Method Article
An Immature Murine Model of Reversible Unilateral Ureteral Obstruction
In This Article
Summary
The present protocol describes a step-by-step, reproducible model of unilateral ureteral obstruction.
Abstract
Unilateral ureteral obstruction (UUO) is a common cause of chronic kidney disease (CKD), leading to the progression of renal interstitial fibrosis and ultimately resulting in irreversible kidney damage. The alleviation of UUO is crucial. Several animal models of reversible unilateral ureteral obstruction (RUUO) have been established in the literature, enabling the observation of structural changes and functional damage while also simulating physiological and pathophysiological changes following the relief of ureteral obstruction. In this study, a reversible obstruction model was established in the unilateral murine ureter using a silicone tube. Significant renal damage was observed prior to obstruction relief, with partial recovery noted afterward. Unlike UUO, this model prevents progressive hydronephrosis, leading to distinct pathological outcomes. This simple surgical procedure demonstrates a high success rate and holds promise as a classical model for investigating reversible obstructive nephropathy and potential treatments for renal interstitial fibrosis. Furthermore, it provides a practical platform for studying the mechanisms of recovery from obstructive nephropathy, renal cell regeneration, and tissue remodeling.
Introduction
Urethral obstruction significantly contributes to renal interstitial fibrosis and chronic kidney disease (CKD), potentially leading to irreversible structural damage and functional impairments in the kidney1. While unilateral ureteral obstruction (UUO) is widely used to study kidney injury and CKD, it does not accurately replicate the spontaneous recovery mechanisms that occur after the removal of an obstruction. The UUO model involves ligating the left ureter with sutures, resulting in permanent obstruction, ureteral dilation, hydronephrosis, compression of the renal parenchyma, and cortical thinning. Histological examination typically reveals tubular dilation, tubular epithelial cell necrosis, and progressive interstitial inflammation and fibrosis2. This model primarily investigates renal interstitial fibrosis and irreversible kidney function loss due to persistent obstruction.
However, many renal diseases encountered in clinical practice, such as obstruction caused by ureteral calculi or tumors, are reversible. The reversible unilateral ureteral obstruction (RUUO) model allows for the partial restoration of kidney structure and urinary tract function, ultimately resolving hydronephrosis. Recovery can be assessed through imaging techniques, histological examination, and biomarker analysis to quantify the reduction in kidney injury and fibrosis3. This model closely mimics the recovery phase of obstructive nephropathy in clinical settings and is more suitable than UUO for studying key processes such as inflammation, immune responses, cell regeneration, and tissue remodeling4,5,6,7,8.
The RUUO model enables researchers to analyze renal repair and regeneration following injury relief, addressing the limitations of UUO in dynamic studies. By comparing different time points before and after obstruction, researchers can investigate molecular pathways involved in injury and repair, including inflammation, apoptosis, fibrosis, and regeneration. This approach enhances understanding of renal recovery mechanisms and identifies potential therapeutic targets2,3,4,5,8,9,10. While renal fibrosis is often considered irreversible, clinical observations suggest that early relief of obstruction during initial fibrosis stages may halt or even reverse disease progression. The RUUO model provides a valuable experimental platform for investigating this phenomenon11.
Moreover, the RUUO model facilitates the study of fibrosis reversal following obstruction relief, offering insights into recovery mechanisms and potential antifibrotic therapies3,4. Consequently, this model is highly practical for translational research. The primary objective of this experimental model is to induce obstructive nephropathy through ureteral cannulation, followed by standardized relief at a predefined time point to ensure consistency. It is optimized for simplicity, reproducibility, and safety, making it an effective tool for experimental research.
Protocol
This animal study adhered to the guidelines of the Declaration of Helsinki and was approved by the Research Ethics Committee of the Children's Hospital of Chongqing Medical University. A total of 27 male Sprague Dawley (SD) rats were commercially obtained and housed in the Laboratory Animal Center of the Children's Hospital of Chongqing Medical University (SPF, license number: SYXK (Chongqing) 2007-0016). The rats were maintained under controlled temperature conditions with a 12-h light/dark cycle and had ad libitum access to food and water.
The protocol was conducted on male SD rats aged 6-8 weeks and is applicable to rats of all ages with bilateral ureters. In this study, fifteen 6-week-old male SD rats were randomly assigned to three groups: the native group (n = 5), the UUO group (n = 5), and the RUUO group (n = 5). Additionally, five 8-week-old SD rats (n = 5) were included as an additional control group. To establish the RUUO model, 12 rats were used, with 7 additional rats procured to account for potential risks such as intraoperative and postoperative mortality, surgical failures, incomplete obstruction, and unsuccessful reversal. This ensured a minimum of 5 rats per group for subsequent analyses.
All surgical procedures were conducted in strict accordance with institutional and national guidelines for laboratory animal care and use. Surgical staff adhered to personal protective equipment (PPE) protocols, including surgical masks, gloves, and gowns. Sterile surgical instruments were used for each procedure and were autoclaved before and after use to maintain sterility. Waste materials, including sharps and biological specimens, were disposed of in compliance with hazardous waste management protocols to mitigate contamination risks and ensure safety.
1. Animal and instrument preparation
- Conduct all procedures using sterile (autoclaved) instruments and consumables. Cut the sterilized silicone tube (inner diameter: 1.5 mm, outer diameter: 2.5 mm) into approximately 1 cm segments. Make a longitudinal incision along one side of the tube wall for subsequent use.
- Anesthetize the rats via intraperitoneal injection of pentobarbital (40 mg/kg) (following institutionally approved protocols). Confirm adequate anesthesia by checking for the absence of reflex responses, such as the pedal withdrawal reflex, upon toe pinch. Apply veterinary ophthalmic ointment to the eyes to prevent corneal drying during anesthesia.
- Depilate the rat's abdomen from the xiphoid process to the pubic symphysis and extend bilaterally to the midline.
- Position the rat in a supine position on a heated surgical pad and secure its limbs with rubber ropes.
- Drape a sterile fenestrated sheet to maintain a sterile field. Prepare the skin with povidone-iodine solution. Make a midline skin incision along the abdomen, extending from the subxiphoid region to just below the umbilicus, to provide adequate exposure of the kidneys and upper ureters.
- Incise the subcutaneous tissues and fascia along the midline using surgical scissors. Dissect the skin and underlying tissues meticulously layer by layer, and fully expose the retroperitoneal space using tissue forceps.
2. Obstructive surgery for reversible unilateral ureteral obstruction
- Retract the bowel to the right side of the abdominal cavity using a sterile swab to facilitate direct visualization of the left ureter. Cover the ureter with saline-soaked gauze to prevent desiccation.
- Dissect and mobilize the left ureter using microscopic forceps, freeing approximately 1.5 cm from the surrounding tissues.
- Place a 1 cm long silicone tube (inner diameter: 1.5 mm, outer diameter: 2.5 mm) beneath the freed ureter. Use forceps to ensure complete encasement within the tube.
- Ligate the silicone tube and the middle portion of the ureter using 3-0 silk thread to induce ureteral obstruction. Avoid excessive ligation force. Gradually pull the silicone tube along the ureter's longitudinal axis to ensure secure but non-slipping ligation.
- Reposition the bowel within the peritoneal cavity carefully, ensuring proper alignment without tension or obstruction.
- Suture the abdominal muscle and fascial layers using a 2-0 non-absorbable suture with a curved cutting needle in a continuous fashion to provide adequate tensile strength. Close the skin with a 4-0 non-absorbable suture, ensuring anatomical alignment and even tension to promote healing and minimize the risk of wound dehiscence.
- Disinfect the incision site with povidone-iodine solution. Allow the rats to recover under controlled conditions at a constant temperature for 7 days.
3. Relief surgery of reversible unilateral ureteral obstruction
- Prepare the necessary animals and instruments, ensuring a sterile setup for abdominal reopening and full exposure of the abdominal cavity.
- Dissect the knot of the silicone tube carefully using a scalpel blade. Remove the tube and irrigate the abdominal cavity with normal saline to minimize adhesion and infection risk.
- Reposition the intestine and suture the abdominal wall incision in layers using 4-0 non-absorbable sutures. Sterilize the incision site with povidone-iodine solution. Place the rat in a controlled-temperature environment for a 7-day postoperative recovery period.
- On postoperative day 14, anesthetize the rats (following the procedure mentioned in step 1.2) and collect kidney samples by transversely sectioning the kidneys into two halves9.
- Store one half in 4% paraformaldehyde for histopathological examination, and rapidly freeze the other half in liquid nitrogen for storage at −80 °C for subsequent molecular analysis. Collect blood samples for biochemical analyses.
- Perform euthanasia via CO2 asphyxiation followed by cervical dislocation following ethical guidelines.
4. Follow-up assessments
- Track body weight post-RUUO to assess overall recovery. Compare weight changes with the control and UUO groups.
- Measure kidney weight and kidney volume to evaluate renal recovery.
- Monitor serum creatinine (Scr) levels as an indicator of renal function improvement9.
- Inject methylene blue into the renal pelvis to confirm ureteral patency. Observe ureteral peristalsis and coloration to assess post-obstruction recovery.
- Perform H&E staining to assess tubular integrity and renal structure after RUUO8,10.
- Conduct Masson's trichrome staining to evaluate renal interstitial fibrosis regression8,10.
- Compare renal damage scores3,8between the UUO and RUUO groups to quantify tissue recovery.
Results
The effects of UUO and its subsequent release (RUUO) on body weight, kidney weight, kidney volume, and serum creatinine (Scr) levels were evaluated, as summarized in Table 1. Data are presented as mean ± standard deviation (SD), with n = 5 per group.
At 6 weeks, the native group exhibited a mean body weight of 234 g ± 16 g, kidney weight of 0.9107 g ± 0.0475 g, and kidney volume of 0.8962 cm³ ± 0.0502 cm³. By 8 weeks, the control group showed significant increases in body weight (291 g ± 20 g, P < 0.05), kidney weight (1.1443 g ± 0.0687 g, P < 0.05), and kidney volume (1.1340 cm³ ± 0.0392 cm³, P > 0.05), with a Scr level of 18.07 µmol/L ± 2.17 µmol/L.
The 8-week UUO group exhibited substantial renal enlargement, with kidney weight (2.5535 g ± 0.2587 g, P < 0.01) and kidney volume (2.8533 cm³ ± 0.3870 cm³, P < 0.01) significantly increased. Body weight was slightly lower than in the control group (280 g ± 17 g, P < 0.05), and Scr levels also increased (20.02 µmol/L ± 1.36 µmol/L, P < 0.05), indicating impaired renal function.
In the 8-week RUUO group, kidney weight (1.5178 g ± 0.1305 g, P < 0.05) and kidney volume (1.6183 cm³ ± 0.0906 cm³, P < 0.05) were lower than in the UUO group but remained elevated compared to the control group, with a notable significance observed only in kidney volume (P < 0.05). Scr levels (16.42 µmol/L ± 4.03 µmol/L, P < 0.05) were reduced compared to the UUO group but did not fully return to baseline. Body weight (288 g ± 12 g, P > 0.05) was higher than in the UUO group but remained slightly lower than in the control group.
The morphology of the kidneys and renal pelvis underwent significant changes following ureteral obstruction. Above the obstruction site, the ureters and renal pelvis exhibited marked dilation, with kidney enlargement and noticeable thinning of the renal cortex and medulla. Following recanalization of the ureteral obstruction, the dilation of the ureters and renal pelvis was substantially reduced, though slight residual dilation was observed (Figure 1).
To assess ureteral patency, 0.1 mL of methylene blue was injected into the renal pelvis using an insulin needle (0.33 mm × 12.7 mm). Complete obstruction was confirmed when the dye failed to traverse the ureteral blockage. The results of methylene blue injection into the renal pelvis and ureters of rats with RUUO prior to obstruction removal are shown in Figure 2A. Following removal of the silicone tube, the ureter at the ligation site exhibited a ruddy color (Figure 2B) and demonstrated normal peristalsis, suggesting that this technique resulted in minimal ureteral damage.
Histological analysis of the normal kidney revealed dense, healthy tubules surrounding the glomeruli (Figure 3, left). Fourteen days after UUO, the proximal tubule epithelium exhibited vacuolation and degeneration, loss of brush borders, and exfoliation. Necrotic cells were observed within the lumens. Distal tubules appeared dilated, some tubules were absent, and collecting ducts were distended, leading to thinning of the renal cortex (Figure 3).
Masson's trichrome staining was used to assess renal interstitial fibrosis, with blue-stained collagen fibers indicating fibrotic changes12. This method effectively differentiates cells, collagen fibers, and other tissue components. In the UUO model, after 14 days, a substantial increase in collagen fibers was observed in the renal interstitium, indicating pronounced interstitial fibrosis (Figure 4). In contrast, the RUUO model demonstrated a noticeable reduction in collagen fibers, suggesting that fibrosis was either mitigated or delayed.
According to the renal damage score (Figure 5), the UUO group exhibited severe renal injury, while the RUUO group demonstrated moderate renal injury. As depicted in Figure 5, the renal damage score was significantly higher in the UUO group compared to the native group (P < 0.0001). The RUUO group showed a significantly lower renal injury score compared to the UUO group (P < 0.05); however, the score remained significantly elevated compared to the native group (P < 0.001). Overall, renal injury in the RUUO group was significantly alleviated compared to the UUO group (P < 0.05).
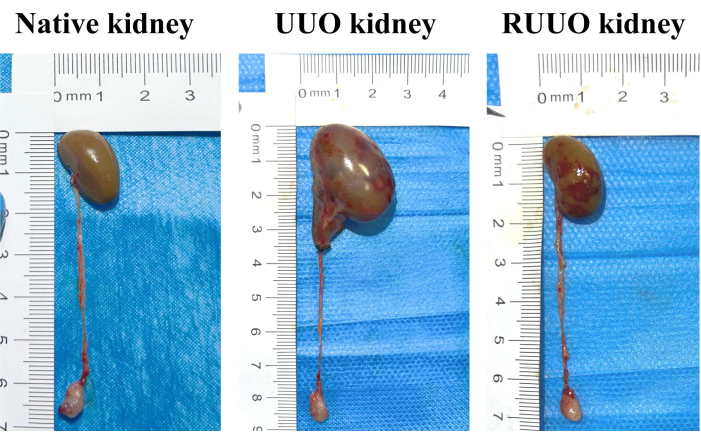
Figure 1: Representative histology of native, UUO (14-day), and RUUO kidneys. Compared to the native kidney, 14 days of UUO resulted in a notable increase in kidney volume, characterized by marked dilation of the renal pelvis and proximal ureter, along with a decrease in the renal cortex and medulla thickness. In contrast, the kidney volume in the RUUO group (right panel) was significantly reduced compared to the UUO group (middle panel), with distinct constriction of the renal pelvis and ureter. Please click here to view a larger version of this figure.
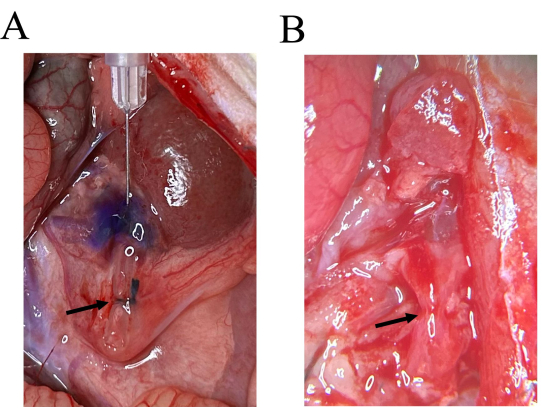
Figure 2: Ureter visualization with methylene blue injection and after silicone tube removal. (A) On the 7th day of RUUO, following the injection of methylene blue into the renal pelvis, staining was observed in the ureter above the silicone tube, while the portion below the ligation site remained unstained. (B) The ureter appears reddish post-removal of the silicone tube. Please click here to view a larger version of this figure.

Figure 3: Representative histology of native, UUO, and RUUO kidneys. The UUO kidney exhibits brush border loss in the proximal tubules, significant tubular lumen dilation, degeneration, exfoliation, and disintegration of tubular epithelial cells, as well as basement membrane exposure, indicative of pronounced acute tubular injury. The number of dilated renal tubules in the RUUO kidney is notably lower than in the UUO kidney, providing evidence of partial tubular recovery. Upper panels: Scale bar = 100 µm; lower panels (inset): Scale bar = 16 µm. Please click here to view a larger version of this figure.
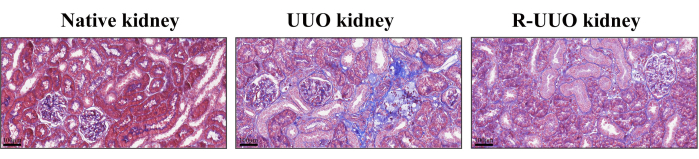
Figure 4: Masson's trichrome staining of native, UUO, and RUUO kidneys. Blue-stained areas indicate collagen fibers. The UUO kidney exhibited a higher density of collagen fibers compared to the native kidney. In contrast, the RUUO kidney showed a significant reduction in collagen fiber density compared to the UUO kidney, indicating an alleviation of interstitial fibrosis. Upper panels: Scale bar = 100 µm. Please click here to view a larger version of this figure.
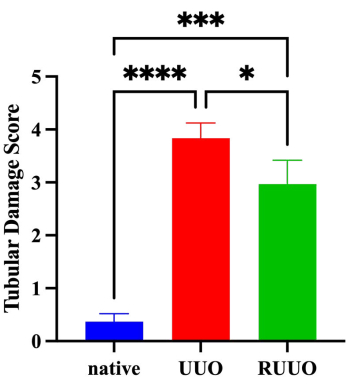
Figure 5: Tubular damage scores in native, UUO, and RUUO kidneys. Based on hematoxylin and eosin (HE) staining, histological changes in kidney tissue were scored using a semi-quantitative scale for tubular necrosis: 0 = normal kidney, 1 = minimal necrosis (≤5% involvement), 2 = mild necrosis (5%-25% involvement), 3 = moderate necrosis (25%-50% involvement), 4 = severe necrosis (50%-75% involvement), and 5 = most severe necrosis (>75% involvement). The y-axis represents renal injury scores, while the x-axis represents experimental groups. Compared to the native group, the UUO group exhibited a significantly higher renal injury score (P < 0.0001). In contrast, the RUUO group had a significantly lower renal injury score compared to the UUO group (P < 0.05). However, when compared to the native group, the RUUO group still showed a significantly elevated renal injury score (P < 0.001). Overall, the UUO group demonstrated severe renal injury, while the RUUO group exhibited moderate renal injury. Renal injury in the RUUO group was significantly alleviated compared to the UUO group (P < 0.05). Statistical significance is indicated as follows: *P < 0.05, ***P < 0.001, and ****P < 0.0001. Please click here to view a larger version of this figure.
| Group | Body Weight (g) | Kidney Weight (g) | Kidney Volume (cm³) | Serum Creatinine (μmol/L) |
| Native (6 weeks) | 234 ± 16a | 0.91 ± 0.05a | 0.90 ± 0.05a | N/A* |
| Control (8 weeks) | 291 ± 20*b | 1.14 ± 0.07*b | 1.13 ± 0.04a | 18.07 ± 2.17*b |
| UUO (8 weeks) | 280 ± 17*c | 2.55 ± 0.26**c | 2.85 ± 0.39**c | 20.02 ± 1.36*c |
| RUUO (8 weeks) | 288 ± 12*b | 1.52 ± 0.13*b | 1.62 ± 0.09**b | 16.42 ± 4.03a |
Table 1: Body weight, kidney weight, kidney volume, and serum creatinine level in different groups. Data are presented as mean ± standard deviation (SD), with n = 5 per group. Group names indicate the rats' age at the time of measurement and the corresponding disease model. Statistical comparisons were performed using the Kruskal-Wallis test, followed by Dunn's multiple comparisons test. Different superscript letters (a, b, c) denote statistically significant differences among groups (P < 0.05), where groups sharing the same letter are not significantly different, while groups with different letters indicate significant differences. For direct comparisons against the control group (except for serum creatinine), statistical significance is indicated as P < 0.05 by (*) and P < 0.01 by (**). *N/A: Serum creatinine was not measured in the native (6-week-old) group. Compared to the native group, the control group exhibited significant increases in both body weight and kidney weight (P < 0.05), indicating notable growth and development in 8-week-old rats and their kidneys. Kidney volume also increased in the control group, though the difference was not statistically significant (P > 0.05). Compared to the control group, the UUO group showed a statistically significant reduction in body weight (P < 0.05), while kidney weight and kidney volume increased significantly (P < 0.01). The serum creatinine level also increased significantly in the UUO group (P < 0.05). In comparison to the UUO group, the RUUO group demonstrated a statistically significant increase in body weight (P < 0.05) and significant reductions in kidney weight, kidney volume, and serum creatinine level (P < 0.05), with a decrease in serum creatinine being particularly notable. Compared to the control group, the RUUO group exhibited a significant increase in kidney volume (P < 0.01) and a significant reduction in serum creatinine level (P < 0.05), while differences in body weight and kidney weight were not significant. Overall, the UUO group displayed increased kidney weight, kidney volume, and serum creatinine level compared to the control group, while the RUUO group showed recovery in these parameters, with all differences being statistically significant (P < 0.05).
Discussion
This model employs a silicone tube to encircle the ureter, providing structural support, followed by ligation with a silk thread to induce complete ureteral obstruction through compression. After seven days, the ligation and silicone tube are removed to facilitate kidney decompression and the restoration of urinary tract integrity and functionality.
Silicone tubing, manufactured from silicone elastomers, offers excellent flexibility, biocompatibility, chemical resistance, and thermal stability. Medical-grade silicone rubber is specifically designed for applications requiring superior mechanical properties, including long-term implantation, whereas general silicone tubing encompasses a range of commercially available variants that may differ in elasticity, hardness, and biocompatibility profiles13. Given the short experimental duration (7-14 days) and the absence of sustained mechanical stress in this study, silicone tubing was selected for establishing the rat ureteral obstruction models due to its adequate biocompatibility, appropriate elasticity, and cost-effectiveness. Therefore, both materials exhibit functional equivalence and can be used interchangeably in this study.
The duration of obstruction is a critical factor in RUUO kidney recovery, with studies indicating that renal damage can be partially mitigated if the obstruction is relieved on day seven, whereas no significant recovery is observed beyond day 147. The optimal duration of ureteral obstruction in the RUUO model was determined to be seven days to prevent excessive damage and ensure that the results were not unduly influenced by prolonged obstruction. By comparing kidneys subjected to 14 days of obstruction with those relieved after seven days, the progression of obstructive nephropathy in the RUUO and UUO models was visually examined. Methylene blue was injected into the renal pelvis to confirm complete ureteral obstruction before catheter removal. The 7th day of obstruction was selected for testing instead of the first day due to the marked dilation of the renal pelvis by this time, which facilitated needle insertion and reduced the risk of ureteral damage from insulin needle use on day 1.
The method for alleviating obstruction is straightforward, effective, and designed to minimize potential ureteral injury. This model ensures complete obstruction while improving the success rate of recanalization and reducing the likelihood of ureteral damage. The RUUO model is characterized by its simplicity, reproducibility, and ease of implementation, making it a valuable tool for studying renal fibrosis, renal regeneration, and associated mechanisms14,15,16.
Building upon the UUO model, the RUUO model addresses the irreversible limitations of its predecessor. By integrating the pathological progression of UUO-induced renal interstitial fibrosis with the subsequent recovery following obstruction relief, the RUUO model replicates the recovery phase of acute kidney injury (AKI) and renal fibrosis. This model facilitates the simulation of clinically obstructed kidney treatment processes and provides a more comprehensive exploration of cell regeneration and extracellular matrix (ECM) remodeling compared to the traditional UUO model.
Studying UUO in humans offers valuable insights into the mechanisms of renal injury, fibrosis, and potential therapeutic interventions. UUO is widely used to mimic obstructive nephropathy caused by conditions such as ureteropelvic junction obstruction (UPJO), kidney stones, or tumors2. Renal fibrosis, characterized by excessive ECM deposition, is a hallmark of chronic kidney disease, and UUO models are instrumental in studying the role of ECM in fibrosis progression. This model reliably replicates the kidney injury process, particularly the development of fibrosis, providing a foundation for investigating the mechanisms underlying renal fibrosis.
Research indicates that relieving obstruction can partially restore and preserve renal hemodynamics and function while mitigating fibrosis progression to some extent17. This has significant implications for kidney injury repair. However, delayed obstruction relief often results in poor recovery and further kidney deterioration, potentially leading to renal failure18. Additionally, UUO has been associated with hypertension, with delayed intervention correlating to a higher incidence and severity of this condition. These findings underscore the critical importance of early intervention in minimizing renal damage and managing blood pressure. Delayed relief not only exacerbates fibrosis and renal function decline but also increases the risk of hypertension.
In this context, the RUUO model serves as a valuable tool for investigating the dynamic nature of kidney damage and recovery. Studies using the RUUO model demonstrate that while ureteral de-obstruction can mitigate some injury, persistent fibrosis and long-term damage remain, as observed in both animal and clinical models19. The RUUO model is, therefore, essential for understanding how early intervention may help limit irreversible renal damage and slow the progression to chronic kidney disease and associated complications such as hypertension.
Several researchers have proposed different models of RUUO10,14,20,21,22,23. However, many existing recanalization models have limitations, including complex surgical procedures, high technical skill requirements, and inconsistent outcomes. For instance, Ulm22 described the ureteral psoas embedding method, which facilitates successful recanalization and is relatively simple to perform. However, its effectiveness can be influenced by factors such as the animal's posture, movement, and variations in psoas muscle tension. Park et al.23 employed a non-invasive microvascular clip to induce obstruction for 10 days, followed by clip removal using the same surgical approach. While this method offers advantages such as biocompatibility, ease of operation, minimal procedural duration, and reproducibility, it may lead to intestinal adhesions and a lower success rate in ureteral recanalization.
The use of a silicone tube provides an effective alternative, as its softness and biocompatibility allow for ureteral obstruction without damaging surrounding tissues. This approach minimizes tissue injury and inflammatory responses while enabling obstruction relief without permanent ureteral damage. Consequently, the RUUO model remains reversible, stable, and suitable for long-term studies.
The vesicoureteral reimplantation method employed by Hesketh et al.10 is well-suited for studying pathophysiological changes following vesicoureteral reimplantation. However, this technique involves a complex surgical procedure with a prolonged operation time and risks of ureteral rupture and detachment due to excessive tension. Furthermore, the shortened ureter may not successfully anastomose with the bladder trigone, leading to abnormal reconnection and potential surgical failure. These challenges limit its applicability, particularly in small animal models.
Yao et al.16 introduced a technique involving ureteral clamping using a folded polyethylene tube, which reduces ureteral damage and provides stable occlusion. However, the smooth surface of the polyethylene tube may result in ligature slippage due to peristalsis and animal movement, potentially causing obstruction. Chevalier et al.9 inserted a silicone tube into the left ureter of neonatal mice, which was removed after five days of vascular clamping. While this model effectively induces obstruction, the use of silk thread ligation as an alternative to vascular clips produced comparable results, including significant reductions in kidney and renal pelvis volume. Additionally, this method caused minimal inflammatory and fibrotic responses around the ureter. Although mild ureteral dilation was observed seven days after obstruction relief, it was considerably less severe than that seen with vesicoureteral reimplantation10. This approach also prevents vesicoureteral reflux, making it a viable alternative for studying RUUO.
A key consideration in this approach is minimizing ureteral damage during surgery, as ureteral injury can induce inflammation, potentially leading to adhesion, closure, and recanalization failure. Vesicoureteral reimplantation poses risks such as direct ureteral damage, anastomotic inflammation, and potential closure due to surgical errors. Additionally, inadequate drainage of urine sediment before reimplantation may contribute to recanalization failure. Using a silicone tube to encase the ureter externally provides protection, while ligation with a vascular clip or silk thread through the silicone tube induces ureteral obstruction. However, vascular clamps may increase the risk of intestinal adhesions and lower recanalization success rates. Although repositioning the clamp every two days during ureteral obstruction has been reported to improve recanalization success rates by 70%17, this method significantly prolongs procedural time and introduces technical challenges. By contrast, silk thread ligation through a silicone tube offers a simpler approach with reduced inflammation. The primary concern remains whether silk thread ligation ensures complete obstruction and uniformity across experimental subjects.
In this study, methylene blue was injected into the ureter to confirm complete obstruction. In the UUO group, all rats achieved successful obstruction (100% success rate), with no mortality observed under standard conditions. In the RUUO group (n = 12), six rats were excluded from analysis due to complications: one died intraoperatively from anesthesia-induced respiratory depression, two succumbed to postoperative complications (suspected bleeding or infection) on days 6 and 13, two had incomplete obstruction, and one experienced failed recanalization due to surgical complications. Consequently, the survival rate for the RUUO group was 75% (95% CI: 46.77%-91.11%), the success rate of complete obstruction was 80% (95% CI: 49.0%-94.3%), and the recanalization success rate was 90% (95% CI: 59.6%-98.2%). All proportions were calculated using the respective denominators after exclusions, and 95% confidence intervals were determined using the Wilson score method.
Recovery refers to the restoration of physiological function following pathological changes or functional impairment. It includes not only the reversal of pathological alterations but also the return of organ function to near-normal levels. In contrast, preservation refers to interventions initiated before or early in the disease process to prevent further deterioration rather than fully reversing existing damage24. Differentiating between recovery and preservation requires comprehensive baseline kidney function assessments, along with dynamic monitoring of disease progression and obstruction resolution. This study was limited to short-term observations and did not incorporate systematic monitoring of pathological markers during recovery. Specifically, in vivo, longitudinal biomarkers were not used to track the true reversal of pathological mechanisms. Although improvements in kidney tissue and renal function were observed, it remains unclear whether these changes represent complete pathological reversal. Future studies should include fibrosis biomarkers and inflammatory mediators to assess disease resolution dynamically.
Additionally, due to limitations in experimental equipment and techniques, this study did not measure proteinuria and albuminuria, parameters well-established as indicators of renal pathophysiology24. Their absence may limit direct comparisons with classical kidney disease models. Future research should incorporate standardized urine protein analysis alongside serum creatinine and blood urea nitrogen measurements to provide a more comprehensive evaluation of renal function and damage.
Surgical relief of obstruction is a primary clinical strategy for treating obstructive kidney diseases25. Although this study does not fully establish the complete reversal of renal pathological mechanisms, it provides a valuable research model for investigating pathological and physiological changes following ureteral obstruction release. It also enables assessment of whether kidney function is fully restored or only partially preserved.
In summary, this model is applicable to rats of different ages, though special care is required for neonatal rats undergoing RUUO, including appropriate silicone tube selection and ureteral damage prevention. Excessive ligation should be avoided to prevent recanalization failure. The model demonstrates comparable recanalization efficacy while being simple, stable, and feasible, making it well-suited for studying inflammatory and immune processes, kidney regeneration, and related mechanisms, with significant potential for further research.
Disclosures
None.
Acknowledgements
This work was supported by the Program for Youth Innovation in Future Medicine, Chongqing Medical University (W0056), Chongqing Science and Health Joint TCM Technology Innovation and Application Development Project (2020ZY023877).
Materials
| Name | Company | Catalog Number | Comments |
| Forceps | Shanghai Medical Devices Co.,Ltd | 20220032 | |
| Gauze | Sichuan Kelun Co., Ltd | 20172140152 | |
| Hematoxylin and Eosin Stain Kit | Solarbio | G1120 | |
| Insulin needles | KDL Medical Devices | 20193140938 | |
| Masson’s Trichrome Stain Kit | Solarbio | G1340 | |
| Medical Cotton balls | Sichuan Kelun Co., Ltd | 20170037 | |
| Medical Cotton sticks | Sichuan Kelun Co., Ltd | 20172140026 | |
| Methylene blue | Tianjin Dengfeng Chemical Reagent Factory | 14038-43-8 | |
| Microscopic forceps | Suqian Shifeng Medical Devices Co., Ltd | S50985 | |
| Needle holders | Suqian Shifeng Medical Devices Co., Ltd | S7005 | |
| Povidone-iodine Solution | Sichuan Kelun Co., Ltd | 514001 | |
| Saline | Sichuan Kelun Co., Ltd | 20220004 | |
| SD Rats | SPF(Beijing)Biotechnology Co.,Ltd | D025 | |
| Silicone tubing | Taizhou Chunshi New Materials Co., Ltd | CS356 | |
| Silk suture | Qiangsheng Medical Devices Co.,Ltd | SA84G | |
| Surgical blade | Huanan Yunyue Medical Devices Co.,Ltd | CE0434 | |
| Surgical scissors | Shanghai Medical Devices Co.,Ltd | J21130 | |
| Syringe | Tongmai medical devices | 20183140304 | |
| Tissue Forceps | Jiangxi Yuyuan Medical Equipment Co., Ltd | J36030 |
References
- Chaves, L. D., et al. Contrasting effects of systemic monocyte/macrophage and CD4+ t cell depletion in a reversible ureteral obstruction mouse model of chronic kidney disease. Clin Dev Immunol. 2013, 836989 (2013).
- Chevalier, R. L., Forbes, M. S., Thornhill, B. A. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int. 75 (11), 1145-1152 (2009).
- Aranda-Rivera, A. K., et al. Sulforaphane protects from kidney damage during the release of unilateral ureteral obstruction (RUUO) by activating nuclear factor erythroid 2-related factor 2 (nrf2): Role of antioxidant, anti-inflammatory, and antiapoptotic mechanisms. Free Radic Biol Med. 212, 49-64 (2024).
- Narváez Barros, A., et al. Reversal unilateral ureteral obstruction: A mice experimental model. Nephron. 142 (2), 125-134 (2019).
- Cochrane, A. L., et al. Renal structural and functional repair in a mouse model of reversal of ureteral obstruction. J Am Soc Nephrol. 16 (12), 3623-3630 (2005).
- Kaeidi, A., et al. The therapeutic approaches of renal recovery after relief of the unilateral ureteral obstruction: A comprehensive review. Iran J Basic Med Sci. 23 (11), 1367-1373 (2020).
- Liu, Y., et al. A porcine model of relief of unilateral ureteral obstruction: Study on self-repairing capability over multiple time points. Mol Cell Biochem. 419 (1-2), 115-123 (2016).
- Puri, T. S., et al. Chronic kidney disease induced in mice by reversible unilateral ureteral obstruction is dependent on genetic background. Am J Physiol Renal Physiol. 298 (4), F1024-F1032 (2010).
- Chevalier, R. L., Kim, A., Thornhill, B. A., Wolstenholme, J. T. Recovery following relief of unilateral ureteral obstruction in the neonatal rat. Kidney Int. 55 (3), 793-807 (1999).
- Hesketh, E. E., et al. A murine model of irreversible and reversible unilateral ureteric obstruction. J Vis Exp. (94), e52559 (2014).
- Klahr, S., Morrissey, J. Obstructive nephropathy and renal fibrosis. Am J Physiol Renal Physiol. 283 (5), F861-F875 (2002).
- Jin, B., et al. Loss of men1 leads to renal fibrosis and decreases HGF-ADAMTS5 pathway activity via an epigenetic mechanism. Clin Transl Med. 12 (8), e982 (2022).
- Fischer, N. G., He, J., Aparicio, C. Surface immobilization chemistry of a laminin-derived peptide affects keratinocyte activity. Coatings (Basel). 10 (6), 560 (2020).
- Song, J., et al. Losartan accelerates the repair process of renal fibrosis in UUO mouse after the surgical recanalization by upregulating the expression of tregs. Int Urol Nephrol. 51 (11), 2073-2081 (2019).
- Song, J., et al. Regulatory t cells accelerate the repair process of renal fibrosis by regulating mononuclear macrophages. Am J Med Sci. 361 (6), 776-785 (2021).
- Yao, Y., et al. Interferon-γ improves renal interstitial fibrosis and decreases intrarenal vascular resistance of hydronephrosis in an animal model. Urology. 77 (3), e768-e761.e713 (2011).
- Chevalier, R. L., Thornhill, B. A., Chang, A. Y., Cachat, F., Lackey, A. Recovery from release of ureteral obstruction in the rat: Relationship to nephrogenesis. Kidney Int. 61 (6), 2033-2043 (2002).
- Lucarelli, G., et al. Delayed relief of ureteral obstruction is implicated in the long-term development of renal damage and arterial hypertension in patients with unilateral ureteral injury. J Urol. 189 (3), 960-965 (2013).
- Ito, K., et al. Renal damage progresses despite improvement of renal function after relief of unilateral ureteral obstruction in adult rats. Am J Physiol Renal Physiol. 287 (6), F1283-F1293 (2004).
- Song, J., et al. A modified relief of unilateral ureteral obstruction model. Ren Fail. 41 (1), 497-506 (2019).
- Morrissey, J., et al. morphogenetic protein-7 improves renal fibrosis and accelerates the return of renal function. J Am Soc Nephrol. 13 (suppl_1), S14-S21 (2002).
- Ulm, A. H., Miller, F. An operation to produce experimental reversible hydronephrosis in dogs. J Urol. 88, 337-341 (1962).
- Park, H. C., et al. Postobstructive regeneration of kidney is derailed when surge in renal stem cells during course of unilateral ureteral obstruction is halted. Am J Physiol Renal Physiol. 298 (2), F357-F364 (2010).
- Forni, L. G., et al. Renal recovery after acute kidney injury. Intensive Care Med. 43 (6), 855-866 (2017).
- Varela, S., Omling, E., Borjesson, A., Salo, M. Resolution of hydronephrosis after pyeloplasty in children. J Pediatr Urol. 17 (1), e101-e102.e7 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved