence of delayed graft function3,4. Machine perfusion also has demonstrated revitalization of marginal grafts, which would otherwise not have met the criteria for transplant5. Despite technological advancements, the most common preservation technique remains SCS on ice. Further preclinical experiments and data can help to make machine perfusion a mainstay of kidney preservation.
The porcine model for kidney transplantation is well-established and has been integral to the development of kidney preservation technology, especially machine perfusion. Unlike unilobular rodent kidneys, porcine and human kidneys are both multilobular, similar in size, and share analogous arterial, venous, and urinary anatomy6,7. Therefore, the porcine kidney facilitates a direct translation to the clinical setting, especially in terms of medical devices and drug therapies. Additionally, porcine kidneys show a similar pathophysiology of IRI as human kidneys8, making them ideal for kidney preservation studies.
In order to ensure graft quality and data reproducibility, standardized and clinically translatable surgical techniques for organ and tissue procurement should be followed. Thus, here, we describe an optimized protocol for kidney procurement in a preclinical swine model. This protocol allows the recovery of kidneys and simulates either a living (single kidney) or deceased (dual kidney) donor setting. The single kidney recovery offers the advantage of performing a subsequent autotransplantation. In the dual kidney recovery model, blood collection prior to euthanasia is possible by inserting blood bag needles directly into the aorta, thereby exsanguinating the animal and providing blood for ex vivo machine perfusion.
Protocol
All procedures described were approved by the Institutional Care and Use Committee of Johns Hopkins University, a United States Department of Agriculture (USDA) licensed, Office of Laboratory Animal Welfare (OLAW)-assured, and the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-accredited institution. Animals were maintained in accordance with the United States Department of Agriculture's Animal Welfare Act, the National Institutes of Health's Guide for the Care and Use of Laboratory Animals, and the United States Public Health Service's Policy on Humane Care and Use of Laboratory Animals.
1. Animals and housing
- Use 3-6-month-old clinically healthy 20-40 kg Yorkshire pigs (Sus scrofa domesticus), or comparable, for this protocol.
- House the animals in chain-link fencing runs with slatted flooring.
2. Preoperative procedure and anesthesia
- Fast the animal at least 12 h prior to sedation time.
- Administer oral simethicone (20 mg/kg) at least 12 h prior to surgery and repeat dosing at least 1 h prior to sedation to reduce bowel dilation.
- Administer ketamine (20-30 mg/kg) and xylazine (2-3 mg/kg) in a single syringe intramuscularly to avoid multiple injections.
- Transfer the pig to the preoperative staging area. Place an intravenous catheter in a marginal ear vein. Administer 3-10 mg/kg/h of 0.9% saline or Lactated Ringer's solution throughout the procedure.
- Once the pig is adequately sedated, place an endotracheal tube (6.0-6.5 mm cuffed endotracheal tube for 20-30 kg animal; 6.5-7.0 mm cuffed for 30-40 kg) for delivery of gas anesthesia and mechanical ventilation. If required, facilitate with a dose of propofol (0.8-1.66 mg/kg) intravenously, administered to effect.
- Remove the hair on and surrounding the surgical site using clippers, as well as a dirty scrub of the surgical site, before moving the animal into the operating room.
- Transfer the animal to the operating table. Administer cefazolin (20-22 mg/kg) intravenously 10 min prior to the start of surgery and again every 90 min intraoperatively. Administer pantoprazole (0.5-1 mg/kg) intravenously at the start of surgery.
- Administer a local block subcutaneously with lidocaine (up to 2 mg/kg) to facilitate added analgesia prior to incision.
- Aseptically prep the surgical site, alternating between chlorhexidine or betadine and 70% ethanol or saline, at least three times.
- Continuously monitor fluid volume, heart rate, blood pressure, pulse oximetry, capnography, electrocardiography, and rectal temperature. Record these values every 10-15 min. Use a heated underbody pad and warm-air blanket to prevent hypothermia.
- Prior to the initiation of surgery, confirm that the animal is within the appropriate plane of surgical anesthesia by utilizing a combination of jaw tone, palpebral reflex, and parameters listed in the previous step.
3. Kidney harvest procedure
- Prepare the animal for surgery, following the steps described in Section 2.
- Following sterile disinfection and draping of the surgical field, perform a median laparotomy (25-30 cm) to gain optimal access to both kidneys. Insert a standard abdominal retractor.
- Cover the colon and small bowel with towels soaked in warm saline. Retract the bowels to either the right side for access to the left kidney or to the left side for access to the right kidney.
- Open the peritoneum overlying the kidney and dissect around the kidney to free any adhesions. Dissect the ureter until 10-12 cm of length is obtained.
- Dissect the renal vein and artery until their origin from the inferior vena cava and the aorta, respectively.
- After complete renal dissection, tie the ureter distally with 2-0 silk ligature and cut proximally to the tie. Leave the proximal ureter end open to allow for urine drainage.
- Heparinize the animal with intravenous heparin (100 IU/kg) and wait for 2 min to ensure adequate heparinization of the kidney. This step should be repeated prior to the resection of each kidney.
- Clamp the renal artery and renal vein close to the aorta and inferior vena cava, respectively, with two Satinsky vascular clamps. Remove the kidney graft by cutting the renal artery and vein close to the clamps.
- Immediately cannulate the renal artery with a 3 mm blunt tip perfusion cannula. Flush the kidney with ice-cold University of Wisconsin (UW) solution or Custodiol Histidine-tryptophan-ketoglutarate (HTK) preservation solution.
- Remove the perfusion cannula after flushing with UW or HTK and place the kidney in a sterile organ bag filled with the same ice-cold preservation solution that was used for flushing the kidney. Place this bag within a second sterile organ bag. Subsequently store the organ or initiate machine perfusion.
- Ligate the renal artery stump with a 2-0 silk ligature. Close the renal vein stump with a two-layer running suture with 6-0 polypropylene.
4. Blood harvest procedure
- If the procedure is terminal and all other surgical interventions have been performed, proceed with harvesting blood for machine perfusion and euthanasia by exsanguination.
- Retract the bowels to the right side and identify the infrarenal abdominal aorta. Free any large adhesions or tissue covering the vessel.
- Insert the blood collection bag needle directly into the aorta. Hang the bag below the animal to facilitate filling. Once the bag is full (approximately 450 mL of blood), remove the needle from the aorta and hold pressure on the puncture site.
- For a second blood bag, insert the new needle 1-2 cm proximally to the previous puncture site. Repeat with multiple blood bags as needed, moving 1-2 cm proximally with each needle.
- After all necessary blood has been collected, induce cardiac arrest with intravenous injection of pentobarbital sodium/phenytoin sodium at a dose of at least 78 mg/kg of the pentobarbital component. If there is concern that pentobarbital will interfere with subsequent assays of the kidney, use potassium chloride (KCl) (75-100 mg/kg IV) as an alternative chemical euthanasia method if it is administered to pigs under anesthesia. Verify death by lack of heartbeat, absent corneal reflex, and a significant decrease in body temperature.
NOTE: The remaining organs and tissue of the sacrificed pig can be used for other research or educational purposes in compliance with the 3R principles.
Results
Our research group has broad experience spanning nearly 15 years with porcine models of both solid organ transplantation and vascularized composite allotransplantation9,10,11,12,13,14,15,16,17. Here, we describe the results of our porcine kidney recovery experiments (n = 139). We have utilized the procured kidneys for transplantation, machine perfusion, or primary cell culture and have thus far not experienced any complications with this surgical procedure model (Figure 1). We define successful kidney retrieval as a surgery without technical failure, as well as the absence of adverse outcomes related to anatomy or surgical technique. Examples of the gross appearance of a kidney graft are shown in Figure 2, representing successful kidney reperfusion via machine perfusion or transplantation.
Depending on the training background and the laboratory's familiarity with swine models, we recommend performing at least one to three preliminary experiments with all personnel involved in the procedures. In the current set of studies, the average total procedural time - from midline incision to euthanasia - was 88 min (Table 1). The average time from midline incision to retrieval of both kidney grafts was 73 min. In addition to support from veterinary staff, including a veterinarian and one or two veterinary technicians, a minimum of three additional surgical and laboratory personnel are required. Depending on experience level, another surgical assistant and one or two additional support staff, including doctoral students or laboratory technicians, may be recommended to ensure efficiency with the graft retrieval, organ flushing, and blood collection in order to minimize the warm ischemia time and risk of clotting during blood collection.
As in humans, anatomical variations are possible in porcine kidneys (Figure 3, Table 2). The standard anatomy - one renal artery and one renal vein - was seen in 64% of kidneys. The most common variations were one artery and two veins (26%), two arteries and one vein (5%), one artery and three veins (4%), and two arteries and two veins (1%). Approximately half (46%) of the pigs had typical anatomy bilaterally. Atypical anatomy was seen both unilaterally (22% left atypical, 14% right atypical) and bilaterally (19%). When choosing a kidney for transplantation, we recommend avoiding any atypical anatomy. If both kidneys have two veins, opt for the kidney with longer veins and reconstruct on the back table to a single lumen prior to transplant. When choosing a kidney for machine perfusion, the venous anatomy is less important if the machine utilizes pooled venous drainage. We recommend avoiding two-artery anatomy for single-pump machine perfusion, as the differences in resistance between the arteries will create uneven circulation.
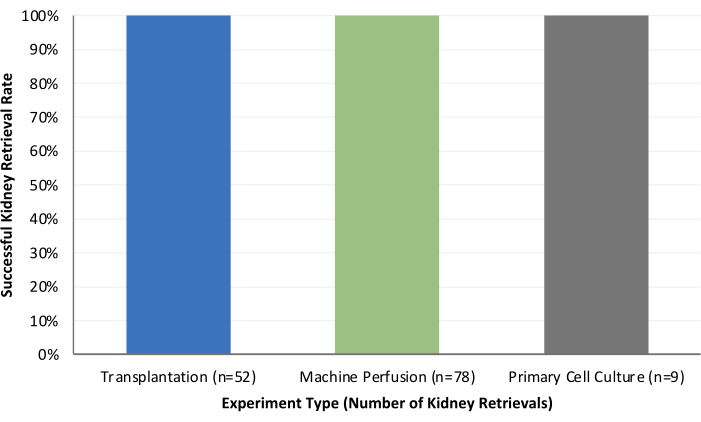
Figure 1: Summary of successful kidney retrieval rates for transplantation, machine perfusion, and primary cell culture experiments. Please click here to view a larger version of this figure.

Figure 2: Examples of kidney graft gross appearance. (A) Native right kidney in situ.(B) Kidney graft after flushing with ice-cold preservation solution. (C) Kidney graft after machine reperfusion. (D) Kidney graft after transplantation and reperfusion. Please click here to view a larger version of this figure.
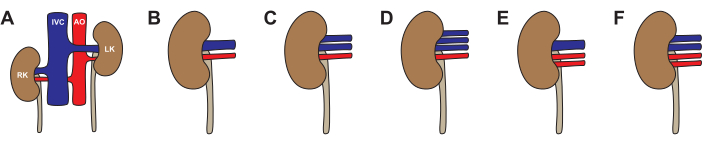
Figure 3: Variations in pig kidney anatomy. (A,B) Typical anatomy with one renal artery and one renal vein for each kidney. (C) Atypical anatomy with one renal artery and two renal veins. (D) Atypical anatomy with one renal artery and three renal veins. (E) Atypical anatomy with two renal arteries and one renal vein. (F) Atypical anatomy with two renal arteries and two renal veins. Abbreviations used: RK- right kidney; IVC- inferior vena cava; AO- aorta; LK- left kidney. Please click here to view a larger version of this figure.
| Experimental Task or Step | Average Time (min) | Surgeon | Surgical Assist | Veterinarian | Veterinary Technician | Researcher | Total Number of Personnel |
| Pre-Operative | 60 | 1 | 2 | 1 | 4 | ||
| Graft Retrieval Surgery (One Kidney) | 41 | 1 | 1 | 1 | 1 | 1 | 5 |
| Graft Retrieval Surgery (Both Kidneys) | 73 | 1 | 1 | 1 | 1 | 1 | 5 |
| Organ Flushing (Per Kidney) | 6 | 1 | 1 | 1 | 1 | 4 | |
| Blood Collection | 15 | 1 | 1 | 1 | 1 | 1 | 5 |
| Euthanasia | N/A | 1 | 1 | 2 |
Table 1: Human resources and time requirements for performing porcine kidney procurement surgery.
| Bilateral Kidney Anatomy | Number of Pigs | Percentage of Pigs |
| Both Typical | 17 | 46% |
| Right Typical, Left Atypical | 8 | 22% |
| Left Typical, Right Atypical | 5 | 14% |
| Both Atypical | 7 | 19% |
| 37 | 100% | |
| Unilateral Kidney Anatomy | Number of Kidneys | Percentage of Kidneys |
| 1 Artery, 1 Vein | 47 | 64% |
| 1 Artery, 2 Veins | 19 | 26% |
| 1 Artery, 3 Veins | 3 | 4% |
| 2 Arteries, 1 Vein | 4 | 5% |
| 2 Arteries, 2 Veins | 1 | 1% |
| 74 | 100% |
Table 2: Summary of anatomic variations found in pig kidneys.
Discussion
The porcine model for kidney transplantation has been vital to the development and optimization of machine perfusion technology. Given the anatomical, immunological, and pathophysiological similarities to human kidneys6,7,8, porcine kidneys offer a facilitated translation to clinical testing and practice.
The model of porcine kidney procurement can be utilized for a variety of preclinical experiments. In a living donor model, a single kidney can be recovered and preserved, with subsequent autotransplantation and removal of the contralateral kidney18,19. In a deceased donor model, both kidneys can be procured , and used for preservation studies, machine perfusion studies, or allotransplantation. The blood collection procedure outlined in Section 4 is key for machine perfusion studies utilizing a perfusate containing whole blood or blood products, especially extended machine perfusion necessitating perfusate exchange20,21,22. By cannulation of the aorta directly, a consistent and rapid flow of blood is obtained, fully exsanguinating the animal in a minimum amount of time. Multiple blood bags can be filled by inserting each needle proximally to the previous, thereby minimizing blood leakage from the previous puncture site.
Both hypothermic machine perfusion (HMP) and normothermic machine perfusion (NMP) are emerging methods for kidney preservation and optimization. When compared to SCS, both HMP and NMP have been shown to improve outcomes and decrease the incidence of delayed graft function3,4,5. Further research is needed to determine the optimal method, but some preclinical studies have indicated that NMP provides better outcomes23. Machine perfusion provides an opportunity for viability testing and prediction of marginal grafts21, as well as the potential to repair previously unusable grafts24. Additional studies have demonstrated machine perfusion as a venue for drug delivery and bioengineering, including the delivery of stem cells25 and the re-cellularization of a porcine kidney with human endothelial cells26. Pig-to-human kidney xenotransplantation is a recent breakthrough27,28, and further advances may lie in machine perfusion.
Variations in renal anatomy are an inevitable hurdle when performing multiple kidney recoveries. A kidney with two renal arteries is an uncommon variant. In our experience, the contralateral kidney has normal arterial anatomy and can be used in lieu of the atypical one. Venous abnormalities are more common than arterial ones but also more amenable to reconstruction. The potential variations and reconstruction possibilities of venous anatomy have been previously described19,29and are beyond the scope of this paper.
Despite its efficacy, the above protocol is limited by the time and resources required to successfully complete a complex large animal surgery, including personnel, surgical equipment, animal housing, and pre- and post-operative care. Additionally, a young, healthy porcine kidney is a limited model to evaluate older adult donors, donors with underlying renal disease, and donors with comorbidities including hypertension and diabetes.
This optimized and reproducible protocol for porcine kidney procurement allows for translatable preclinical research in kidney transplantation, preservation, and machine perfusion.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors would like to acknowledge the many veterinary technicians at Johns Hopkins University School of Medicine for their technical assistance. We would also like to express our gratitude to Drs. Jessica Izzi and Amanda Maxwell, and the numerous veterinary residents, including Drs. Mallory Brown, Jessica Plunkard, and Alexis Roach for providing our animals with excellent clinical care and veterinary oversight. Finally, we would like to acknowledge all members of the Vascularized Composite Allotransplantation (VCA) Laboratory at the Johns Hopkins School of Medicine who have assisted in any capacity with kidney procurement or other organ procurement procedures performed in our laboratory. This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R44DK136396.
Materials
| Name | Company | Catalog Number | Comments |
| 70% Ethanol Solution | Fisher Scientific | 04-355-122 | |
| Adson Tissue ForceA2:D30ps, 4.75", 1 x 2 Teeth | Wexler Surgical | FL0081.1 | |
| Bair Hugger Animal Health Overbody Blanket | 3M | 53777 | |
| Bair Hugger Warming Unit | 3M | 77500 | |
| Balfour Abdominal Retractor w/ Fixed Side Blades, 4" Deep, 10" Maximum Spread | MPM Medical Supply | 124-7017 | |
| Betadine Solution (5% Providone-iodine) | MWI Animal Health | NDC-67618-155-01 | |
| Cefazolin for Injection, USP | MWI Animal Health | NDC-63323-237-10 | |
| Chlorhexidine Solution | MWI Animal Health | NDC-30798-624-31 | |
| Custodial HTK Organ Preservation Solution | Essential Pharmaceuticals | 25767073545 | |
| DeBakey Tissue Forceps, 7.75", 2 mm Tips | Wexler Surgical | FL0789.1 | |
| EUTHASOL (pentobarbital sodium and phenytoin sodium) | Virbac | NDC-051311-050-01 | |
| Heparin Sodium Injection, USP | MWI Animal Health | NDC-71288-402-10 | |
| Hot Dog Temperature Management Controller | Augustine Surgical Inc. | WC71V | |
| Hot Dog Veterinary Underbody Warming Mattress | Augustine Surgical Inc. | V106 | |
| Invisishield Isolation Bag, 20" x 20" | Medline | DYNJSD1003 | |
| Jacobson Micro Needle Holder, Straight Jaws, Round Handle, 7.25" | Wexler Surgical | NL0729.11 | |
| Ketamine Hydrochloride Injectable Solution | NexGen Pharmaceuticals | NC-0256 | |
| Lap Sponges 18" x 18" | Medline | MDS231318LF | |
| Metzenbaum Dissection Scissors, 7" Curved | Wexler Surgical | SL5011.1S | |
| Non-Conductive Suction Tubing with Scalloped Connectors, 1/4" x 10' | Medline | DYND50251 | |
| Pantoprazole Sodium for Injection | MWI Animal Health | NDC-55150-202-00 | |
| Perfusion Cannula, Free-Flow, 3 mm Blunt Tip | MED Alliance Solutions | PER-3003S | |
| Rigid Bulb Tip Yankauer | Medline | DYND50130 | |
| Satinsky Clamp, 30 mm Angled DeBakey Atraumatic Jaws, Curved Shanks, 10" | Wexler Surgical | AL2150.1 | |
| Scalpel Handle #3 | World Precision Instruments | 500236 | |
| Servator B UW (University of Wisconsin) | Global Transplant Solutions | JFISERB10A r2 | |
| Single Collection Unit Prefilled CPDA-1, 450 mL | Jorgensen Laboratories | JO520 | |
| Sofsilk Suture Tie, 2-0, Black, 18" | Covidien | S-195 | |
| Surgical Scalpel Blade No. 10 | World Precision Instruments | 500239 | |
| Surgipro II Suture, 6-0, Blue, 30", Double Armed, CV-22 Needle | Covidien | VP-733-X | |
| Three-Quarter Surgical Drape | Medline | DYNJP2414 | |
| Valleylab Electrosurgical Pencil with Stainless Steel Electrodes | Covidien | CVNE2516H | |
| Valleylab Force FXc Electrosurgical Generator | Covidien | MFI-MDT-FORCE-FXC | |
| Valleylab Polyhesive Adult Patient Return Electrode | Covidien | E7507-SD | |
| Xylazine Hydrochloride Injectable Solution | NexGen Pharmaceuticals | NC-0334 |
References
- Merion, R. M., et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 294 (21), 2726-2733 (2005).
- Lentine, K. L., et al. OPTN/SRTR 2022 annual data report: Kidney. Am J Transplant. 24 (2S1), S19-S118 (2024).
- Hosgood, S. A., et al. Normothermic machine perfusion versus static cold storage in donation after circulatory death kidney transplantation: a randomized controlled trial. Nat Med. 29 (6), 1511-1519 (2023).
- Malinoski, D., et al. Hypothermia or machine perfusion in kidney donors. N Engl J Med. 388 (5), 418-426 (2023).
- Hamar, M., Selzner, M. Ex vivo machine perfusion for kidney preservation. Curr Opin Organ Transplant. 23 (3), 369-374 (2018).
- Pereira-Sampaio, M. A., Favorito, L. A., Sampaio, F. J. Pig kidney: anatomical relationships between the intrarenal arteries and the kidney collecting system. Applied study for urological research and surgical training. J Urol. 172 (5 Pt 1), 2077-2081 (2004).
- Bagetti Filho, H. J., Pereira-Sampaio, M. A., Favorito, L. A., Sampaio, F. J. Pig kidney: anatomical relationships between the renal venous arrangement and the kidney collecting system. J Urol. 179 (4), 1627-1630 (2008).
- Giraud, S., et al. Contribution of large pig for renal ischemia-reperfusion and transplantation studies: the preclinical model. J Biomed Biotechnol. 2011 (21), 532127 (2011).
- Girard, A. O., et al. Hickman catheter use for long-term vascular access in a preclinical swine model. J Vis Exp. (193), e65221 (2023).
- Gerling, K. A., et al. A novel sutureless anastomotic device in a swine model: A proof of concept study. J Surg Res. 291, 116-123 (2023).
- Etra, J. W., et al. Latissimus Dorsi myocutaneous flap procedure in a swine model. J Invest Surg. 34 (12), 1289-1296 (2021).
- Etra, J. W., et al. A skin rejection grading system for vascularized composite allotransplantation in a preclinical large animal model. Transplantation. 103 (7), 1385-1391 (2019).
- Al-Rakan, M., et al. Ancillary procedures necessary for translational research in experimental craniomaxillofacial surgery. J Craniofac Surg. 25 (6), 2043-2050 (2014).
- Santiago, G. F., et al. Establishing cephalometric landmarks for the translational study of Le Fort-based facial transplantation in Swine: enhanced applications using computer-assisted surgery and custom cutting guides. Plast Reconstr Surg. 133 (5), 1138-1151 (2014).
- Ibrahim, Z., et al. Cutaneous collateral axonal sprouting re-innervates the skin component and restores sensation of denervated Swine osteomyocutaneous alloflaps. PLoS One. 8 (10), e77646 (2013).
- Ibrahim, Z., et al. A modified heterotopic swine hind limb transplant model for translational vascularized composite allotransplantation (VCA) research. J Vis Exp. (80), e50475 (2013).
- Wachtman, G. S., et al. Biologics and donor bone marrow cells for targeted immunomodulation in vascularized composite allotransplantation: a translational trial in swine. Transplant Proc. 43 (9), 3541-3544 (2011).
- Kaths, J. M., et al. Heterotopic renal autotransplantation in a porcine model: A step-by-step protocol. J Vis Exp. 108, e53765 (2016).
- Liu, W. J., et al. Orthotopic kidney auto-transplantation in a porcine model using 24 hours organ preservation and continuous telemetry. J Vis Exp. (162), e61591 (2020).
- Steinhauser, C., et al. Assessment of hemodynamic and blood parameters that may reflect macroscopic quality of porcine kidneys during normothermic machine perfusion using whole blood. World J Urol. 42 (1), 471 (2024).
- Kaths, J. M., et al. Normothermic ex vivo kidney perfusion for graft quality assessment prior to transplantation. Am J Transplant. 18 (3), 580-589 (2018).
- Urcuyo, D., et al. Development of a prolonged warm ex vivo perfusion model for kidneys donated after cardiac death. Int J Artif Organs. 40 (6), 265-271 (2017).
- Vallant, N., et al. A comparison of pulsatile hypothermic and normothermic ex vivo machine perfusion in a porcine kidney model. Transplantation. 105 (8), 1760-1770 (2021).
- Hosgood, S. A., Saeb-Parsy, K., Hamed, M. O., Nicholson, M. L. Successful transplantation of human kidneys deemed untransplantable but resuscitated by ex vivo normothermic machine perfusion. Am J Transplant. 16 (11), 3282-3285 (2016).
- Vallant, N., Wolfhagen, N., Sandhu, B., Hamaoui, K., Papalois, V. Delivery of mesenchymal stem cells during hypothermic machine perfusion in a translational kidney perfusion study. Int J Mol Sci. 25 (9), 5038 (2024).
- Uzarsk, J. S., et al. Sustained in vivo perfusion of a re-endothelialized tissue engineered kidney graft in a human-scale animal model. Front Bioeng Biotechnol. 11, 1184408 (2023).
- Anand, R. P., et al. Design and testing of a humanized porcine donor for xenotransplantation. Nature. 622 (7982), 393-401 (2023).
- Pan, W., et al. Cellular dynamics in pig-to-human kidney xenotransplantation. Med. 5 (8), 1016-1029 (2024).
- Golriz, M., et al. Pig kidney transplantation: an up-to- date guideline. Eur Surg Res. 49 (3-4), 121-129 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved