Method Article
Mass Cytometry Analysis of Systemic and Local Immune Responses in Hepatocellular Carcinoma
* These authors contributed equally
In This Article
Summary
This protocol outlines a comprehensive mass cytometry (cytometry by time-of-flight [CyTOF]) analysis method for evaluating both systemic and local immune responses in hepatocellular carcinoma (HCC). The approach aims to provide insights into the immune landscape of HCC, offering a deeper understanding of the tumor microenvironment and the associated immune mechanisms.
Abstract
Hepatocellular carcinoma (HCC) is one of the most common and deadliest forms of liver cancer worldwide. Despite advances in treatment, the prognosis for HCC patients remains poor due to the complex interplay of genetic, environmental, and immunological factors driving its progression. Understanding the immune landscape of HCC is crucial for developing effective therapies, particularly in the field of immunotherapy, which holds great promises for improving patient outcomes. This study employs mass cytometry (cytometry by time-of-flight [CyTOF]) technology to investigate both systemic and local immune responses in patients with HCC. By analyzing peripheral blood and tumor samples, the research aims to identify unique immune cell populations, and their functional states associated with HCC progression. The findings provide a comprehensive overview of the immune landscape in HCC, highlighting potential biomarkers and therapeutic targets. This approach offers valuable insights into the immune mechanisms underlying HCC and paves the way for the development of more effective immunotherapies for this malignancy.
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer and a significant global health issue due to its high incidence and mortality rates1. According to the World Health Organization, HCC ranks as the fifth most common cancer and the second leading cause of cancer-related deaths worldwide2. It is particularly prevalent in regions with high rates of chronic hepatitis B and C infections, such as East Asia and sub-Saharan Africa3. Major risk factors include viral hepatitis, cirrhosis, and metabolic syndrome4. HCC requires long-term treatment, imposing substantial physical and financial burdens, underscoring the need for effective prevention, early detection, and innovative treatment strategies5.
The immune system plays a crucial role in the development of HCC. The liver is an immunologically active organ with an abundance of immune cells, including liver-resident macrophages, natural killer (NK) cells, and T cells, which are essential for monitoring and eliminating abnormal cells6. However, HCC can evade immune surveillance by expressing immunosuppressive molecules, recruiting immunosuppressive cells, and altering the tumor microenvironment7,8. This immune escape not only promotes tumor growth and metastasis but also affects the response to immunotherapy9,10.
Systemic and local immune responses in the tumor microenvironment are key factors influencing cancer progression and therapeutic outcomes. Systemic immune responses involve circulating immune cells that can recognize and attack distant tumor cells, such as peripheral T cells, NK cells, and monocytes that can target tumor cells throughout the body. Local immune responses focus on immune cell activity within the tumor microenvironment, including tumor-infiltrating lymphocytes (TILs), tumor-associated macrophages (TAMs), and regulatory T cells (Tregs). While TILs often exert cytotoxic effects against tumor cells, TAMs and Tregs typically contribute to an immunosuppressive environment that supports tumor growth11,12. Tumor cells and stromal cells can reshape the tumor microenvironment to promote immunosuppression and evade immune surveillance. The interaction between systemic and local immune responses determines the overall effectiveness of anti-tumor immunity11. Understanding this interaction can aid in developing more effective immunotherapy strategies.
Traditional flow cytometry and immunohistochemistry, while widely used in immunological studies, exhibit significant limitations when it comes to analyzing complex immune landscapes due to their inability to perform comprehensive, high-dimensional analysis. Flow cytometry is highly effective for detecting surface and functional markers at the single-cell level; however, its capacity for simultaneous multi-marker analysis is restricted, often limited by spectral overlap and practical constraints on the number of fluorescent tags that can be used13,14. Immunohistochemistry, on the other hand, provides valuable insights into the tissue context of specific markers, but it is similarly hampered by the limited number of analyzable markers and the inherent difficulties of achieving robust, quantitative, high-dimensional assessments15.
To effectively characterize complex immune environments, high-dimensional techniques like mass cytometry (cytometry by time-of-flight [CyTOF]) are essential. Mass cytometry is an advanced technology that employs mass spectrometry to analyze multiple protein markers in single cells. It enables multiparametric analysis of individual cells without the spectral overlap issues seen in traditional flow cytometry16. By using metal-tagged antibodies, it can measure dozens of markers simultaneously, offering a comprehensive and unbiased view of cellular phenotypes and functions17. For example, Gadalla et al. developed a CyTOF panel with more than 40 parameters for the analysis of peripheral blood mononuclear cells (PBMC) and tumor tissue, demonstrating its advantage in high-dimensional immunophenotyping18. Traditional flow cytometry, with its limited number of detectable parameters, was unable to identify these rare cell populations exhibiting unique phenotypes. In contrast, mass cytometry enabled a comprehensive evaluation of the functional states of these cells, providing a more detailed and robust characterization. Behbehani et al. utilized mass cytometry to analyze bone marrow samples from patients with myelodysplastic syndromes (MDS), successfully identifying and characterizing rare aberrant hematopoietic progenitor cells18. The ability of mass cytometry to simultaneously detect over 40 surface and intracellular markers significantly enhanced the detection of these low-frequency cell subsets19. These capabilities overcome traditional limitations and provide deeper insights into immune landscapes, driving progress in immunology and therapeutic development. The ability to comprehensively profile cellular phenotypes and functions at the single-cell level greatly advances the understanding of immunological processes and aids in the development of targeted therapies.
Mass cytometry provides comprehensive insights into the systemic and local immune cell populations in HCC by simultaneously detecting multiple protein markers. This technology can distinguish between various types of T cells within the tumor microenvironment, such as effector T cells, regulatory T cells (Tregs), and exhausted T cells, elucidating their specific roles in tumor progression. By utilizing mass cytometry, researchers can identify immune markers associated with HCC prognosis20. For instance, T cell subsets with high Programmed Cell Death Protein 1 (PD-1) expression can serve as predictors of a patient's response to immune checkpoint inhibitors21. Additionally, it facilitates the discovery of new therapeutic targets by identifying specific immunosuppressive molecules, thereby providing a foundation for personalized treatment strategies. Technology's ability to detect multiple markers and its single-cell resolution make it particularly advantageous for uncovering novel therapeutic targets and designing combination immunotherapies. This advanced approach holds significant potential for improving treatment outcomes in HCC patients by offering a detailed understanding of the immune landscape and enabling the development of tailored therapeutic interventions.
This study aims to utilize mass cytometry to analyze the systemic and local immune cell profiles of patients with HCC. The objectives are to characterize the immune cell populations, correlate these characteristics with clinical outcomes and therapeutic responses, and identify specific immune markers and cell subsets associated with HCC prognosis. By elucidating the roles of various immune cells in treatment responses, this study seeks to provide a foundation for personalized treatment strategies. The findings are expected to optimize existing immunotherapies and offer valuable insights for developing new treatments, ultimately aiming to improve overall survival and quality of life for HCC patients.
Protocol
The steps for blood and HCC sample collection, peripheral blood mononuclear cells (PBMCs) isolation, single-cell dissociation, and staining are outlined in the following plan. The experimental reagents and materials are all listed in the Table of Materials. All experiments were carried out with the approval of the Ethics Committee of the First Affiliated Hospital, School of Medicine, Zhejiang University, ensuring that the collection of tumor samples did not interfere with pathological diagnosis. Written informed consent was obtained from all human subjects.
1. Isolation of PBMCs
- Draw a blood sample from the vein of HCC patients and use a tube filled with an anticoagulant to prevent the blood from clotting. Blood samples were obtained from 4 patients (2 males and 2 females) aged 50-60 years, with an average age of 55 years. For each patient, collect 10-20 mL of peripheral blood to ensure sufficient volume for subsequent PBMCs isolation while minimizing patient discomfort.
- Draw a specific volume of blood (e.g., 6 mL) and add an equal volume of peripheral blood lymphocyte separation liquid (Ficol-paque or Lymphoprep) to a 15 mL centrifuge tube.
NOTE: The total volume should ideally not exceed 12 mL to allow space for proper mixing and to prevent overflow; the maximum recommended volume for a 15 mL centrifuge tube is 14 mL. - Tilt the centrifuge tube at 45° and slowly add the blood along the wall of the tube, carefully laying the blood slowly on top of peripheral blood lymphocyte separation liquid to avoid mixing the two layers.
NOTE: A pipet can be used to slowly add blood along the wall of the tube. - Place the centrifuge tube in the centrifuge and set the acceleration at 8 and deceleration at 3. Centrifuge at 450 x g, 4 °C, for 30 min. After centrifugation, 4 layers are formed in the test tube, from top to bottom: plasma layer, PBMCs layer (white membrane layer), Ficol-Paque layer, and red blood cell and granulocyte layer.
- Using a pipette, carefully collect the PBMCs layer into a new sterile centrifuge tube. Add 3 times the volume of PBS and centrifuge at 500 x g at 4°C for 5 min. After centrifugation, a pellet is formed at the bottom of the tube.
- Discard the supernatant using a pipette, add 1 mL of 2% Fetal Bovine Serum-Phosphate Buffered Saline (FBS-PBS) to resuspend the cells, and then add 3 mL of Red Blood Cell (RBC) lysis buffer. Centrifuge at 500 x g at 4°C for 5 min.
- Ensure there are no red blood cells at the bottom of the tube, indicating that the red blood cells have been completely lysed. Resuspend the cells in 2% FBS-PBS and proceed directly with the subsequent experiments, or add cell cryopreservation solution and store at -80°C for 2-3 months.
NOTE: As a general guideline, for resuspension, use 0.5 mL of 2% FBS-PBS per 1 x 106 cells. The cell count is determined using a hemocytometer or an automated cell counter after trypan blue staining to distinguish live cells from dead cells. This ratio helps ensure optimal cell recovery and viability during subsequent procedures.
2. Isolation of tumor tissue cells
NOTE: The method for the isolation of tumor tissue cells was adapted from Song et al.22.
- After resecting the HCC, guided by a pathologist, use a sterile scalpel to excise a portion of the tumor tissue. The tissue block should be approximately 1 cm3 in size. Rinse off any surface stains with pre-chilled 1x PBS. Immerse the tissue in a 15 mL centrifuge tube containing about 5 mL of RPMI 1640 medium containing 10% FBS. Place the tube on ice and transport it back to the laboratory for further processing.
- Thoroughly rinse off blood stains and manually remove fatty connective tissue using forceps to ensure complete tissue cleaning with pre-cooled 2% FBS-PBS. Transfer the tissue to a tissue-culture-treated dish containing a digestion solution. Secure the tumor tissue with forceps and cut it into pieces smaller than 1 mm3 with a scalpel. Transfer the tissue digestion solution to a 50 mL centrifuge tube, adding more tissue digestion solution until the total volume is approximately 15 mL.
- Place the centrifuge tube containing the tissue digestion mixture in a shaker. Tilt or flatten it and secure it for digestion at 150 rpm and 37 °C for 1 h. Filter the digestion solution through a 70 µm filter.
- During this procedure, use a 1 mL syringe plunger to grind the tissue fragments. Rinse the filter with a 2% FBS-1640 solution. Take the filtrate and transfer it to a 15 mL centrifuge tube.
- Centrifuge the filtrate at 500 x g for 5 min at 4 °C. After centrifugation, discard the supernatant. Cautiously re-suspend the pellet in approximately 10 mL of 36% Percoll solution. Then, centrifuge it again at 500 x g for 5 min at 4 °C.
- Gently aspirate 1 mL of RBC lysis buffer with a pipette to resuspend the cell suspension. Transfer the suspension to a new 15 mL centrifuge tube and add the RBC lysis buffer to reach a total volume of 10 mL. Let the mixture sit at room temperature for 10 min.
NOTE: A new centrifuge tube is used to prevent any impurities on the tube wall from re-entering the cell suspension, thereby reducing the risk of decreased cell yield. - Centrifuge the cell suspension at 500 x g for 5 min at 4 °C, then resuspend the cells in an appropriate volume of 2% FBS-PBS. As a general guideline, use 0.5 mL of 2% FBS-PBS per 1 x 106 cells for resuspension.
NOTE: The cell count is determined by using a hemocytometer or an automated cell counter after trypan blue staining to distinguish live cells from dead cells. The resuspension volume depends on the required cell quantity for subsequent experiments. This ratio helps ensure optimal cell recovery and viability during subsequent procedures.
3. Cisplatin staining
- Take 3 x 106 cells from the PBMCs obtained in step 1.7 and the tumor cells isolated in step 2.6, respectively. After cell recovery, resuspend them in 1 mL of PBS without Ca2+ and Mg2+. Add cisplatin to a final concentration of 0.5 µM, mix well, and incubate at room temperature for 2 min.
NOTE: Cisplatin staining is performed to distinguish dead cells from live cells based on membrane integrity. Dead cells with compromised membranes will uptake cisplatin and show a positive signal, while live cells remain unstained23. The number of cells must be at least 1 x 106. - Centrifuge the tubes at 500 x g for 5 min at room temperature, discard the supernatant, then add 1 mL of cell staining buffer to each tube containing the cell suspensions prepared in step 3.1 to stop the reaction. Centrifuge at 500 x g for 5 min at room temperature, discard the supernatant, and ensure that the cell pellet is not distributed linearly along the wall of the tube after centrifugation.
4. Fc receptor blocking
- Prepare a 50 µL block mix in advance for each sample: Combine 48 µL of cell staining buffer, then add 2 µL of Fc blocking solution (cell staining buffer: Fc blocking solution=9:1).
- Suspend the cells from step 3.2 in the above mixture and let them sit at room temperature for 10 min.
5. Incubation of membrane protein antibodies
- For each cell sample obtained from step 4.2, prepare the membrane protein antibody mix by adding 1.1 µL units of each antibody. Then, add cell staining buffer to reach a final volume of 55 µL. At this point, the total volume is approximately 100 µL.
NOTE: The mix includes antibodies targeting key membrane proteins, such as CD163, CCR3, CD141, CD117, and CD4524,25. These antibodies were selected for their specificity in identifying membrane protein markers and were obtained from commercially available sources (see Table of Materials for details, including clone numbers, manufacturers, and catalog numbers). - Add 50 µL of prepared antibody mix to each tube sample, bring the total volume to 100 µL. Gently swirl the samples and incubate them at room temperature for 15 min.
- Add 2 mL of cell staining buffer to each sample, centrifuge at 500 x g for 5 min at room temperature, and discard the supernatant. Repeat this step 2x.
- Discard the supernatant and briefly vortex the remaining liquid with the cell pellet to resuspend and thoroughly disperse the cell.
6. Nucleus protein staining
- Once the cells have been fully resuspended, add 500 µL of the mixed solution (fixation: fixation/permeabilization = 3:1) to each sample from step 5.4. Gently mix the samples and incubate at room temperature for 30 min.
- Dilute the permeabilization buffer (10x) with deionized water. After incubation, centrifuge at 500 g for 5 min at room temperature and discard the supernatant. Add 1000 µL of 1x permeabilization buffer to each tube to wash the cells. Centrifuge at room temperature at 1,000 x g for 5 min, then discard the supernatant.
- Resuspend antibodies in 1x permeabilization buffer. Discard the supernatant, and add 50 µL of the antibody mixture to each tube of cells. Gently pipette the cells to mix, then incubate at room temperature for 30-45 min.
- Add 1000 µL of 1x permeabilization buffer to each tube, centrifuge at roomtemperature at 1,000 x g for 5 min, and discard the supernatant.
- Add 1000 µL of cell staining buffer to each tube to resuspend the cells again, centrifuge at room temperature at 1,000 x g for 5 min, and discard the supernatant.
7. Cell fixation
- Prepare a 1.6% formaldehyde solution in PBS, with 1 mL needed per sample.
- Add 1 mL of 1.6% formaldehyde solution to each sample from step 6.5, vortex to mix thoroughly, and incubate at room temperature for 10 min.
NOTE: While formaldehyde is commonly used for cell fixation, alternative reagents such as 4% paraformaldehyde (PFA) or methanol can also be used. The choice of fixative should be based on the specific requirements of the experiment and the cellular features to be observed. - Centrifuge at room temperature at 800 x g for 5 min and discard the supernatant.
8. Nuclear Intercalation Staining
- Prepare cell intercalation solution: Dilute Cell-ID Intercalator-Iridium (Ir) with Fix and permeabilization buffer to a final concentration of 125 nM. Prepare 1 mL of the solution per sample.
- Add 1 mL of the prepared cell intercalation solution to each fixed sample from step 7.3. Mix gently and vortex immediately. This helps to minimize the formation of cell aggregates.
- Incubate at room temperature for 1 h or at 4°C overnight. Centrifuge at 500 g for 5 min at room temperature, and discard the supernatant.
9. Preparation of cell suspension
- Add 1000 µL of cell staining buffer to the tube from step 8.3 and centrifuge at 800 x g for 5 min. Discard the supernatant. Repeat this step 2x.
- Add 450 µL to 900 µL of deionized water to resuspend the cells. Count the cells using a hemocytometer or an automated cell counter after trypan blue staining. After counting, proceed with data collection and analysis using mass cytometry.
10. Mass cytometry and data analysis
- Acquire mass cytometry data using a CyTOF system and save it as Flow Cytometry Standard (FCS) files. Ensure proper instrument calibration and quality control to reduce background noise and batch effects as per the manufacturer's instructions.
- Preprocess the CD45+ cell population in the FCS file using the associated software. Remove debris based on cell size (forward scatter, FSC) and granularity (side scatter, SSC). Exclude doublets by sequential gating of FSC-A/FSC-H or SSC-A/SSC-H plots. Eliminate dead cells using viability staining, such as cisplatin exclusion. Gate the CD45+ population to focus on immune cells and export the gated population for further analysis.
- Transform the data with a cofactor of 5 and identify the main clusters using clustering software. Perform clustering based on the Spanning-tree Progression Analysis of Density-normalized Events (SPADE) algorithm, which groups cells with similar marker expression profiles into clusters26. Use Hierarchical Stochastic Neighbor Embedding (HSNE) for dimensionality reduction and identification of distinct clusters26.
- Perform re-clustering of the major clusters using the cytofkit package in R software for unsupervised clustering. Identify sub-clusters with PhenoGraph using default parameters.
- Apply Uniform Manifold Approximation and Projection (UMAP) for dimensionality reduction. Perform statistical analysis using the Wilcoxon test, considering a P-value < 0.05 as statistically significant. Visualize the results using ggplot2.
Results
To elucidate the immunological characteristics associated with HCC, a comprehensive analysis of immune cell populations was conducted. Paired PBMCs and HCC tissue samples were collected from 4 patients with HCC. Mass cytometry profiling was performed to examine immune cell populations at the single-cell proteomic level, using two antibody panels for both PBMCs and HCC tissue samples.
After quality control, 45,326 cells were included in the mass cytometry analysis. The PhenoGraph clustering algorithm, in conjunction with t-SNE, was employed to generate two-dimensional graphs and partition the cells into distinct phenotypes. Major immune cell subsets were identified based on lineage markers such as CD3 (T cells), CD4 (CD4+ T cells), CD8 (CD8+ T cells), CD56 (NK cells), CD19 (B cells) and CD14 (monocytes)27,28. Immune cell clusters were characterized using mass cytometry technologies.
In PBMC samples, as shown in Figure 1A, a total of 14 cell types were identified, including CD4 T cells, CD8 T cells, NK cells, B cells, monocytes, central memory CD8 T cells (CD8Tcm), macrophages, plasmacytoid dendritic cells (pDCs), basophils, eosinophils, effector CD8 T cells (CD8Teff), CD141+ conventional dendritic cells (CD141+ cDCs), neutrophils, and CD1c+ conventional dendritic cells (CD1c+ cDCs). The detailed marker expression pattern for each cell type is depicted in Figure 1B. Furthermore, Figure 1C illustrates the distribution of these cell types within each sample. In the PBMC samples, distinct proportions of each cell type were identified. Notably, sample D showed a higher proportion of CD4 T cells compared to the other samples. Samples A and B showed a significant enrichment of B cells. Additionally, CD141+ conventional dendritic cells (CD141+ cDCs) were predominantly found in sample C. These findings highlight the unique distribution and abundance of specific cell types in different samples, providing insights into the heterogeneity of the immune landscape in HCC.
Similarly, in tissue samples, as shown in Figure 2A, 8 cell types were identified, including monocytes, T cells, neutrophils, NK cells, B cells, pDCs, eosinophils, and myeloid dendritic cells (mDCs). The marker expression pattern for each cell type is provided in Figure 2B and Figure 2C visually represents the distribution of these cell types within the samples. The tissue samples showed a consistent pattern of cell type proportion across all patients. This suggests a shared immunological characteristic in terms of the relative abundance of these cell types in HCC. Understanding this consistent pattern provides valuable insights into the underlying immune landscape and its potential implications for the pathogenesis of HCC.
The immune cell atlas constructed through this analysis offers valuable insights into the immune landscape of HCC, shedding light on the cellular and systemic adaptations associated with the disease.
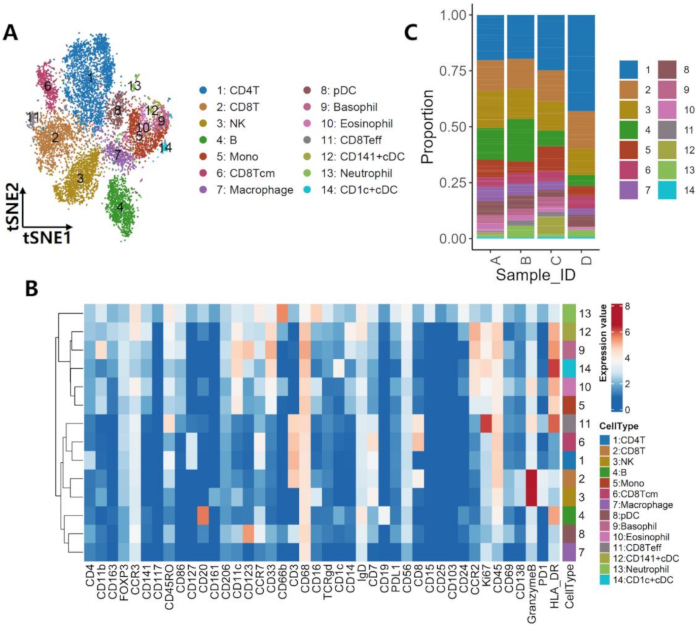
Figure 1: Multi-omics profiling of the PBMCs ecosystem. (A) The 14 cell clusters identified from PBMCs samples and illustrated on a t-SNE plot. (B) Protein markers for the cell clusters shown in (A). (C) Distribution pattern of the subsets across 4 samples based on mass cytometry data. Please click here to view a larger version of this figure.

Figure 2: Multi-omics profiling of the HCC tissue ecosystem. (A) The 8 cell clusters identified from HCC tissue samples and illustrated on a t-SNE plot. (B) Protein markers for the cell clusters shown in (A). (C) Distribution pattern of the subsets across 4 samples based on mass cytometry data. Please click here to view a larger version of this figure.
Discussion
This study leverages mass cytometry technology to provide an in-depth analysis of both systemic and local immune responses in HCC. The application of mass cytometry in this context enables the simultaneous detection of multiple markers at a single-cell level, offering a detailed immunophenotypic characterization that is crucial for understanding the complex immune landscape of HCC. Mass cytometry has revolutionized immunological studies by facilitating high-dimensional single-cell analysis. This technique employs rare metal isotope tags conjugated to antibodies, allowing the simultaneous measurement of over 40 parameters in a single run. The capability is particularly advantageous in studying HCC, where the tumor microenvironment (TME) is characterized by a high degree of cellular heterogeneity and intricate immune interactions18,29.
One significant advantage of mass cytometry over traditional flow cytometry is its enhanced multiplexing capability. While conventional flow cytometry is limited by spectral overlaps when using fluorescent markers, mass cytometry employs metal isotopes, which do not suffer from this issue. This enables the simultaneous detection of a larger number of markers without the need for complex compensation algorithms30. This capability is essential in HCC research, where profiling various immune cell populations and their states is critical for understanding tumor-immune interactions. Mass cytometry provides high-dimensional data at single-cell resolution, allowing for a comprehensive analysis of immune cells within the TME31. This level of detail is crucial for identifying rare cell populations and understanding their roles in tumor progression and immune evasion. For example, mass cytometry can differentiate between subsets of T cells, macrophages, and other immune cells, providing insights into their functional states and interactions within the tumor18.
A sequence of critical steps in the protocol ensures the reliability and reproducibility of the data obtained. During blood layering over the separation liquid, careful and slow addition is essential to maintain the integrity of the layers and avoid mixing, which is crucial for the successful isolation of PBMCs32. Similarly, enzymatic digestion of tumor tissues requires careful timing to balance dissociation and cell viability33. Proper handling during these stages ensures high recovery and purity of PBMCs and tumor cells, which is essential for downstream staining and mass cytometry analysis. Furthermore, the cisplatin staining process plays a pivotal role in accurately distinguishing live from dead cells; improper timing or concentration can lead to false-positive or false-negative results, impacting data quality34. In addition, Fc receptor blocking minimizes non-specific antibody binding, ensuring precise identification of cell surface markers, while the fixation and permeabilization steps must be carefully controlled to preserve cellular integrity and intracellular antigens critical for accurate mass cytometry results35.
Mass cytometry's high-dimensional analysis capabilities make it an invaluable tool for biomarker discovery in HCC. By profiling the immune landscape at a single-cell level, researchers can identify potential biomarkers associated with disease progression, therapeutic response, and overall prognosis36. The distinct immune cell distributions observed across PBMC and tissue samples in this study provide critical information for patient stratification. For example, patients with higher levels of effector CD8 T cells may respond better to therapies that enhance cytotoxic T cell activity, while those with elevated levels of immunosuppressive cells, such as Tregs, may benefit from combination therapies to effectively modulate the immune environment. This stratified approach could lead to more personalized and effective treatment strategies for HCC.
Mass cytometry provides detailed insights into the immune cell populations and their functional states within the TME, adding to the identification of potential targets for immunotherapy37. These biomarkers can be validated and used to develop targeted therapies and personalized treatment strategies30. The identification of immunosuppressive cell populations, such as Tregs and myeloid-derived suppressor cells (MDSCs) can inform the development of therapies aimed at modulating these cells to enhance anti-tumor immunity38. Mass cytometry enables comprehensive immune profiling, which is essential for understanding the complex interactions within the TME39. This includes characterizing the spatial distribution of immune cells, their phenotypic and functional states, and their interactions with tumor cells40. Such detailed profiling can reveal novel insights into the mechanisms of immune evasion and resistance, guiding the development of combination therapies that target multiple pathways.
Mass cytometry technology offers significant advantages in analyzing systemic and local immune responses in HCC. Its enhanced multiplexing capabilities, high dimensionality, and single-cell resolution provide detailed insights into the immune landscape of HCC41. By leveraging this detailed immunophenotypic data, researchers can gain a deeper understanding of the mechanisms of immune evasion in HCC and develop more effective immunotherapeutic strategies to improve patient outcomes.
Despite the advantages of mass cytometry technology and its application in profiling the immune landscape of HCC, it also has limitations. The multi-step isolation and staining process can lead to cell loss, particularly for fragile immune cell populations. Fixation may alter epitope recognition, potentially affecting marker detection accuracy. Furthermore, mass cytometry data analysis is sensitive to batch effects, which could introduce artifacts. Finally, the need for a substantial number of viable cells limits the applicability of the protocol to small tumor samples42. Future optimizations are needed to address these limitations and enhance the methodology's robustness. Integrating mass cytometry data with other high-dimensional techniques in future studies will further advance the understanding of immune responses in HCC and guide the development of innovative therapies.
Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (grant 2019YFA0803000 to J.S.), the Excellent Youth Foundation of Zhejiang Scientific (grant R22H1610037 to J.S.), the National Natural Science Foundation of China (grant 82173078 to J.S.), the Natural Science Foundation of Zhejiang Province (grant 2022C03037 to J.S.).
Materials
| Name | Company | Catalog Number | Comments |
| 1×PBS | HyClone | SH30256.01 | |
| 10×PBS | HyClone | SH30256.01 | |
| 100 mm×20 mm tissue-culture-treated culture dish | Corning | 430167 | |
| 1000 mL pipette tips | Rainin | 30389218 | |
| 15 mL centrifuge tube | NEST | 601052 | |
| 200 mL pipette tips | Rainin | 30389241 | |
| 40 mm nylon cell strainer/70-mm nylon cell strainer | Falcon | 352340 | |
| 50 mL centrifuge tube | NEST | 602052 | |
| 70 μm syringe fifilter | Sangon Biotech | F613462-9001 | |
| Anti-Human CCR2 Antibody (clone: K036C2) | BioLegend | 357224 | |
| Anti-Human CCR3 Antibody (clone: 5E8) | BioLegend | 310724 | |
| Anti-Human CCR7 Antibody (clone: G043H7) | BioLegend | 353240 | |
| Anti-Human CD103 Antibody (clone: Ber-ACT8) | BioLegend | 350202 | |
| Anti-Human CD115 Antibody (clone: 9-4D2-1E4) | BioLegend | 347314 | |
| Anti-Human CD117 Antibody (clone: 104D2) | BioLegend | 313201 | |
| Anti-human CD11b Antibody (clone: 1CRF44) | BD | 562721 | |
| Anti-human CD11c Antibody (clone: B-ly6) | BD | 563026 | |
| Anti-Human CD123 Antibody (clone: 6H6) | BioLegend | 306002 | |
| Anti-Human CD127 Antibody (clone: A019D5) | BioLegend | 351337 | |
| Anti-Human CD13 Antibody (clone: WM19) | BioLegend | 301701 | |
| Anti-Human CD138 Antibody (clone: MI15) | BioLegend | 356535 | |
| Anti-human CD14 Antibody (clone: HCD14) | BioLegend | 325604 | |
| Anti-Human CD141 Antibody (clone: M80) | BioLegend | 344102 | |
| Anti-Human CD15 Antibody (clone: QA19A61) | BioLegend | 376302 | |
| Anti-human CD16 Antibody (clone: B7311) | BD | 561313 | |
| Anti-Human CD161 Antibody (clone: HP-3G10) | BioLegend | 339902 | |
| Anti-Human CD163 Antibody (clone: GHI/61) | BioLegend | 333603 | |
| Anti-Human CD169 Antibody (clone: 7-239) | BioLegend | 346002 | |
| Anti-human CD19 Antibody (clone: HIB19) | BioLegend | 302226 | |
| Anti-Human CD1c Antibody (clone: L161) | BioLegend | 331501 | |
| Anti-Human CD20 Antibody (clone: 2H7) | BioLegend | 302301 | |
| Anti-Human CD206 Antibody (clone: 15-2) | BioLegend | 321151 | |
| Anti-Human CD24 Antibody (clone: ML5) | BioLegend | 311129 | |
| Anti-Human CD25 Antibody (clone: BC96) | BioLegend | 302624 | |
| Anti-human CD3 Antibody (clone: UCHT1) | BD | 555916 | |
| Anti-Human CD31 Antibody (clone: W18200D) | BioLegend | 375902 | |
| Anti-Human CD32 Antibody (clone: FUN-2) | BioLegend | 303232 | |
| Anti-Human CD326 Antibody (clone: CO17-1A) | BioLegend | 369812 | |
| Anti-Human CD33 Antibody (clone: WM53) | BioLegend | 303402 | |
| Anti-human CD4 Antibody (clone: L200) | BD | 563094 | |
| Anti-Human CD45 Antibody (clone: HI30) | BD | 563716 | |
| Anti-Human CD45RO Antibody (clone: UCHL1) | BioLegend | 304220 | |
| Anti-human CD56 Antibody (clone: 5.1H11) | BioLegend | 362510 | |
| Anti-Human CD64 Antibody (clone: S18012C) | BioLegend | 399502 | |
| Anti-Human CD66b Antibody (clone: 6/40c) | BioLegend | 392917 | |
| Anti-human CD68 Antibody (clone: Y1/82A) | BioLegend | 333808 | |
| Anti-Human CD69 Antibody (clone: FN50) | BioLegend | 310902 | |
| Anti-Human CD7 Antibody (clone: 4H9/CD7) | BioLegend | 395602 | |
| Anti-human CD8 Antibody (clone: RPA-T8) | BD | 557750 | |
| Anti-Human CD80 Antibody (clone: W17149D) | BioLegend | 375402 | |
| Anti-Human CD86 Antibody (clone: BU63) | BioLegend | 374202 | |
| Anti-Human FOXP3 Antibody (clone: 206D) | BioLegend | 320101 | |
| Anti-Human HLA_ABC Antibody (clone: W6/32) | BioLegend | 311426 | |
| Anti-human HLA-DR Antibody (clone: L243) | BioLegend | 307650 | |
| Anti-Human IgD Antibody (clone: IA6-2) | BioLegend | 348211 | |
| Anti-Human Ki67 Antibody (clone: Ki-67) | BioLegend | 350501 | |
| Anti-Human PD_L2 Antibody (clone: MH22B2) | BD | 567783 | |
| Anti-Human PD1 Antibody (clone: EH12.2H7) | BioLegend | 329951 | |
| Anti-Human PDL1 Antibody (clone: MIH2) | BioLegend | 393602 | |
| Anti-human TCR-γδ Antibody (clone: B1) | BD | 740415 | |
| Cell cryopreservation solution | Thermo Fisher | A2644601 | |
| Cell-lD Cisplatin | Standard BioTools | 201064 | |
| Cell-lD Intercalator-lr | Standard BioTools | 201192A | |
| Collagenase, Type IV | Gibco | 17104019 | |
| Constant-temperature shake | FAITHFUL | FS-50B | |
| CyTOF System | Fluidigm Corporation | Helios | |
| Cytosplore | Cytosplore Consortium | 2.3.1 | |
| Dispase II | Gibco | 17105041 | |
| DNase I | Merck | DN25 | |
| Eppendorf centrifuge | Eppendorf | 5702 | |
| EQ Four Element Calibration Beads | Standard BioTools | 201078 | |
| FBS | Gibco | 16000-044 | |
| Ficoll-paque | Cytiva | 17-1440-02 | |
| Finnpipette | Thermo Scientific | 4700870 | |
| Fixation buffer | Thermo Scientific | FB001 | |
| FlowJo | BD Life Sciences | 10.1 | |
| Formaldehyde solution | Thermo Scientific | 28906 | |
| Granzyme B Antibody, anti-human/mouse (clone: QA16A02) | BioLegend | 396413 | |
| Heparin Tubes | BD | 367874 | |
| Human BD Fc Block 2.5 mg/mL | BD | 564220 | |
| MACS Tissue Storage Solution | Miltenyi | 130-100-008 | |
| Maxpar Fix and Perm Buffer | Standard BioTools | 201067 | |
| Maxpar metal-coniugated antibodies | Standard BioTools | Various | |
| Maxpar PBS | Standard BioTools | 201058 | |
| Maxpar Water | Standard BioTools | 201069 | |
| Maxpare Cell Staining Buffer | Standard BioTools | 201068 | |
| Metal-conjugated Anti-Human α-SMA Antibody (clone: 1A4) | Miltenyi Biotec | 130-098-145 | |
| Percoll | Merck | P4937-500ML | |
| Permeabilization buffer | Thermo Scientific | 00833356 | |
| RBC lysis buffer | BD | 555899 | |
| Refrigerated centrifuge | Eppendorf | 5910ri | |
| RPMI 1640 medium | GE HealthCare | SH30027.0 | |
| Scalpel | APPLYGEN | TB6298-1 | |
| Sterile Pasteur pipette | ZDAN | ZD-H03 | |
| Tissue digestion solution | Yeasen Biotech | 41423ES30 | |
| Tuning Solution | Standard BioTools | 201072 | |
| Vortex Mixer | Thermo Scientific | 88882012 |
References
- Zhang, C. H., Cheng, Y., Zhang, S., Fan, J., Gao, Q. Changing epidemiology of hepatocellular carcinoma in Asia. Liver Int. 42 (9), 2029-2041 (2022).
- Renne, S. L., et al. Hepatocellular carcinoma: A clinical and pathological overview. Pathologica. 113 (3), 203-217 (2021).
- Sayiner, M., Golabi, P., Younossi, Z. M. Disease burden of hepatocellular carcinoma: A global perspective. Dig Dis Sci. 64 (4), 910-917 (2019).
- Zhang, X., et al. Risk factors and prevention of viral hepatitis-related hepatocellular carcinoma. Front Oncol. 11, 686962 (2021).
- Zou, H., et al. Economic burden and quality of life of hepatocellular carcinoma in greater China: A systematic review. Front Public Health. 10, 801981 (2022).
- Li, Y., You, Z., Tang, R., Ma, X. Tissue-resident memory t cells in chronic liver diseases: Phenotype, development and function. Front Immunol. 13, 967055 (2022).
- Shen, K. Y., Zhu, Y., Xie, S. Z., Qin, L. X. Immunosuppressive tumor microenvironment and immunotherapy of hepatocellular carcinoma: Current status and prospectives. J Hematol Oncol. 17 (1), 25 (2024).
- Oura, K., Morishita, A., Tani, J., Masaki, T. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: A review. Int J Mol Sci. 22 (11), 5801 (2021).
- Chen, Y., et al. Effect of infiltrating immune cells in tumor microenvironment on metastasis of hepatocellular carcinoma. Cell Oncol. 46 (6), 1595-1604 (2023).
- Du, Q., An, Q., Zhang, J., Liu, C., Hu, Q. Unravelling immune microenvironment features underlying tumor progression in the single-cell era. Cancer Cell Int. 24 (1), 143 (2024).
- Xu, L., et al. Reshaping the systemic tumor immune environment (stie) and tumor immune microenvironment (time) to enhance immunotherapy efficacy in solid tumors. J Hematol Oncol. 15 (1), 87 (2022).
- Mao, X., et al. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol Cancer. 20 (1), 131 (2021).
- Roederer, M. Spectral compensation for flow cytometry: Visualization artifacts, limitations, and caveats. Cytometry. 45 (3), 194-205 (2001).
- Sahaf, B., Rahman, A., Maecker, H. T., Bendall, S. C. . Biomarkers for immunotherapy of cancer: Methods and protocols. , (2020).
- Turaç, G., et al. Combined flow cytometric analysis of surface and intracellular antigens reveals surface molecule markers of human neuropoiesis. PLoS One. 8 (6), e68519 (2013).
- Wu, K., et al. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front Immunol. 11, 1731 (2020).
- Elaldi, R., et al. High dimensional imaging mass cytometry panel to visualize the tumor immune microenvironment contexture. Front Immunol. 12, 666233 (2021).
- Gadalla, R., et al. Validation of cytof against flow cytometry for immunological studies and monitoring of human cancer clinical trials. Front Oncol. 9, 415 (2019).
- Ijsselsteijn, M. E., Van Der Breggen, R., Farina Sarasqueta, A., Koning, F., De Miranda, N. A 40-marker panel for high dimensional characterization of cancer immune microenvironments by imaging mass cytometry. Front Immunol. 10, 2534 (2019).
- Zhang, Q., et al. Mass cytometry-based peripheral blood analysis as a novel tool for early detection of solid tumours: A multicentre study. Gut. 72 (5), 996-1006 (2023).
- Zheng, B., et al. Trajectory and functional analysis of pd-1(high) cd4(+)cd8(+) t cells in hepatocellular carcinoma by single-cell cytometry and transcriptome sequencing. Adv Sci. 7 (13), 2000224 (2020).
- Song, J., et al. Protocol for isolating single cells from human pancreatic cancer tissues and analyzing major immune cell populations using flow cytometry. STAR Protoc. 4 (4), 102679 (2023).
- Simoni, Y., Chng, M. H. Y., Li, S., Fehlings, M., Newell, E. W. Mass cytometry: A powerful tool for dissecting the immune landscape. Curr Opin Immunol. 51, 187-196 (2018).
- Comi, M., et al. Coexpression of cd163 and cd141 identifies human circulating il-10-producing dendritic cells (dc-10). Cell Mol Immunol. 17 (1), 95-107 (2020).
- Fan, L., et al. High-dimensional single-cell analysis delineates peripheral immune signature of coronary atherosclerosis in human blood. Theranostics. 12 (15), 6809-6825 (2022).
- Sheng, J., et al. Human endogenous retrovirus activation contributes to biliary atresia pathogenesis through re-education of resident macrophages. Biorxiv. , (2022).
- Terekhova, M., et al. Single-cell atlas of healthy human blood unveils age-related loss of nkg2c(+)gzmb(-)cd8(+) memory t cells and accumulation of type 2 memory t cells. Immunity. 56 (12), 2836-2854.e9 (2023).
- Sathaliyawala, T., et al. Distribution and compartmentalization of human circulating and tissue-resident memory t cell subsets. Immunity. 38 (1), 187-197 (2013).
- Lee, S., Vu, H. M., Lee, J. H., Lim, H., Kim, M. S. Advances in mass spectrometry-based single cell analysis. Biology. 12 (3), 395 (2023).
- Iyer, A., Hamers, A. A. J., Pillai, A. B. Cytof for the masses. Front Immunol. 13, 815828 (2022).
- Yuan, X., Wang, J., Huang, Y., Shangguan, D., Zhang, P. Single-cell profiling to explore immunological heterogeneity of tumor microenvironment in breast cancer. Front Immunol. 12, 643692 (2021).
- Serban, G. M., Mănescu, I. B., Manu, D. R., Dobreanu, M. Optimization of a density gradient centrifugation protocol for isolation of peripheral blood mononuclear cells. Acta Marisiensis - Seria Medica. 64 (2), 83-90 (2018).
- Shcherbakova, A., et al. Factors affecting cell viability during the enzymatic dissociation of human endocrine tumor tissues. Biology (Basel). 13 (9), 665 (2024).
- Devine, R. D., Alkhalaileh, H. S., Lyberger, J. M., Behbehani, G. K. Alternative methods of viability determination in single cell mass cytometry. Cytometry A. 99 (10), 1042-1053 (2021).
- Gonzalez, V. D., Huang, Y. W., Fantl, W. J. Mass cytometry for the characterization of individual cell types in ovarian solid tumors. Methods Mol Biol. 2424, 59-94 (2022).
- Zabransky, D. J., et al. Profiling of syngeneic mouse hcc tumor models as a framework to understand anti-pd-1 sensitive tumor microenvironments. Hepatology. 77 (5), 1566-1579 (2023).
- Levine, L. S., et al. Single-cell analysis by mass cytometry reveals metabolic states of early-activated cd8(+) t cells during the primary immune response. Immunity. 54 (4), 829-844.e5 (2021).
- Lv, B., et al. Immunotherapy: Reshape the tumor immune microenvironment. Front Immunol. 13, 844142 (2022).
- Zhou, Z., et al. Deciphering the tumor immune microenvironment from a multidimensional omics perspective: Insight into next-generation car-t cell immunotherapy and beyond. Mol Cancer. 23 (1), 131 (2024).
- Hsieh, W. C., et al. Spatial multi-omics analyses of the tumor immune microenvironment. J Biomed Sci. 29 (1), 96 (2022).
- Wang, Z., et al. Gdf15 induces immunosuppression via cd48 on regulatory t cells in hepatocellular carcinoma. J Immunother Cancer. 9 (9), e002787 (2021).
- Krams, S. M., Schaffert, S., Lau, A. H., Martinez, O. M. Applying mass cytometry to the analysis of lymphoid populations in transplantation. Am J Transplant. 17 (8), 1992-1999 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved