Method Article
Optimized and Simplified Technique for the Production and Culture of Precision-Cut Liver Slices
In This Article
Summary
A protocol for the production and culture of Precision-cut Liver Slices (PCLS) for the study of mouse livers. The article focuses on key aspects of the protocol, which only requires standard laboratory equipment with access to a vibratome and allows survival of PCLS for a minimum of 4 days.
Abstract
This protocol presents a simple system for the creation and culture of Precision-cut Liver Slices (PCLS). PCLS contains all cells in an intact environment and, therefore, resembles a mini model of the whole organ. They enable the study of live tissues while replicating their complex phenotypes. This protocol allows the preparation of slices from mouse livers using a vibratome and standard laboratory equipment. Protocols for producing and culturing PCLS lack standardization and can vary quite drastically depending on the tissue of interest, the type of vibratome used, and the need for oxygen. These can be difficult to reproduce in some laboratories that have only access to a basic vibratome and common tissue culture facilities. We have put together a protocol focusing on the importance of some key steps within the varied protocols already available. This protocol, therefore, emphasizes the importance of the embedding method, the cutting orientation, a dynamic versus a static system, and the relevance of a minimum volume of culture. This protocol can be established and reproduced in a simple manner in most laboratories that have access to a basic tissue slicer. Taken together and following this protocol, PCLS can stay alive for a minimum of 4 days. PCLS is a simple, economical, and reproducible model to study pathophysiological and therapeutic screening for organs such as the liver.
Introduction
Precision-cut tissue slices (PCTS) are thin sections of organs. They allow the preservation of the architecture of the organ replicating a mini-organ while preserving the 3-dimensional aspect of neighboring cells and extracellular matrix. It is an appealing model due to its easy access, cost-saving, and less labor-intensive characteristics while preserving the tissue architecture.
PCTS fill a gap between in vitro cell studies and in vivo animal research, overcoming most disadvantages of both models. PCTS has been generated from various organs, such as the liver1, intestines2,3, colon2, brain4,5, lung6,7,8, kidney9,10, spleen11,12, heart13,14 but also tumors15,16. They can also originate from various animals, such as mouse1, rat17,18 but also pig19 and human surgical wastes15,20,21. Although PCTS require the use of animals, implying ethical related issues, the organ from one animal can generate multiple PCTS, thereby reducing the number of animals in agreement with the NC3Rs guidelines (Reduction, Replacement, Refinement)22 while limiting interindividual variations.
The development of improved tissue slicers, e.g., vibratomes23, has allowed a transition from manually cut slices characterized by heterogeneous thickness and poor survival rate to reproducible thinner slices with better preserved structural integrity.
However, protocols for PCTS and, more specifically, Precision-cut Liver Slices (PCLS) preparation and culture vary significantly in the literature and lack standardization, especially for essential parameters such as slicing equipment, medium content, and culture conditions. The protocols can also vary noticeably depending on the tissue of origin. Some of the protocols will require oxygenation of the buffer or culture with some complicated bioreactor systems24. They usually focus individually on different technical aspects or are designed for different tissues and can often be costly and more challenging to reproduce in the average laboratory in a cost-efficient manner.
Here, this protocol puts together some key points such as the embedding method, the direction of cutting, the use of transwells25, a dynamic culture system26 and the importance of a minimal volume of culture. Some of these steps have previously been optimized independently or in a different context, such as fibrosis27 or tumor response28. This protocol also emphasizes the importance of embedding using certain types of slicers and the orientation of cutting, which are both difficult parameters to master and often neglected in the literature. This simple method generates PCLS maintained in culture for a minimum of 4 days with an easy set-up and using standard laboratory equipment with access to a rudimentary tissue slicer.
Protocol
Wild-type CD57Bl/6J mice were purchased from Charles River Laboratories. Mice had free access to food and water, housed in individually ventilated cages with controlled temperature and humidity conditions and with a 12 h light cycle. Animals aged 3 weeks were sacrificed, and livers were promptly harvested without perfusion. All animal work was approved following local ethical review by the University College London Animal Welfare and Ethical Review Board and performed under Home Office project license PP9223137 and in accordance with the Home Office (Animals) Scientific Procedures Act (1986) and ARRIVE guidelines. All efforts were made to limit harm to animals in accordance with standard practice at the Biological Services Unit at University College London.
1. Set up for the experiment
- On the day before harvest, perform the following steps.
- Prepare 1 L of Krebs-Henseleit buffer (KREBS) by dissolving one vial of Krebs powder into 1 L of sterile water. Cool it down to 4 °C and keep it on wet ice.
- Disinfect the tray with 70% ethanol and rinse with sterile PBS. Keep the tray wrapped in tin foil in the fridge overnight to help maintain a cold environment while cutting.
- Spray all other removable parts with ethanol, rinse with sterile PBS, leave to dry, and keep them sterile. Autoclave the blades and keep them sterile until use.
- Prepare 4% w/v low-melting agarose in sterile water. Once resuspended and melted, store the agarose in the fridge at 4 °C.
- On the day of harvest and before the harvest of the liver, perform the following steps.
- Melt the 4% low melting agarose and keep it in a water bath at 37 °C until use. Make sure the agarose has cooled down to 37 °C and that all bubbles have dissipated before use.
- Prepare the culture plates by adding respectively 2.6 mL, 1.5 mL, and 0.7 mL per well into 6, 12, and 24 well plates. Add 8 µm porous inserts to each well. Place the plates into a humidified incubator set to 37 °C, 5% CO2, and 21% O2 level. This will help adjust the pH while warming up the media, so it is ready for culture.
- Culture the slices with porous 8 µm inserts to allow access to both faces of the slice. Prepare the media as follows: Add to the William's Medium E (WME), 2 mM L-glutamine supplement, 10% Fetal Bovine Serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin, 10 μg/mL Gentamycin, 25 mM D-Glucose solution and 15 mM HEPES solution.
2. Collection of liver and preparation (15 min)
- Sterilize all instruments prior to harvest.
- Anaesthetize the mouse according to local procedures for the care of animals for scientific purposes by using an isoflurane mask. Before opening the abdominal cavity, pinch between the toes to ensure the animal is properly anesthetized. If the liver can be removed quickly, the mouse can be euthanized by CO2 asphyxiation or cervical dislocation. As the procedure is a terminal procedure, do not use eye ointment as this would not affect the animal.
- Spray the abdomen with 70% ethanol. Optional: Shave the mouse to prevent contamination with hair.
- Open the abdominal cavity with sterile forceps and scissors by cutting the skin and peritoneum from the middle of the abdomen. Dissect the liver gently from other organs or vessels and avoid damaging the lobes.
- Store the whole liver immediately in ice-cold Krebs Buffer. Perform all further steps on the ice at 4 °C and proceed to the liver preparation as quickly as possible to prevent further cell death.
3. Embedding of the liver lobes (25 min for each liver lobe)
- Transfer the liver into a Petri dish on ice containing ice-cold Krebs buffer, making sure that the whole liver is fully submerged.
- Separate each lobe individually using blunt forceps and a sharp, sterile knife to avoid damaging the lobes.
- Choose the first lobe for sectioning and keep the remaining lobes in an ice-cold Krebs buffer until they are ready for embedding and slicing.
- Cut all edges to get a more manageable lobe with straight edges ready for embedding. This will also help remove some of the fibrous Glisson's capsule to further facilitate sectioning. Do this while keeping the liver surfaces wet in an ice-cold Krebs buffer.
- Place a 3 cm Petri dish (or similar) on wet ice and pour the 4% low-melting agarose (already in a 37 °C water bath) into it. Keep well on ice to allow the agarose to cool down in an upward direction while preventing the lobe from sinking to the bottom and embedding it uniformly.
- Leave the agarose to further cool down for 30 s and place the trimmed lobe into it. The lobe will settle in the middle of the agarose block. The embedding process requires practice and optimization to suit every lab condition and personal experience.
- Place the embedded lobe, still on ice, in the fridge for 5 min. The agarose should then be clearly set.
- Cut off the outside of the agarose block. Dislodge the agarose block from the dish.
- Cut the agarose into a more manageable size, ensuring that the upper side and the side glued to the vibratome platform are parallel to the upper edge of the lobe.
- Start the slicing process as quickly as possible but ensure that the agarose block is kept in an ice-cold Krebs buffer and on ice.
4. Liver slices production (40 min per lobe)
- Set the vibratome for cutting at a thickness of 250 µm, with a speed of 5 and a frequency of 7 Hz. These are guiding parameters; depending on the type of vibratome used, this might require optimization.
- Spray the vibratome and bench areas with 70% ethanol before cutting to keep the environment as sterile as possible.
- Place the tray onto the vibratome and pour ice all around it. Place the blades onto the vibratome at an angle of 10° downwards and below horizontal.
- Fill the vibratome tank with ice-cold Krebs buffer. Place a thin layer of cyanoacrylate glue onto the platform.
- Dry the edge of the agarose block that will be glued onto the platform using a sterile absorbent tissue.
- Place the agarose block onto the removable platform. Position the lobe upright to allow it to be cut transversally. Although the size of the slices is reduced, this drastically facilitates the cutting process by limiting the pressure against the lobe while cutting.
- Wait 1 min for the glue to set and submerge the removable platform into the tank and ensure the agarose block is completely covered with Krebs buffer.
- Program the vibratome for cutting by setting the start and stop positions. Start cutting thicker slices initially until the liver lobe is reached.
- Discard the first slice, as it might not be cut to the right thickness. To avoid damaging the slices, use a spatula to collect the liver slices instead of forceps or brushes.
NOTE: A small brush disinfected with 70% ethanol and rinsed with sterile PBS can also be used to gently guide the tissue during the cutting process. - Repeat the process until the required number of slices is reached. The lobe might occasionally dislodge itself from the agarose, preventing further use. Collect the slices into ice-cold Krebs buffer until culture.
5. Incubation of liver slices
- Transfer the slices, using a spatula, into the prepared wells containing the media and inserts.
- Place the plates onto an orbital shaker, set the speed at 130 rpm, and incubate using a conventional cell culture incubator with 5% CO2 and 21% O2 at 37 °C. The final volume of culture is 2.6 mL, 1.5 mL, and 0.7 mL per well in 6, 12, and 24-well plates, respectively.
- Place one empty plate below the culture plate containing the slices in culture to allow any excessive heat originating from the shaker's platform to be dissipated. Change the medium every 48 h.
6. Cell survival assay
- Transfer the slices into a 48-well plate containing 400 µL of prewarmed complete Williams' Medium E media and add 80 µL of MTS (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) tetrazolium reagent.
- Following incubation for 1 h at 37 °C, 5% CO2 onto a shaker, transfer 200 µL of media into a 96-well plate, and measure the absorbance at 490 nm using a multi-well plate reader. Use slices left on the bench in PBS and at room temperature for 24 h as negative controls.
7. Histology staining
- Deparaffinize and rehydrate the sections using xylene and ethanol, followed by deionized water. Stain the sections with Hematoxylin solution for 3 min.
- Rinse with deionized water for 5 min. Dip quickly 10x into acid ethanol (1 mL of concentrated HCL and 400 mL of 70% ethanol).
- Rinse 2x into deionized water and blot excess water. Dip the sections into Eosin for 30 s.
- Dehydrate into 95% ethanol, then 100% ethanol for 5 min, 3x each. Dip sections into xylene 3x for 15 min each. Place coverslips on slides.
Results
At harvest, perfusion of the animal is purposely omitted to ensure rapid processing of the organ and prevent organ damage. The liver is extracted quickly following incision and immediately placed in an ice-cold organ-protective buffer, e.g., Krebs buffer24,29. Although slicing fresh liver tissue without embedding has been previously described1, embedding of the liver in low-melting agarose30 (Figure 1) combined with an organ-protective buffer will enable optimal cutting conditions on the vibratome, reducing tissue damage and increasing reproducibility in section thickness. Tissue thickness is critical as thin sections allow more cell layers to access nutrients and oxygen31 and reduce cell death. However, sections that are too thin become difficult to cut homogeneously. Conversely, slices thicker than 400 µm will show a lower penetration rate of nutrients. The sections were incubated in a liquid-air interface using an insert (Figure 1) and incubated with 5% CO2 and 21% O2 at 37 °C on a shaker. Sections are to be incubated in a culture medium within 3 h following harvest, after which cell death occurs rapidly32.
To determine the viability of PCLS, cell viability was assessed by 4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay, which requires NAD(P)H-dependent dehydrogenases, i.e., metabolically active cells, to reduce MTS. MTS values have been normalized to the respective slice weight. To optimize PCLS viability, a minimal volume of culture medium was essential to sustain viability after 24 h of incubation. A volume of 0.7 mL in 24 well plates showed a significant reduction of viability by TMS assay (p = 0.02) compared to 1.5 mL in 12 well plates and 2.6 mL in 6 well plates (Figure 2A). These volumes were chosen to allow the sections to be slightly covered, but they might need adjustment depending on the type of inserts and plates used. As others33, 12 well plates are used as the best compromise for optimal survival within a smaller volume of culture medium.
Shaking is essential and increases PCLS viability by 50% at 24 h post-incubation compared to a static culture (Figure 2B). Shaking creates a critical air-liquid interface, optimized with the use of transwells, allowing access to nutrients and oxygen to both faces of the section. The uptake of oxygen and nutrients is also increased by the constant flow created by the shaking movement, which also passes through the transwell membrane.
The MTS assay was assessed from 1 h incubation up to day 6 of incubation. Cell viability remained constant from day 0 to day 4 post-incubation before observing a significant decrease (p = 0.05) at day 6 (Figure 2C). The PCLS morphology assessed by hematoxylin and eosin (H&E) staining showed no change of bile ducts and architecture up to 5 days post-incubation (Figure 3A-D). Compared to day 0 (Figure 3A), PCLS showed no histological difference at day 1 (Figure 3B) and day 2 (Figure 3C) post-incubation, with nuclear hyperchromasia, mild inflammatory infiltrate, vacuolization in favor of a moderate cell death process at day 5 post-incubation (Figure 3D). Taken together, this PCLS culture protocol enables viability for at least 4 days, consistent with studies using slices in similar conditions31.
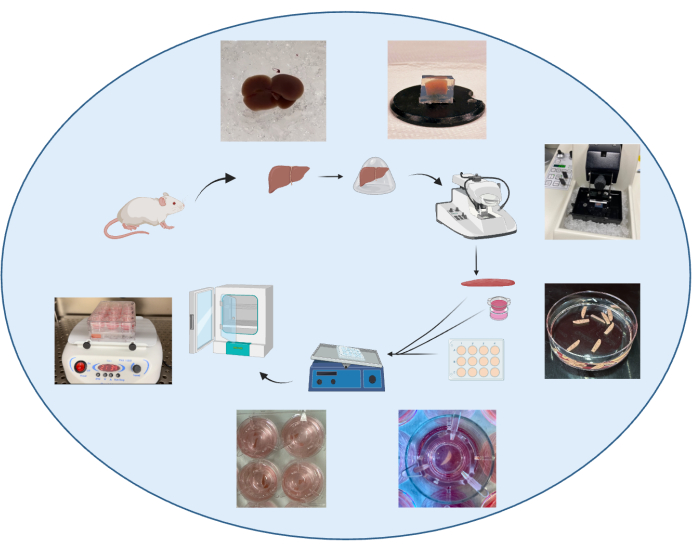
Figure 1: Schematic summarizing the protocol for generating PCLS. This figure has been modified from34. Please click here to view a larger version of this figure.

Figure 2: Optimized protocol of PCLS culture shows satisfactory viability for 5 days. (A) Effect of well size on cell proliferation (n=3). (B) Effect of shaking on cell proliferation (n=6 per condition). (C) MTS Cell proliferation assay from liver sections from d0 to d6 days of incubation (n=5 per timepoint). OD arbitrary unit, normalized to slice fresh weight. Graph shows mean ± SD. Unpaired 2-tailed Student's t-test, ns=not significant, *p<0.05, **p<0.01. This figure has been modified from34. Please click here to view a larger version of this figure.
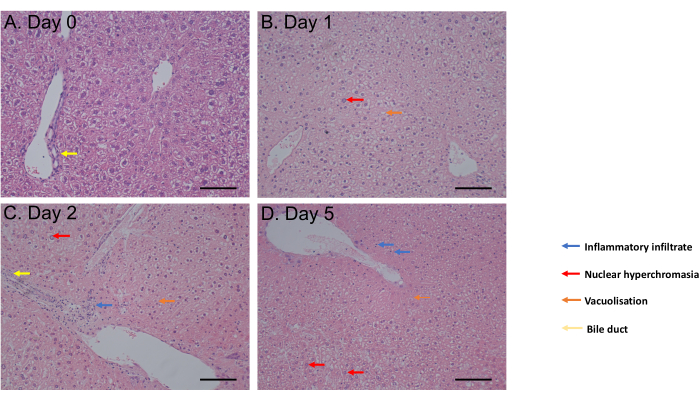
Figure 3: Histology results. (A-D) Representative images of histology of liver PCLS following H&E staining. Scale bar = 100 µM. This figure has been modified from34. Please click here to view a larger version of this figure.
Discussion
We demonstrate that producing and culturing PCLS can be easily achieved while ensuring a half-life of at least 4 days. This protocol recapitulates five critical steps: the embedding method if this type of vibratome is used, the orientation of cutting, a dynamic system of culture, a minimal volume of culture, and the use of inserts.
Protocols for the production and culture of PCLS are commonly available. However, they do lack standardization; they might focus on similar and specific points of the protocol but can be difficult to replicate in a simple manner or in most laboratories that have access to a basic vibratome. The types of vibratomes or tissue slicers are wide. They will vary in cost and technical specificities, such as having an integrated cooling system or not, but their common feature is their cutting system using an oscillating razor blade. The main difference with regard to slicing tissues is the requirement for embedding. For obvious reasons and the impact of embedding on viability, it should ideally be avoided. One example of a slicer of reference that does not require embedding is the Krumdieck slicer35. This type of slicer allows the tissue to be cut in a cooled buffer while using a core, producing evenly sized slices while avoiding embedding. However, such apparatus tends to be more costly than more basic vibratomes and less commonly used or available in most laboratories. Vibratomes such as the one used in this protocol tend to be already available for the cutting of chemically fixed tissues but will require embedding of the liver lobes. Some have shown that cutting liver slices can be achieved without embedding and using a similar vibratome1; however, in our experience, this has proven difficult to reproduce. Also, while using this type of vibratome, liver slicing without a 3D supporting agarose gel causes damaged slices and uneven thickness and, therefore, increases cell death. This protocol involves cutting the liver lobe transversely instead of sagittally. The cutting step is a difficult technique to master, and to our knowledge, the cutting orientation is an important detail that is never focused on. The orientation of the lobe during cutting can drastically facilitate the cutting process while reducing the pressure on the liver. The use of hydrogel could also be considered as an improved benefit36.
The next important criterion is the need for higher volumes of culture to increase viability. Higher volumes have already been suggested to provide more nutrients and dilute more toxic bile acid products37. Adding a dynamic system with shaking and combined with the use of Transwells improves access to nutrients and potentially oxygen to both faces of the section by creating a constant flow18,38,39. The use of transwell and the advantage of a dynamic system have already been proven in different contexts, such as human tumor liver slice responses28 and for the modeling of fibrosis26,27. This protocol confirms their advantage in a broader physiological aspect.
Williams' Medium E is commonly chosen as a standard cell culture medium for PCLS40,41. Supplemented media with glucose and serum has been described with potential benefit in preserving the viability and functionality of slices42. Glucose concentration in media usually varies between 4 nM to 36 nM43,44, but no consensus has been found on the effect of higher glucose concentration on viability or the oxidative response. The addition of insulin or dexamethasone35 is claimed to improve long-term viability, but no consensus has been reached as the addition of such supplements could potentially induce secondary insulin resistance with a downstream effect on viability45 .
Previous data shows that sections thinner than 200 μm become difficult to cut homogenously and can show oxidative stress, while slices thicker than 400 µm show a low penetration rate of nutrients18,19,46. Also, based on PCLS appearance, effects on texture, and ease of cutting, a thickness of 250 µm is favored. The penetration of the nutrients or therapeutic agent in the inner cell layers of the PCLS is also greatly improved using transwells as part of the dynamic system 18,32. As opposed to the use of the Krumdieck slicer which has the advantage of producing evenly sized slices through the integration of a core cutting system, the protocol can be adapted by resizing the slices in equal dimensions post slicing. However, the variability in size, weight, or protein content should be considered in the experiment and its impact on the culture environment and, therefore, on viability and biomarkers. For this reason, the MTA assay readings, while using this protocol, are normalized to the fresh weight of each slice. Also, thickness heterogenicity can be observed, but unfortunately, it is likely to be observed using all types of slicers. The user could consider discarding the least homogeneous slices by assessing their aspect, but this is still considered an unreliable option and remains a drawback of PCTS. The main limitation associated with this model remains the relative short-term viability, but it falls within the timeframe already published24,31. The oxygen availability could be enhanced to increase such viability. Some previously published protocols required complex culture media and oxygen concentration higher than 80%, upregulating the metabolism and providing longer viability1,24,35,38. It is also difficult to directly compare oxygen levels used to oxygenate PCLS and oxygen levels used to culture cell lines. Data on the effects of oxygen on PCLS physiology is very limited18,47, and higher oxygen concentration is likely to modify the pathophysiology and the phenotype substantially by generating toxic reactive oxygen species48.
In conclusion, short-lived PCLS can be produced with limited equipment and used as a reliable ex vivo model. Tissue architecture is crucial in liver physiology, and PCLS allowing it to be preserved is another example of why this model should be considered in a more prevailing way. Precision-cut slices should, therefore, become a more recognized tool in scientific research.
Disclosures
There is no competing interest to be disclosed.
Acknowledgements
The authors thank Mirabela Bandol, Samantha Richards, Louise Fisher, Rebecca Towns, and the staff from UCL Biological Services for their help with breeding and maintenance of the animal colonies. This work was supported by funding from the United Kingdom Medical Research Council Clinician Scientist Fellowship MR/T008024/1 (JB) and the NIHR Great Ormond Street Hospital Biomedical Research Centre (JB). The views expressed are those of the author(s) and not necessarily those of the NHS or the NIHR.
Materials
| Name | Company | Catalog Number | Comments |
| 3 cm petri dish | Any | any suitable for cell culture | |
| 6, 12, 24 well culture plate | Any | any suitable for cell culture | |
| Cyanoacrylate super glue | Any | ||
| D-Glucose | Gibco | A24940 | |
| Eosin | Merck | HT110316 | |
| Ethanol | Any | ||
| Fetal Bovine serum | ThermoFisher | 26400044 | |
| Gentamycin | Gibco | 15750060 | |
| Hematoxylin | Merck | 51275 | |
| HEPES | Gibco | H0887 | |
| inserts 8um, for 12 well plates | Strastedt | 83.3931.800 | |
| inserts 8um, for 24 well plates | Strastedt | 83.3932.800 | |
| inserts 8um, for 6 well plates | Strastedt | 83.3930.800 | |
| KREBS | Merck | K3753 | |
| Laminar Flow Hood | Hepa air filtration | ||
| Low melting agarose | ThermoFisher | 16520050 | |
| MTS tetrazolium reagent | Abcam | ab197010 | |
| multi-well plate reader | Any | ||
| PBS tablets | ThermoFisher | P4417 | |
| Penicillin/Streptomycin | Gibco | 15140-122 | |
| Scalpel blade | Any | ||
| Surgical forceps | Any | with a flat square-tip | |
| Surgical scissors | Any | ||
| Vibratome | Leica | VT1000 S | |
| William’s Medium E with GlutaMAX (WME) | ThermoFisher | W4125 |
References
- Pearen, M. A., et al. Murine precision-cut liver slices as an ex vivo model of liver biology. J Vis Exp. (157), e60992 (2020).
- De Kanter, R., et al. Prediction of whole-body metabolic clearance of drugs through the combined use of slices from rat liver, lung, kidney, small intestine and colon. Xenobiotica. 34 (3), 229-241 (2004).
- van de Kerkhof, E. G., de Graaf, I. A., Ungell, A. L., Groothuis, G. M. Induction of metabolism and transport in human intestine: validation of precision-cut slices as a tool to study induction of drug metabolism in human intestine in vitro. Drug Metab Dispos. 36 (3), 604-613 (2008).
- Nogueira, G. O., Garcez, P. P., Bardy, C., Cunningham, M. O., Sebollela, A. Modeling the human brain with ex vivo slices and in vitro organoids for translational neuroscience. Front Neurosci. 16, 838594 (2022).
- Ucar, B., Stefanova, N., Humpel, C. Spreading of aggregated alpha-Synuclein in sagittal organotypic mouse brain slices. Biomolecules. 12 (2), 163 (2022).
- Liu, G., et al. Precision cut lung slices: an ex vivo model for assessing the impact of immunomodulatory therapeutics on lung immune responses. Arch Toxicol. 95 (8), 2871-2877 (2021).
- Klouda, T., Kim, H., Kim, J., Visner, G., Yuan, K. Precision cut lung slices as an efficient tool for ex vivo pulmonary vessel structure and contractility studies. J Vis Exp. (171), e62392 (2021).
- Viana, F., O'Kane, C. M., Schroeder, G. N. Precision-cut lung slices: A powerful ex vivo model to investigate respiratory infectious diseases. Mol Microbiol. 117 (3), 578-588 (2022).
- De Kanter, R., et al. Drug-metabolizing activity of human and rat liver, lung, kidney and intestine slices. Xenobiotica. 32 (5), 349-362 (2002).
- Stribos, E. G. D., Seelen, M. A., van Goor, H., Olinga, P., Mutsaers, H. A. M. Murine precision-cut kidney slices as an ex vivo model to evaluate the role of transforming growth factor-beta1 signaling in the onset of renal fibrosis. Front Physiol. 8, 1026 (2017).
- James, K., Skibinski, G., Hoffman, P. A comparison of the performance in vitro of precision cut tissue slices and suspensions of human spleen with special reference to immunoglobulin and cytokine production. Hum Antibodies Hybridomas. 7 (4), 138-150 (1996).
- Tatibana, M., Kita, K., Asai, T. Stimulation by 6-azauridine of carbamoyl phosphate synthesis for pyrimidine biosynthesis in mouse spleen slices. Eur J Biochem. 128 (2-3), 625-629 (1982).
- Camelliti, P., et al. Adult human heart slices are a multicellular system suitable for electrophysiological and pharmacological studies. J Mol Cell Cardiol. 51 (3), 390-398 (2011).
- Liu, Z., et al. Comparative analysis of adeno-associated virus serotypes for gene transfer in organotypic heart slices. J Transl Med. 18 (1), 437 (2020).
- Zimmermann, M., et al. Human precision-cut liver tumor slices as a tumor patient-individual predictive test system for oncolytic measles vaccine viruses. Int J Oncol. 34 (5), 1247-1256 (2009).
- Philouze, P., et al. CD44, gamma-H2AX, and p-ATM expressions in short-term ex vivo culture of tumour slices predict the treatment response in patients with oral squamous cell carcinoma. Int J Mol Sci. 23 (2), 877 (2022).
- Tigges, J., et al. Optimization of long-term cold storage of rat precision-cut lung slices with a tissue preservation solution. Am J Physiol Lung Cell Mol Physiol. 321 (6), L1023-L1035 (2021).
- Olinga, P., et al. Comparison of five incubation systems for rat liver slices using functional and viability parameters. J Pharmacol Toxicol Methods. 38 (2), 59-69 (1997).
- Ou, Q., et al. Physiological biomimetic culture system for pig and human heart slices. Circ Res. 125 (6), 628-642 (2019).
- Martin, C. Human lung slices: New uses for an old model. Am J Respir Cell Mol Biol. 65 (5), 471-472 (2021).
- Sewald, K., Danov, O. Infection of human precision-cut lung slices with the Influenza virus. Methods Mol Biol. 2506, 119-134 (2022).
- . Available from: https://www.nc3rs.org.uk (2023)
- Iulianella, A. Cutting thick sections using a vibratome. Cold Spring Harb Protoc. 2017 (6), (2017).
- Szalowska, E., et al. Effect of oxygen concentration and selected protocol factors on viability and gene expression of mouse liver slices. Toxicol In Vitro. 27 (5), 1513-1524 (2013).
- Kenerson, H. L., Sullivan, K. M., Labadie, K. P., Pillarisetty, V. G., Yeung, R. S. Protocol for tissue slice cultures from human solid tumors to study therapeutic response. STAR Protoc. 2 (2), 100574 (2021).
- Paish, H. L., et al. A bioreactor technology for modeling fibrosis in human and rodent precision-cut liver slices. Hepatology. 70 (4), 1377-1391 (2019).
- Dewyse, L., et al. Improved precision-cut liver slice cultures for testing drug-induced liver fibrosis. Front Med (Lausanne). 9, 862185 (2022).
- Jagatia, R., et al. Patient-derived precision cut tissue slices from primary liver cancer as a potential platform for preclinical drug testing. EBioMedicine. 97, 104826 (2023).
- Koch, A., et al. Murine precision-cut liver slices (PCLS): a new tool for studying tumor microenvironments and cell signaling ex vivo. Cell Commun Signal. 12, 73 (2014).
- Brugger, M., et al. High precision-cut liver slice model to study cell-autonomous antiviral defense of hepatocytes within their microenvironment. JHEP Rep. 4 (5), 100465 (2022).
- Dewyse, L., Reynaert, H., van Grunsven, L. A. Best practices and progress in precision-cut liver slice cultures. Int J Mol Sci. 22 (13), 7137 (2021).
- Lerche-Langrand, C., Toutain, H. J. Precision-cut liver slices: characteristics and use for in vitro pharmaco-toxicology. Toxicology. 153 (1-3), 221-253 (2000).
- van de Kerkhof, E. G., de Graaf, I. A., de Jager, M. H., Meijer, D. K., Groothuis, G. M. Characterization of rat small intestinal and colon precision-cut slices as an in vitro system for drug metabolism and induction studies. Drug Metab Dispos. 33 (11), 1613-1620 (2005).
- Perocheau, D., et al. Ex vivo precision-cut liver slices model disease phenotype and monitor therapeutic response for liver monogenic diseases. F1000Res. 12, 1580 (2023).
- de Graaf, I. A., et al. Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat Protoc. 5 (9), 1540-1551 (2010).
- Bailey, K. E., et al. Embedding of precision-cut lung slices in engineered hydrogel biomaterials supports extended ex vivo culture. Am J Respir Cell Mol Biol. 62 (1), 14-22 (2020).
- Graaf, I. A., Groothuis, G. M., Olinga, P. Precision-cut tissue slices as a tool to predict metabolism of novel drugs. Expert Opin Drug Metab Toxicol. 3 (6), 879-898 (2007).
- Leeman, W. R., van de Gevel, I. A., Rutten, A. A. Cytotoxicity of retinoic acid, menadione and aflatoxin B(1) in rat liver slices using Netwell inserts as a new culture system. Toxicol In Vitro. 9 (3), 291-298 (1995).
- Schumacher, K., et al. Perfusion culture improves the maintenance of cultured liver tissue slices. Tissue Eng. 13 (1), 197-205 (2007).
- Jetten, M. J., et al. Interindividual variation in response to xenobiotic exposure established in precision-cut human liver slices. Toxicology. 323, 61-69 (2014).
- Duryee, M. J., et al. Precision-cut liver slices from diet-induced obese rats exposed to ethanol are susceptible to oxidative stress and increased fatty acid synthesis. Am J Physiol Gastrointest Liver Physiol. 306 (3), G208-G217 (2014).
- Starokozhko, V., et al. Maintenance of drug metabolism and transport functions in human precision-cut liver slices during prolonged incubation for 5 days. Arch Toxicol. 91 (5), 2079-2092 (2017).
- Vickers, A. E., et al. Organ slice viability extended for pathway characterization: an in vitro model to investigate fibrosis. Toxicol Sci. 82 (2), 534-544 (2004).
- Boess, F., et al. Gene expression in two hepatic cell lines, cultured primary hepatocytes, and liver slices compared to the in vivo liver gene expression in rats: possible implications for toxicogenomics use of in vitro systems. Toxicol Sci. 73 (2), 386-402 (2003).
- Zhang, W. Y., et al. Amelioration of insulin resistance by scopoletin in high-glucose-induced, insulin-resistant HepG2 cells. Horm Metab Res. 42 (13), 930-935 (2010).
- Smith, J. T., et al. Effect of slice thickness on liver lesion detection and characterisation by multidetector CT. J Med Imaging Radiat Oncol. 54 (3), 188-193 (2010).
- Drobner, C., Glockner, R., Muller, D. Optimal oxygen tension conditions for viability and functioning of precision-cut liver slices. Exp Toxicol Pathol. 52 (4), 335-338 (2000).
- Hart, N. A., et al. Oxygenation during hypothermic rat liver preservation: an in vitro slice study to demonstrate beneficial or toxic oxygenation effects. Liver Transpl. 11 (11), 1403-1411 (2005).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved