Method Article
Validation of Therapeutic Agent Conjugation to Polyvinyl Alcohol-Coated Medical Devices
In This Article
Summary
Here, we present a protocol for derivatizing medical devices with protein therapeutics by conjugating protein therapeutics to a polyvinyl alcohol membrane casted on medical devices. Conjugation of protein-based therapeutics allows for localized and concentrated delivery and is a cost-effective approach that requires vastly lower drug amounts for dosing while minimizing off-target effects.
Abstract
Protein-based therapeutics are often limited by their route of administration and inability to confine them to their site of action. One innovative approach we have developed is to covalently bind protein therapeutics to medical devices, allowing more localized and highly concentrated delivery of these agents to their intended site of action. This study aims to evaluate if glucagon-like peptide-2 (GLP-2) can be covalently bound to the vaginal expansion sleeve (VES) and intestinal expansion sleeve (IES) devices in clinically relevant and measurable quantities.
Expansion sleeves were coated with polyvinyl alcohol (PVA) and crosslinked with glutaraldehyde/sulfuric acid vapor to create a chemically active surface capable of binding amine-containing therapeutics such as GLP-2. A standard curve was created by binding 250 µg, 100 µg, 50 µg, 25 µg, and 0 µg of GLP-2 to PVA-coated wells of a 24-well plate. An ELISA standard was created using a rabbit anti-GLP-2 antibody followed by a goat anti-rabbit IgG alkaline phosphatase secondary antibody plus an alkaline phosphatase blue microwell substrate. Colorimetry (yellow to blue at 620 nm) was proportional to the concentration of GLP-2 bound antibodies, enabling calculation of the bound concentration of GLP-2 on the PVA-coated sleeves. The addition of 50 µg of GLP-2 to IES/VES devices bound an average of 22.69 ± 9.32 µg/cm2 of GLP-2 with an external IES/VES surface area (9.425 cm2), indicating that 44% of added GLP-2 was immobilized on the PVA coated IES/VES sleeves.
Current human GLP-2 dosing is 50 µg/Kg. Because each sleeve carries 22 µg, this is approximately 44% of a systemic dose in a single device. This methodology makes it possible to add dramatically lower doses of therapeutic agents to get the same effect as systemic administration of the GLP-2 drug while also avoiding systemic effects.
Introduction
Protein-based therapeutics such as enzymes, antibodies, cytokines, hormones, and growth factors have been shown to be highly effective in the treatment of several diseases1. If we improve our understanding of how to provide these therapeutic agents, we could significantly advance the biotechnology for applying these substances as medicines/medical treatments1. The efficacy of protein therapeutics is limited by their biodistribution, cost, and off-target effects. Several approaches have been attempted to improve the pharmacokinetics and pharmacodynamics of protein therapeutics, including PEGylation, glycosylation, lipidation, and fusion of other proteins2. 'Targetability' is another concern because most of the current protein therapeutics have multiple sites of action, resulting in off-target effects. Specifically, targeting these drugs to desired sites could lower the required therapeutic dose and reduce side effects2. If one could restrict drug delivery to the intended site of action, it might be possible to radically improve treatment while also limiting the undesirable 'off-target' side effects of these drugs.
Multiple medical devices are currently being used that contain therapeutics, such as drug-eluting stents with an antiproliferative drug coating containing clopidogrel, vincristine, and vinblastine to help prevent restenosis and the need for repeat revascularization after cardiac catheterization3. Additionally, biodegradable drug-eluting ureteral stents bearing paclitaxel, doxorubicin, gemcitabine, and other antiproliferative agents have been created to help treat upper tract urothelial carcinoma4. As these stents degrade, the anti-tumor drugs are released locally in a controlled manner, prolonging the drug delivery without the need for additional dosing4. This methodology allows for the long-term effect of the anti-tumor drugs at the intended site of action while also preventing the need for systemic exposure, limiting the drug action on non-tumor cells, and reducing drug toxicity. Also, the biodegradable nature of the stent can also prevent the need for a stent removal procedure4.
Short bowel syndrome (SBS) is another condition where the use of protein-based therapeutics has shown significant improvement in patients5. SBS can occur through various mechanisms but results in inadequate intestinal length, causing malabsorption and malnutrition. Therapies, such as glucagon-like peptide 2 (GLP-2) analogs, can be used before moving to surgical management of this condition5. Human GLP-2, a systemic nutrient peptide hormone, is produced in the distal small intestine and colon by L-type endocrine cells. GLP-2 induces proliferation of intestinal crypt cells, promotes repair of damaged mucosa, inhibits apoptosis of intestinal epithelial cells, and improves intestinal bloody supply5. GLP-2 analogs, such as teduglutide, have been approved for patients 1 year or older for treatment of SBS to help augment the natural intestinal adaptation6. Naturally, intestinal adaptation can take years and results in delayed gastric emptying to increase absorptive time and increased diameter by intestinal dilation to increase the surface area for absorption7. GLP-2 analogs have the same pharmacological activity as the natural GLP-2 while having a more stable structure, allowing a longer half-life and stronger affinity to targets5. Currently, the annual cost of teduglutide is $295,000, and patient dosing is 0.05 mg/kg per day5. Teduglutide side effects include nausea, vomiting, diarrhea, abdominal pain, weight loss, gastrointestinal polyps, and increased growth of existing tumors5.
Previously, our lab has shown that deployment of an intestinal expansion sleeve (IES) (Figure 1) was able to produce an immediate 36.2% elongation of small bowel ex vivo and a 30.2% elongation of small bowel after a one-month deployment in vivo6,8. This device could become a therapy for SBS patients that could shorten parenteral nutrition dependence. The ability to bioconjugate therapeutics to the IES device could further improve the distraction enterogenesis capacity by adding the intestinal mucosal growth agonist GLP-2 to the device. Polyvinyl alcohol (PVA) membranes are hydrophilic polymers that can be prepared by dissolving PVA into water, casting it onto a structural support, and then crosslinking with glutaraldehyde using sulfuric acid as a catalyst9. These hydrogel nanoparticles have been shown to have potential as drug delivery systems through drug diffusion, hydrogel matrix swelling, and chemical reactivity of the drug/matrix10. PVA has been FDA-approved for clinical use in humans due to its excellent biocompatibility and safety11. PVA polymerization results in terminal groups that allow for bioconjugation12.
IES/VES devices are 3 cm in length with an external surface area of 9.425 cm2 that is capable of casting with PVA membranes. These PVA membranes can be bioconjugated with GLP-2 to allow for localized and concentrated delivery of therapeutic agents. Lowering the required dose of the drug and bypassing the need for systemic administration, avoiding negative off-site effects of treatment. This can help mitigate the cost of these expensive drugs by requiring less dosage, and the delayed release of the drug can decrease the frequency of dosing, further lowering costs. Coating the IES device with GLP-2 could lower required dosages, save patients money, avoid systemic administration, prevent negative effects of treatment, and maintain a continuous delivery of the drug to the target site. This report evaluates the extent to which a therapeutic protein, like GLP-2, can be covalently bound to medical devices such as IES/VES devices in physiologically meaningful quantities to provide the local benefit of these drugs while avoiding off-target effects.
Protocol
NOTE: Sections 1 and 2 are completed at the same time. Coating with PVA and crosslinking the PVA membrane should be done at the same time for both the expansion sleeves and the 24-well plate. This allows them to be ready at the same time to create the standard curve with the 24-well plate to use absorbance to calculate the GLP-2 concentration of each sleeve.
1. Creating GLP-2 coated IES and VES devices
- Make the 5% PVA solution by adding 5 g of PVA into 100 mL of deionized water. To help dissolve, consider stirring on a hotplate. Then, make the 1 mg/mL GLP-2 solution by adding 1 mg of GLP-2 into 1 mL of deionized water.
- Precontract intestinal expansion sleeves and vaginal expansion sleeves over a glass pipette, stabilizing the ends with plastic pipettes cut to fit tightly on the glass pipette around the precontracted sleeves.
- Paint the precontracted sleeves with the premade 5% PVA using a small paintbrush. After painting a coat of PVA, allow the sleeves to dry at room temperature (RT) for 10 min, then paint another coat for a total of 5 PVA coats on each sleeve.
- After the 5th coat dries, place the precontracted sleeves into a desiccator under a chemical safety hood to allow them to crosslink at RT. Initiate crosslinking by adding 3 mL of 25% glutaraldehyde and 3 mL of 94%-98% sulfuric acid to separate open beakers in the desiccator for the vapors to react and crosslink the PVA membranes onto the sleeves.
- After crosslinking the sleeves in the chamber for 48 h, take them out of the chamber and allow the glutaraldehyde to evaporate off the sleeves for 15 min.
- Then, add 50 µL of the 1 mg/mL GLP-2 solution by carefully pipetting onto each sleeve. Allow this to dry for 30 min at RT before removing the precontracted sleeves off the glass pipette and adding each sleeve to the respective well on the 24-well plate.
2. Creating the GLP-2 coated 24-well plate
- To make the standard curve of GLP-2 bound to the PVA, pipet 50 µL of 5% PVA onto the bottom of 18 of the intended-to-use wells of a 24-well plate. Leave 6 wells blank to hold the IES and VES devices (3 for each type of device).
- Allow the layer of PVA to dry on the 24-well plate overnight in an oven at 120 °C.
- Place the 24-well plate into a desiccator under a chemical safety hood to allow the PVA to crosslink for 48 h at RT. Initiate crosslinking by adding 3 mL of 25% glutaraldehyde and 3 mL of 94%-98% sulfuric acid to separate open beakers so that the vapors can react inside the chamber to crosslink the PVA onto the 24-well plate.
- After crosslinking for 48 h, remove the 24-well plate from the chamber and allow the glutaraldehyde to evaporate off the plate for 15 min.
- Then, make a concentration gradient by adding 250 µL, 100 µL, 50 µL, 25 µL, and 0 µL of 1 mg/mL GLP-2 solution into their labeled wells, creating the 24-well plate with 3 wells for each concentration of GLP-2 (250 µg, 100 µg, 50 µg, 25 µg, and 0 µg), and 3 wells for each type of sleeve (IES and VES).
3. Creation of standard curve and discerning sleeve GLP-2 concentration
- After the respective GLP-2 dilutions are added to the wells and the drug-coated sleeves are added to the wells, refrigerate the plate overnight at 4 °C to allow for drug binding to occur.
- Next day, add 2 mL of 1 mg/mL of milk powder in deionized water-blocking solution to each well for 1 h. After blocking, wash each well 3 times with PBS for 5 min each wash.
- Make the rabbit anti-GLP-2 antibody solution by adding 40 µL of rabbit anti-GLP-2 antibody into 50 mL of deionized water. Then, add 2 mL of the rabbit GLP-2 antibody solution to each well on the 24-well plate and refrigerate overnight at 4 °C.
- After primary antibody incubation, wash each well another 3x with PBS for 5 min each wash. Make the anti-rabbit IgG alkaline phosphatase produced in goat secondary antibody solution by adding 20 mL of secondary antibody into 40 mL of deionized water. Then, add 2 mL of secondary antibody solution to each well and let it sit for 2 h at RT.
- Aspirate the secondary antibody solution and wash each well with another 3x PBS wash for 5 min each. Then, add the alkaline phosphatase blue microwell substrate to each well.
- Allow the alkaline phosphatase conjugated to the secondary antibody to react with the substrate for 20 min at RT. As the reaction progresses, watch for the color of the solution to change from yellow to blue.
- Stop the reaction by pipetting 200 µL of solution from each well and put it into a 96-well plate labeled the same as the 24-well plate. Once the reagent is removed from the 24-well plate with the PVA membrane conjugated to the alkaline phosphatase enzyme, the reaction will stop, and the color will not change.
- Read the 96-well plate using an absorbance microplate reader at 620 nm wavelength to generate the standard curve and calculate the concentration of GLP-2 on the PVA-coated sleeves.
Results
This protocol describes coating and crosslinking IES and VES devices with PVA to allow for the conjugation of GLP-2. Figure 2 shows the standard curve generated to determine the concentration of GLP-2 on each device. Figure 3 shows the GLP-2 concentration of each IES and VES device, and the concentration of the wells used for the standard curve. The sleeves had an average GLP-2 concentration of 22.69 ± 9.32 µg/cm2, which corresponded to the 50 µg wells of the 24-well plate, which had a GLP-2 concentration of 23.7 µg/cm2. This shows that the addition of 50 µg of GLP-2 results in 45% of the added drug remaining bound to the PVA-coated medical device.
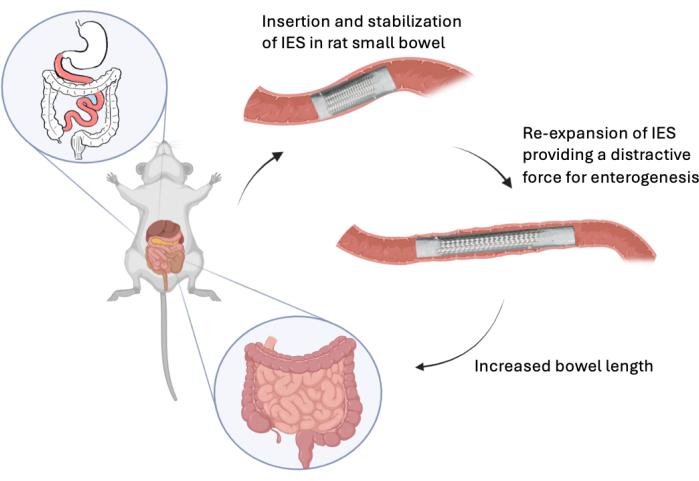
Figure 1: Deployment of IES. This graphic shows the in vivo deployment of a intestinal expansion sleeve in the rat model. Please click here to view a larger version of this figure.
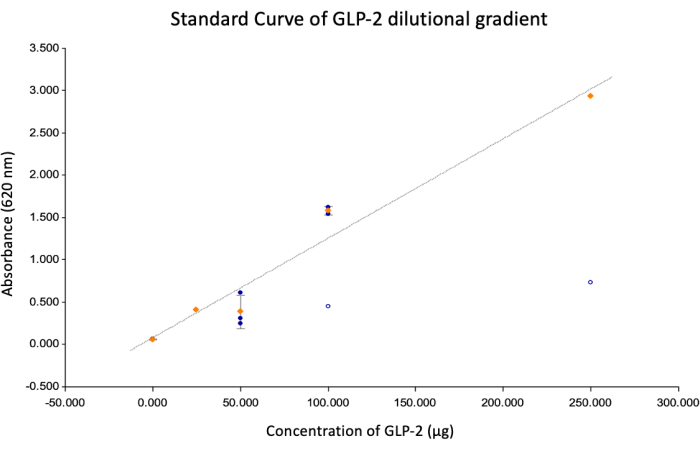
Figure 2: Standard curve. This image shows the standard curve generated using a line graph with the following values: Y = mx + b, Y = absorbance at 620 nm, x = concentration, m = 0.0118, B = 0.0734, R2 = 0.966. The 24-well plate had a surface area of 1.93 cm2, giving the GLP-2 concentrations of 125.95 µg/cm2, 66.38 µg/cm2, 23.77 µg/cm2, 14.58 µg/cm2, and 0.00 µg/cm2 for the 250 µg, 100 µg, 50 µg, 25 µg, and 0 µg wells, respectively. Please click here to view a larger version of this figure.
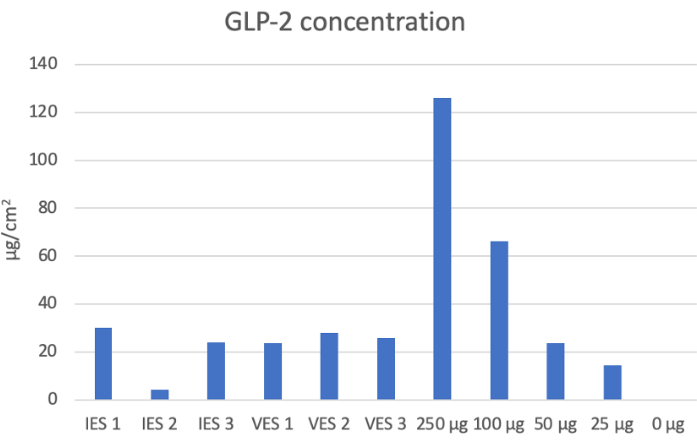
Figure 3: GLP-2 concentration for devices and respective wells. This graph shows the concentrations determined for each IES and VES device and the wells of the 24-well plate used to make the standard curve. After the addition of 50 µg of GLP-2 to each sleeve (external surface area of 9.425 cm2), the amount of GLP-2 bound to the device was 22.69 ± 9.32 µg/cm2. This corresponds to the 23.7 µg/cm2 for the 50 µg well of the standard curve. By adding 50 µg of GLP-2 to these sleeves, 45% of the added drug was bound to the device after being washed multiple times. Please click here to view a larger version of this figure.
Discussion
We present here a methodology to bind protein-based therapeutics, such as GLP-2, to PVA-coated and crosslinked medical devices. The concentration of the bound GLP-2 was determined by the creation of a standard GLP-2 concentration curve on PVA-coated 24-well plates by the addition of rabbit anti-GLP-2 antibody, goat anti-rabbit IgG alkaline phosphatase, and alkaline phosphatase blue microwell substrate. The secondary antibody conjugated to an alkaline phosphatase enzyme allowed for the addition of an alkaline phosphatase blue microwell substrate that starts out yellow, and as the GLP-2 antibody-bound alkaline phosphatase reacts with the substrate, it turns blue. This color change is read at an absorbance of 620 nm to create the standard curve in Figure 2 and calculate the concertation of the GLP-2 bound IES and VES devices using their absorption. After the addition of 50 µg of GLP-2 to these PVA-coated devices, 45% of the added drug was bound to the device.
In this protocol, the critical step is the crosslinking step. PVA-coated medical devices require over 48 h in an airtight chamber to allow the 25% glutaraldehyde and 94%-98% sulfuric acid vapors to crosslink the PVA onto the devices. This crosslinking allows the PVA membrane with reactive groups to be a stable bioreactive surface to bind the protein-based therapeutics for targeted drug delivery. The use of 5 coats was determined empirically since 1-2 coats resulted in re-expanded by 1 h, 3 coats by 24 h, and we wished for the device to take several days to re-expand to provide a distractive force over time for intestinal and vaginal expansion. With a surface area of 9.425 cm2, the IES and VES devices have the potential to bioconjugate a large quantity of therapeutic agents in comparison to their small size. If the crosslinking step fails, the stability of the PVA membrane is diminished, and the ability to retain bioconjugated therapeutics is lowered, as seen by IES 2 in Figure 3.
Covalently binding protein-based therapeutics to PVA-coated medical devices allows for localized and concentrated delivery of these agents to the sites of interest while also avoiding systemic administration and the associated cost and off-target effects. Currently, the typical Teduglutide dose for all patients is 50 µg/kg per day13. Because each sleeve carries 22 µg/cm2, this is approximately 44% of a systemic dose in a single device. With an external surface area of 9.425 cm2, these sleeves are capable of localized delivery of 213.85 µg of GLP-2. This is a 4.3x greater dose than systemic and is localized to the area of interest. Targetability allows for less of the drug to be used by bypassing the need for systemic administration. Currently, annual teduglutide therapy costs $295,000 for the 50 µg/kg per day dosing5. By conjugating teduglutide to the sleeve, the drug dosage required should be decreased, and systemic side effects minimized. Common side effects of teduglutide include abdominal pain (28%), reaction at the injection site (26%), nausea (26%), abdominal distension (17%), headache (16%), and vomiting (14%)13.
A limitation of this study is the pharmacodynamics and pharmacokinetics of the time-release of GLP-2 from the bioconjugated PVA-coated medical device. The current trial documented that GLP-2 can be bound to the crosslinked PVA devices, but we do not yet have information about how long the drug remains bound before being released from the PVA membrane and the extent of the total release of the GLP-2 from the site of implantation. We know that after several washing steps, GLP-2 remains attached to the device, but the extent of conjugation to the device and how that will affect release are unknown. In future studies, we could measure the release in supernatants from IES and VES stored in biofluids to model in vivo release or recover these devices at different times after in vivo implantation to measure the remaining GLP-2. Additionally, we plan to measure if any factors, such as pH, temperature, and moisture, affect the stability and release rate of the drug from the device. Future experiments would determine this by implanting a bioconjugated PVA sleeve and measuring drug concentration surrounding the sleeve at different times post-deployment and under different set conditions. Without knowing the pharmacodynamics of the PVA-coated sleeve, we are unable to determine how the localized GLP-2 dosing would compare to typical systemic dosing. Also, the maximum binding capacity of the PVA-coated sleeves is still unknown. After adding 50 µg, it was determined that 45% of the added drug remained bound to the sleeve. This could mean that we have already exceeded the maximum binding capacity. Future studies adding different concentrations of GLP-2 to each sleeve are warranted to determine the true maximal binding capacity of these devices and the release rate with varying doses.
Here, we validate the capability of therapeutic conjugation to our PVA-coated medical devices. The results show that 44% of added GLP-2 was covalently bound to the IES and VES devices, allowing for highly concentrated and local delivery of protein-based therapeutics such as GLP-2. Localized drug delivery will hopefully help lower treatment costs, increase accessibility, and lower side effects. This methodology can be used for hundreds of current protein-based therapeutics to enhance local drug delivery and improve treatment outcomes.
Disclosures
Jonathan Steven Alexander and Christen J. Boyer hold the patent US10537659B2, 3D-printed polyvinyl alcohol medical devices and methods of activation (2017). The remaining authors have nothing to disclose.
Acknowledgements
This research was funded by R. Keith White, MD, via the Department of Surgery John C. McDonald Chair Fund, W. Reid Grimes, MD for funding via the Department of Surgery Whitney Boggs Endowed Professorship Fund, and LSU LIFT2 grant, HSCS-2022-LIFT-002.
Materials
| Name | Company | Catalog Number | Comments |
| Anti-Rabbit IgG (whole molecule)–Peroxidase antibody produced in goat | Sigma-Aldrich | A0545-1ML | |
| BluePhos Microwell Substrate Kit | Sera Care | 5120-0059 | |
| GenClone 25-107, 24-Well Cell Culture Plates Flat Bottom Wells, TC Treated, 100 Plates/Unit | Genesee Scientific | 25-107 | |
| GLP-2 Recombinant Rabbit Monoclonal Antibody (3K3P5) | Invitrogen | MA5-42869 | |
| Glucagon-like Peptide-2 (GLP-2) (1-33), rat | Echelon Biosciences | 195262-56-7 | |
| Glutaraldehyde, 25% Aqueous Solution | Sigma-Aldrich | 111-30-8 | |
| Nalgene Polypropylene Desiccator with Stopcock | ThermoFisher Scientific | 5310-0250 | |
| Poly(vinyl alcohol) | Sigma-Aldrich | 9002-89-5 | |
| Sulfuric Acid (TraceMetal Grade), Fisher Chemical | Fischer Scientific | A510-P212 |
References
- Conner, K. P., Devanaboyina, S. C., Thomas, V. A., Rock, D. A. The biodistribution of therapeutic proteins: Mechanism, implications for pharmacokinetics, and methods of evaluation. Pharmacol Ther. 212, 107574 (2020).
- Ebrahimi, S. B., Samanta, D. Engineering protein-based therapeutics through structural and chemical design. Nat Commun. 14 (1), 2411 (2023).
- Senst, B., Goyal, A., Basit, H., Borger, J. . Drug Eluting Stent Compounds. , (2024).
- Shan, H., et al. Advances in drug delivery via biodegradable ureteral stent for the treatment of upper tract urothelial carcinoma. Front Pharmacol. 11, 224 (2020).
- Zhu, C., Li, Y. An updated overview of glucagon-like peptide-2 analog trophic therapy for short bowel syndrome in adults. J Int Med Res. 50 (3), 3000605221086145 (2022).
- Clayton, S., et al. Self-expanding intestinal expansion sleeves (IES) for short gut syndrome. Pediatr Surg Int. 38 (1), 75-81 (2022).
- Rosete, B. E., Wendel, D., Horslen, S. P. Teduglutide for pediatric short bowel syndrome patients. Expert Rev Gastroenterol Hepatol. 15 (7), 727-733 (2021).
- Colvin, J. C., et al. Novel Intestinal Expansion Sleeve (IES): promoting distraction enterogenesis in a live animal model. , (2023).
- Kim, K. J., Lee, S. B., Han, N. W. Kinetics of crosslinking reaction of PVA membrane with glutaraldehyde. Korean J Chem Eng. 11 (1), 41-47 (1994).
- Hamidi, M., Azadi, A., Rafiei, P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 60 (15), 1638-1649 (2008).
- Chong, S. F., Smith, A. A., Zelikin, A. N. Microstructured, functional PVA hydrogels through bioconjugation with oligopeptides under physiological conditions. Small. 9 (6), 942-950 (2013).
- Jensen, B. E., Dávila, I., Zelikin, A. N. Poly(vinyl alcohol) physical hydrogels: Matrix-mediated drug delivery using spontaneously eroding substrate. J Phys Chem B. 120 (26), 5916-5926 (2016).
- . Teduglutide for short bowel syndrome. Aust Prescr. 43 (2), 72-73 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved