Method Article
Novel Treatment for Complex Bile Leakage After Liver Transplantation
* These authors contributed equally
In This Article
Summary
Bile leakage is a common complication after liver transplantation that significantly affects patients' prognosis. The protocol presents a new method of combining endoscopic retrograde cholangiopancreatography (ERCP), branch choledochoscope, and percutaneous transhepatic cholangioscopy (PTCS) for treating complex bile leakage after liver transplantation.
Abstract
Bile leakage is a common complication after liver transplantation that may usually be cured with endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic cholangial drainage (PTCD), percutaneous transhepatic cholangioscopy (PTCS)and surgery. We report a novel treatment for biliary leakage lasting 9 months after liver transplantation, during which ERCP, PTCD, PTCS, and surgical therapy were in vain. We have used a new method of multi-endoscopic treatment. First, ERCP and peroral single operator cholangioscopy are used to place a plastic stent as a marker at the distal end of the bile leakage site. Second, PTCS is performed through the PTCD tube. During the operation, the proximal end of the bile leakage site is located by the plastic stent and B ultrasound, and the continuity of the biliary tract is temporarily reconstructed through the guide wire and urinary catheter. Third, a double-headed guidewire is placed, the urinary catheter is removed, the stents are placed, and biliary continuity is reconstructed. In conclusion, we have found a new method combining ERCP, peroral single operator cholangioscopy, and PTCS to treat complex bile leakage after liver transplantation.
Introduction
Liver transplantation has become a standard of care in patients with end-stage liver disease. After liver transplantation, approximately 1/3rd of patients are affected by biliary tract complications, and these result in significant morbidity and decreased patient survival, which is called the Achilles heel of liver transplantation1. Biliary leakage is the second most common complication after liver transplantation, with an incidence of 2%-21%2,3,4. Approaches commonly used for treating biliary complications involve endoscopic retrograde cholangiopancreatography (ERCP), percutaneous transhepatic cholangial drainage (PTCD), percutaneous transhepatic cholangioscopy (PTCS) and surgery5, but they are not all effective.
The goal of this method is to address complex biliary leakage after liver transplantation by combining ERCP, peroral single operator cholangioscopy, and PTCS.
The technical principle of this method is divided into three steps. First, through ERCP and peroral single operator cholangioscopy, the distal end of the biliary leakage is identified under direct vision, and a plastic stent is placed as a marker. Then, through PTCS, under the guidance of the plastic stent, the proximal end of the biliary leakage is searched from the common hepatic duct under direct vision, and the urinary catheter reconstructs the continuity of the biliary tract. Finally, through ERCP, a double-headed guide wire is used, the urinary catheter is removed, and the left and right hepatic duct stents are placed to reconstruct biliary continuity.
Peroral cholangioscopy (POCS) was first reported by Japan in 19766. Chen et al.7 first reported the first-generation single-operator visualization choledochoscope system, SpyGlass, in 2007, and the second-generation SpyGlass (SpyGlass DS) was launched by Boston in 20158. With the development of instruments, the peroral single operator cholangioscopy has become thinner and has more functions.
The biggest advantage of this method is that it can be performed under the direct vision of the peroral single operator cholangioscopy, which increases the success rate and safety of the operation9,10,11,12. Rainer9 reported successful stent placement using a direct biliary vision system in post-liver transplantation patients with ERCP stent implantation failure, and they suggested that direct visualization of the tiny opening at the biliary stricture was the only way to successfully pass the guidewire.
This method is suitable for patients with complex biliary leakage after liver transplantation who cannot be cured by ERCP, PTCD, PTCS, and surgery.
We report the case of a 38-year-old man who had a history of Crohn's disease and vitiligo in addition to acute-on-chronic liver failure brought on by an outbreak of the Hepatitis B virus. Liver function declined over time despite repeated artificial liver treatments. When a 15-year-old child with brain death provided a matching donor liver, bile leakage occurred 14 days after the liver transplantation (Figure 1A). The abdominal cavity drainage tube and PTCD were installed right away due to acute abdominal pain and septic shock. Following drainage, the patient's overall health improved, and the amount of peritoneal effusion was greatly reduced (Figure 1B).

Figure 1: Before and after drainage of bile leakage. (A) The white arrow shows the fluid before drainage. (B) The white arrow shows the fluid after drainage. Please click here to view a larger version of this figure.
After 3 weeks, the intrahepatic bile duct was insufficient to support the guide wire, which easily passed through the intrahepatic bile duct to other locations, so the attempt to reconstruct the biliary tract from top to bottom through the PTCD tube in the left and right hepatic ducts both failed (Figure 2). After 4 weeks, an attempt to reconstruct the biliary tract using ERCP from bottom to top was unsuccessful because the guidewire could not pass through stricture into the intrahepatic bile duct (Figure 3). After 2 weeks, the common bile duct could not be located, and there were numerous stones at the junction of the left and right hepatic ducts in the hilar, making it impossible to reconstruct the biliary tract from top to bottom with PTCS. About 4 weeks later, an attempt to reconstruct the biliary tract by choledochojejunostomy failed because of the obvious inflammation and edema of the tissue surrounding the biliary leak, and the patient's previous Crohn's history greatly increased the risk of postoperative biliary leak with intestinal fistula.

Figure 2: Biliary continuity reconstructed by PTC. (A) The white arrow indicates an attempt from the left hepatic duct. (B) The white arrow indicates an attempt from the right hepatic duct. Please click here to view a larger version of this figure.
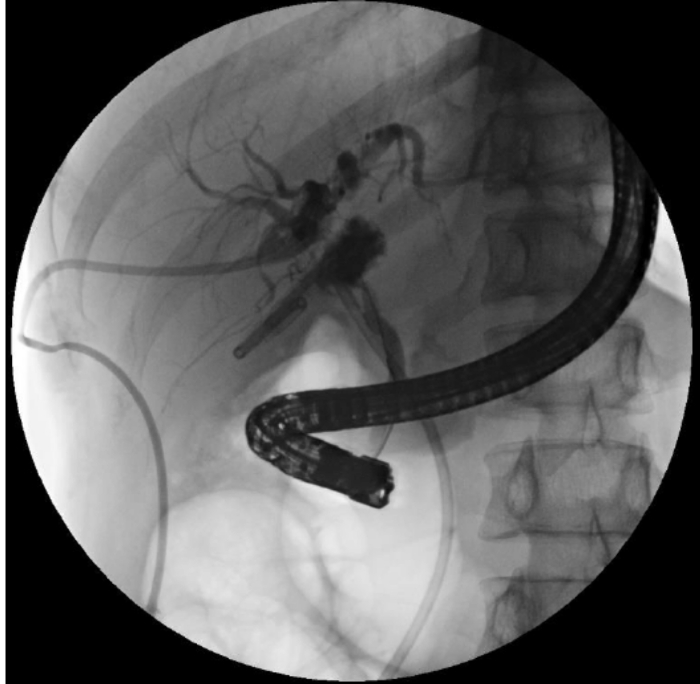
Figure 3: Biliary continuity reconstructed by ERCP. The guidewire could not enter the intrahepatic bile duct. Please click here to view a larger version of this figure.
Here, we have found a new method of combining ERCP, peroral single operator cholangioscopy, and PTCS to treat complex bile leakage after liver transplantation. A plastic stent was first placed to locate the distal end of the bile leakage through ERCP and peroral single operator cholangioscopy. The proximal end of the bile leakage was then located by PTCS and B ultrasound, and finally, the continuity of the biliary tract was reconstructed by ERCP.
Protocol
The study conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. The informed consent was obtained from the patient.
1. Preoperative preparation
- Instruct the patient to fast for 6-8 h before ERCP. Administer Pethidine 1 mg/kg, Anisodamine 20 mg, Midazolam injection 1 mg, Flurbiprofen 100 mg intravenously, and tetracaine hydrochloride orally. When the pharyngeal reflex disappeared, Local anesthesia plus sedation and analgesia were deemed successful.
2. Plastic stent placement
- Ensure the patient is placed in a prone position , and once the depth of anesthesia is satisfactory, start the procedure. Insert the duodenoscope into the mouth, pass it through the esophagus and stomach to reach the duodenum, and find the duodenal papilla.
- Insert the guidewire into the common bile duct.
- Administer iopromide injection (iodine concentration 300 mg/mL) at the rate of 2 mL/s through the ERCP or PTCD tube. Observe intrahepatic and extrahepatic bile ducts for cholangiography after injection, and identify the stricture of the bile duct. Note that the stricture of the bile duct is located in the common hepatic duct and the intrahepatic and extrahepatic bile ducts cannot be simultaneously visualized (Figure 4A).
- Dilate the lower opening of the common bile duct with a 6-8 mm dilatation balloon to 7 mm by injecting iopromide into the balloon.
- Insert the peroral single operator cholangioscopy to observe the bile duct, find strictures that cannot be passed through, and insert the guide wire through stricture, followed by dilation with a 6 mm biliary dilating bougie.
- Dilate the stenosis with a 6-8 mm dilation balloon to 7 mm by injecting iopromide injection (Figure 4B).
- Insert the peroral single operator cholangioscopy again to observe the bile duct. Note the blind bile duct with stones and flocculent material, with no intrahepatic bile duct observed even after iopromide Injection.
- Place a 7F x 7 cm plastic biliary stent into the blind duct through the stricture, and the other end was placed out of the duodenal papilla (Figure 4C).
- Throughout the procedure, provide oxygen through a nasal cannula at 3 L/min and closely monitor the vital signs during ERCP. Then, provide medications for acid inhibition (Esomeprazole 40 mg BID), enzyme inhibition (Octreotide 0.6 mg Q12H), parenteral nutrition (Fat Emulsion, Amino Acids (17) and Glucose (1%) Injection 1920 mL QD), pain inhibition (Flurbiprofen 100mg QD), and infection inhibition (Cefoperazone Sodium and Sulbactam Sodium for Injection 1.5 g Q8H). Perform an amylase test after the operation.
- Complete the operation and instruct the patient to fast until the amylase level drops to normal. Closely observe the patient for abdominal pain, melena, etc.

Figure 4: Plastic stent placed by ERCP. (A) PTCD cholangiography. (B) The white arrow indicates the narrow section. (C) The white arrow indicates plastic stent. Please click here to view a larger version of this figure.
3. Urinary catheter placement
- After 1 week of recovery, instruct the patient to lie down in the supine position and administer anesthesia. Disinfect the epigastrium skin with 0.5% iodophor and cover with sterile towels.
- After confirming proper anesthetization, insert the guidewire along the PTCD tube, remove the PTCD tube, and place a 20F protective sheath tube along the guidewire. Remove the guidewire and place a choledochoscope along the sheath.
- Flush the observed yellow flocculent material in the bile ducts of the right and left liver lobes with normal saline.
- Identify the common hepatic duct by finding the junction of the left and right hepatic ducts under the guidance of B ultrasound. Flush the yellow flocculent material in the opening of the common hepatic duct and dilate the stenosis of the common hepatic duct with 6 mm and 8 mm balloons.
- Identify the common bile duct by the plastic stent placed at step 2 under B-ultrasound guidance. Insert the choledochoscope along the common hepatic duct, and flush and dilate the stenotic segment of the common hepatic duct repeatedly. Open the stenotic segment completely and find the plastic stent placed at step 2.
- Insert the guidewire into the duodenal papilla, remove the sheath, and insert a 12F urinary catheter along the guidewire to the duodenum. Once done, remove the guidewire.
- Stitch the skin with a taper needle and 0-0/T non-absorbable suture and knot the suture on the skin and urinary catheter.
- Return the patient to the ward and give medication for acid inhibition (Esomeprazole 40 mg BID), enzyme inhibition (Octreotide 0.6 mg Q12H), parenteral nutrition (Fat Emulsion, Amino Acids (17) and Glucose (1%) Injection 1920 mL QD), pain inhibition (Flurbiprofen 100 mg QD), and infection inhibition (Cefoperazone Sodium and Sulbactam Sodium for Injection 1.5 g Q8H). Perform an amylase test after the operation.
- Instruct the patient to fast until the amylase level drops to normal. Closely observe the patient for abdominal pain, melena, etc.
4. Biliary stent placement
- After 1 week of recovery, place the biliary stent. Instruct the patient to lie in the prone position and administer anesthesia. Check the plastic stent and urinary catheter to be in place (Figure 5A).
- Upon proper anesthetization, insert the duodenoscope into the mouth, pass it through the esophagus and stomach, reach the duodenum and find the duodenal papilla. Identify the urinary catheter and the plastic stent ends and remove the plastic stent from the mouth.
- Insert the double-headed guide wire along the urinary catheter. Ensure correct entry of guidewire into the duodenum and then clamp with a foreign body clamp, passing through the duodenum, stomach, and esophagus, and removed from the mouth.
- Cut the skin fixation line of the urinary catheter and remove the urinary catheter. Under X-ray fluoroscopy, adjust the other end of the double-headed guide wire into the right hepatic duct by pulling the double-headed guide wire from the mouth side (Figure 5B).
- Insert another guidewire into the left hepatic duct (Figure 5B).
- Place a 7F x 7 cm double pigtail bile duct in the left hepatic duct (Figure 5C), and an 8.5 F x 12 cm bile duct plastic stent in the right hepatic duct, with the other end leading from the duodenal papilla (Figure 5D).
- Throughout the procedure, provide oxygen through a nasal cannula at 3 L/min and closely monitor the vital signs during ERCP. Then, provide medications for acid inhibition (Esomeprazole 40 mg BID), enzyme inhibition (Octreotide 0.6 mg Q12H), parenteral nutrition (Fat Emulsion, Amino Acids (17) and Glucose (1%) Injection 1920 mL QD), pain inhibition (Flurbiprofen 100mg QD), and infection inhibition (Cefoperazone Sodium and Sulbactam Sodium for Injection 1.5 g Q8H). Perform a amylase test after the operation.
- Complete the operation. Instruct the patient to fast until the amylase level drops to normal. Closely observe the patient for abdominal pain, melena, etc.

Figure 5: Biliary continuity reconstructed by ERCP. (A) The white arrow indicates the PTCD tube. The black arrow indicates the plastic stent. (B) The white arrow indicates the double guide wire through the right hepatic duct. The black arrow indicates a guide wire through the left hepatic duct. (C) A plastic stent for the left hepatic duct. (D) The white arrow indicates the plastic stent for the right hepatic duct. The black arrow indicates the plastic stent for the left hepatic duct. Please click here to view a larger version of this figure.
Results
We describe a new method combining ERCP, peroral single operator cholangioscopy, and PTCS to treat complex bile leakage after liver transplantation. To reconstruct biliary continuity, we placed a 7F x 7 cm double pigtail bile duct in the left hepatic duct (Figure 5C) and an 8.5 F x 12 cm bile duct plastic stent in the right hepatic duct (Figure 5D), using a combination of multiple endoscopes.
Through the method, the left and right hepatic duct stents were successfully placed, solving the bile leakage problem. During the 18-month postoperative follow-up to December 2023, Abdominal CT was performed regularly, and no biliary leaks occurred (Figure 6). The patient was hospitalized for a total of 21 days, with a 1-week interval between each procedure. The focus is on preventing complications such as postoperative pancreatitis and hemobilia. Amylase and lysosomal acid lipase A decreased to normal on the 3rd to 4th postoperative day, and hemoglobin did not decrease significantly before and after surgery (Table 1).

Figure 6: Abdominal CT performed during follow-up. (A) The white arrow shows the biliary stents 6 months after operation. (B) The white arrow shows the biliary stents 18 months after operation. Please click here to view a larger version of this figure.
| Plastic stent placement | Urinary catheter placement | Biliary stent placement | ||||||||||
| AMYL | LIPA | WBC | Hb | AMYL | LIPA | WBC | Hb | AMYL | LIPA | WBC | Hb | |
| (U/L) | (U/L) | (109/L) | (g/L) | (U/L) | (U/L) | (109/L) | (g/L) | (U/L) | (U/L) | (109/L) | (g/L) | |
| D0 | 20 | 60 | 5.12 | 83 | 40 | 59 | 4.77 | 93 | 45 | 87 | 5.43 | 87 |
| D1 | 233 | 148 | 4.55 | 84 | 225 | 558 | 4.79 | 95 | 159 | 244 | 6.69 | 88 |
| D2 | 80 | 24 | 5.5 | 81 | 180 | 307 | 5.28 | 91 | 153 | 368 | 4.91 | 83 |
| D3 | 64 | 78 | 6.73 | 86 | 120 | 250 | 4.42 | 97 | 115 | 227 | 5.12 | 89 |
| D4 | 50 | 62 | 3.79 | 89 | 90 | 201 | 7.83 | 94 | 100 | 150 | 4.71 | 86 |
Table 1: Preoperative and postoperative examination results. Abbreviations: AMYL = amylase, LIPA = lysosomal acid lipase A, WBC = White blood cell, Hb = Hemoglobin. D0 The day of surgery, D1 first postoperative day, D2 second postoperative day, D3 third postoperative day, D4 fourth postoperative day.
Furthermore, we have successfully treated multiple patients with complex bile leaks after liver transplantation at our center using this method, which has clinical feasibility (Table 2).
| Sex | Age | Diagnosis | Outcomes of treatments | |||||
| (years) | (After LT) | ERCP | PTCD | PTCS | Reoperation | ERCP+ PTCS + peroral single operator cholangioscopy | ||
| Case1 | male | 38 | Biliary leakage | NO | NO | NO | NO | YES |
| Case2 | female | 42 | Biliary leakage | NO | NO | NO | / | YES |
| Case3 | male | 61 | Biliary leakage | NO | NO | NO | / | YES |
| Case4 | male | 52 | Biliary leakage | NO | NO | NO | / | YES |
| Case5 | male | 47 | Biliary leakage | NO | NO | NO | / | YES |
Table 2: Combined multi-endoscopy in the treatment of complex biliary complications after liver transplantation. Abbreviations: LT = liver transplantation, ERCP = endoscopic retrograde cholangiopancreatography, PTCD = percutaneous transhepatic cholangial drainage, PTCS = percutaneous transhepatic cholangioscopy
Discussion
In the study, we have found a new method of combining ERCP, peroral single operator cholangioscopy, and PTCS for the treatment of complex bile leakage after liver transplantation. The key steps of this method were as follows: first, under the peroral single operator cholangioscopy, the end of the bile leakage was identified, and a plastic stent was placed. Secondly, under the guidance of B-ultrasound, the common hepatic duct strictures were observed and dilated, and the plastic stent was exposed. During these two important steps, it was necessary to perform them gently under the direct vision of the peroral single operator cholangioscopy to avoid iatrogenic complications such as biliary perforation and bleeding.
Clinicians need to choose the appropriate method for bile leakage after liver transplantation according to experience and the situation of patients. Patients with small leakages should be treated with the endoscope, and those with large leakages should be treated with abdominal drainage before the endoscope. Common endoscopic methods include ERCP, PTC, and EUS.
Endoscopic treatment and surgical treatment play an important role in biliary complications after liver transplantation13,14,15. ERCP has been favored and concerned because of its advantages of minimally invasive, repeatable operation, less trauma, and rapid recovery16,17. However, the greatest limitation of ERCP is to insert the guidewire into the target position18. PTC has the advantages of being minimally invasive, convenient, and low surgical failure19, but PTC is an invasive treatment that is prone to postoperative injury, has a high risk of secondary bleeding, and has become a second-line treatment20. Surgery is a salvage treatment21, but the greatest limitation of surgery is the high risk of operation and the shortage of donor liver.
Endoscopic ultrasound-guided biliary drainage (EUS-BD) has become an important technique for biliary drainage when ERCP fails22. EUS-BD is divided into extrahepatic drainage and intrahepatic drainage. Extrahepatic drainage consists of endoscopic ultrasound-guided choledochoduodenostomy (EUS-CDS), and intrahepatic drainage consists of endoscopic ultrasound-guided rendezvous technique (EUS-RV) and endoscopic ultrasound-guided hepaticogastrostomy (EUS-HGS). EUS-BD has several advantages. It is minimally invasive and can be performed directly after the failure of ERCP, with less pain during and after the operation. The major adverse events of EUS-BD include bile leakage, bleeding, cholangitis, sepsis, and peritonitis, with a few common complications including intra-peritoneal stent migration and fatal perforation23,24.
The advantage of this method is the use of peroral single operator cholangioscopy. Compared with ERCP or PTC, it can dilate the biliary stricture under direct vision, improve the success rate9,10,11,12 and the safety of operation, and avoid the occurrence of iatrogenic biliary perforation25. Woo et al.11 reported that direct biliary vision was beneficial for biliary guidewire placement and improved the success rate of stent placement in patients with biliary stricture after liver transplantation. A retrospective study evaluated 30 patients with complex biliary stricture who had previously failed guidewire placement using conventional ERCP, with a 70% success rate of guidewire passage through the stricture using a direct biliary vision system10. Compared with EUS-CDS, this method avoids puncture from the duodenum to the bile duct26, reducing the risk and difficulty of the operation. Compared with EUS-RV and EUS-HGS, this method preserves the normal excretion pathway of bile26 and avoids bile damage to the stomach. The disadvantage of this method is that it needs to combine ERCP, peroral single operator cholangioscopy, and PTCS, which requires high professional skills of endoscopists and cannot be carried out in primary hospitals. In addition, due to no Integrated operating room, three invasive procedures cannot be performed at one time, and each procedure requires a recovery period of 6-7 days before the next invasive procedure can be performed, which prolongs the length of hospital stay, anesthetic risk, and medical cost.
The approach fills the gap in the management of bile leakage after liver transplantation, where ERCP, PTC, and surgery have failed. It avoids the lifelong carrying of a PTCD tube or second liver transplantation, improves patients' prognosis, and has a good clinical prospect for the treatment of complex biliary leakage after liver transplantation.
Disclosures
The authors declare no conflict of interest.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (81873591); the Guangdong Natural Science Foundation (2022A1515011052); the Science and Technology Planning Project of Guangdong Province (2018A050506030); the Science and Technology Program of Guangzhou (201704020073); the Guangdong Provincial Key Laboratory Construction Projection on Organ Donation and Transplant Immunology (2013A061401007 and 2017B030314018); and the Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (2015B050501002).
Materials
| Name | Company | Catalog Number | Comments |
| Biliary stent | Boston Scientific | M00533560 | 7 F*7 cm |
| Branch choledochoscope | Leinzett | LAN-EP-2612 | |
| Disposable electric snare | Boston Scientific | M00562320 | |
| Disposable sphincterotomy knife | Boston Scientific | M00545170 | |
| Electronic choledochoscope | Olympus | / | |
| Electronic duodenum mirror | Olympus | / | |
| Guidewire | Boston Scientific | M00556140 | |
| Guidewire guided the dilated balloon catheter | Boston Scientific | M00558600 | |
| Integrated biliary stent | Boston Scientific | M00539210 | 7 F*7 cm |
| Integrated biliary stent | Boston Scientific | M00539280 | 8.5 F*12 cm |
References
- Duffy, J. P., et al. Long-term patient outcome and quality of life after liver transplantation: Analysis of 20-year survivors. Ann Surg. 252 (4), 652-661 (2010).
- Riediger, C., et al. T-tube or no t-tube in the reconstruction of the biliary tract during orthotopic liver transplantation: Systematic review and meta-analysis. Liver Transpl. 16 (6), 705-717 (2010).
- Wojcicki, M., Milkiewicz, P., Silva, M. Biliary tract complications after liver transplantation: A review. Dig Surg. 25 (4), 245-257 (2008).
- Thuluvath, P. J., Pfau, P. R., Kimmey, M. B., Ginsberg, G. G. Biliary complications after liver transplantation: The role of endoscopy. Endoscopy. 37 (9), 857-863 (2005).
- Macías-Gómez, C., Dumonceau, J. M. Endoscopic management of biliary complications after liver transplantation: An evidence-based review. World J Gastrointest Endosc. 7 (6), 606-616 (2015).
- Nakajima, M., Akasaka, Y., Fukumoto, K., Mitsuyoshi, Y., Kawai, K. Peroral cholangiopancreatosocopy (pcps) under duodenoscopic guidance. Am J Gastroenterol. 66 (3), 241-247 (1976).
- Chen, Y. K., et al. Single-operator cholangioscopy in patients requiring evaluation of bile duct disease or therapy of biliary stones (with videos). Gastrointest Endosc. 74 (4), 805-814 (2011).
- Kulpatcharapong, S., Pittayanon, R. S. J. K., Rerknimitr, R. Diagnostic performance of different cholangioscopes in patients with biliary strictures: A systematic review. Endoscopy. 52 (3), 174-185 (2020).
- Rainer, F., et al. A novel way to avoid reoperation for biliary strictures after liver transplantation: Cholangioscopy-assisted guidewire placement. Endoscopy. 51 (11), E314-E316 (2019).
- Bokemeyer, A., et al. Digital single-operator cholangioscopy: A useful tool for selective guidewire placements across complex biliary strictures. Surg Endosc. 33 (3), 731-737 (2019).
- Woo, Y. S., et al. Spyglass cholangioscopy-assisted guidewire placement for post-ldlt biliary strictures: A case series. Surg Endosc. 30 (9), 3897-3903 (2016).
- Martins, F. P., Seleti, S. M. R., Contini, M. L., Ga, D. E. P., Ferrari, A. P. Is there a place for cholangioscopic evaluation of biliary anastomotic stricture after deceased donor liver transplant. Arq Gastroenterol. 57 (4), 347-353 (2020).
- Magro, B., Tacelli, M., Mazzola, A., Conti, F., Celsa, C. Biliary complications after liver transplantation: Current perspectives and future strategies. Hepatobiliary Surg Nutr. 10 (1), 76-92 (2021).
- Arain, M. A., Attam, R., Freeman, M. L. Advances in endoscopic management of biliary tract complications after liver transplantation. Liver Transpl. 19 (5), 482-498 (2013).
- Tsujino, T., et al. Endoscopic management of biliary complications after adult living donor liver transplantation. Am J Gastroenterol. 101 (10), 2230-2236 (2006).
- López Álvarez, M., Otero, M., Vázquez Millán, M. A., Suárez López, F., Alonso Aguirre, P. Endoscopic treatment of biliary complications after liver transplantation. Rev Esp Enferm Dig. 112 (8), 605-608 (2020).
- Alves, A. R., Gomes, D., Furtado, E., Tomé, L. Efficacy of endoscopic retrograde cholangiopancreatography in the treatment of biliary complications following liver transplant: 10 years of a single-centre experience. GE Port J Gastroenterol. 25 (1), 10-17 (2018).
- Yasen, A., et al. Efficiency of percutaneous transhepatic cholangioscopy in the treatment of biliary complications after liver transplantation. HPB (Oxford). 25 (4), 463-471 (2023).
- Zimmerman, M. A., et al. resolution of biliary complications after living and deceased donor liver transplantation: A report from the adult-to-adult living donor liver transplantation cohort study consortium. Liver Transpl. 19 (3), 259-267 (2013).
- Moy, B. T., Birk, J. W. A review on the management of biliary complications after orthotopic liver transplantation. J Clin Transl Hepatol. 7 (1), 61-71 (2019).
- Wadhawan, M., Kumar, A. Management issues in post living donor liver transplant biliary strictures. World J Hepatol. 8 (10), 461-470 (2016).
- Enochsson, L., et al. Nationwide, population-based data from 11,074 ercp procedures from the swedish registry for gallstone surgery and ercp. Gastrointest Endosc. 72 (6), 1175-1184 (2010).
- Khan, M. A., et al. Endoscopic ultrasound-guided biliary drainage: A systematic review and meta-analysis. Dig Dis Sci. 61 (3), 684-703 (2016).
- Dhir, V., et al. Endoscopic ultrasonography-guided biliary and pancreatic duct interventions. Dig Endosc. 29 (4), 472-485 (2017).
- Derdeyn, J., Laleman, W. Current role of endoscopic cholangioscopy. Curr Opin Gastroenterol. 34 (5), 301-308 (2018).
- Iwashita, T., Doi, S., Yasuda, I. Endoscopic ultrasound-guided biliary drainage: A review. Clin J Gastroenterol. 7 (2), 94-102 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved