Method Article
Production of Nanofibrillar Patterned Collagen for Tissue Engineering
In This Article
Summary
The following protocol presents a shear-based extrusion method for the fabrication of collagen hydrogels with nanoscale patterned fibrils, which can be applied to a broad range of tissue engineering applications.
Abstract
Regenerative biomaterials are designed to facilitate cell-material interactions to guide the repair of damaged tissues and organs. These materials are designed to emulate the biophysical properties of native tissue, providing cellular phenotypic and morphological guidance that contributes to the restoration of the regenerative tissue niche. Collagen, a prevalent extracellular matrix protein, is a common component of these regenerative biomaterials due to its biocompatibility and other favorable properties. The current study describes a novel and straightforward method for the fabrication of engineered nanofibrillar collagen with directed fibril patterning. Through the manipulation of shear stress, temperature, and pH, collagen fibrillogenesis and alignment are precisely controlled without requiring specialized equipment. This approach allows for the creation of nanofibrillar collagen biomaterials that mimic the native structure of tissues exhibiting either anisotropic or isotropic characteristics. The flexibility in collagen nanofibril patterning not only facilitates the study of nanoscale patterning on cell behavior but also offers diverse possibilities for patterned tissue engineering applications.
Introduction
The goal of regenerative medicine is to enhance tissue regeneration and healing through the application of engineered therapeutics. One approach involves the fabrication of biomaterial constructs that closely emulate the composition and structure of native tissues. When designing these constructs, a critical consideration is the native underlying extracellular matrix (ECM), which provides instructional cues for cell proliferation, migration, and differentiation, ultimately driving tissue regeneration1,2. The native ECM is predominantly comprised of fibrillar collagen3,4. In highly organized tissues such as skeletal muscle and tendon, these ECMs exhibit an aligned patterning, which is key to supporting force transmission across the tissue as well as motor function5,6.
The utility of collagen as a biomaterial for tissue engineering applications extends beyond its extensive prevalence in native tissues, as it also displays good biocompatibility, biodegradability, and cell affinity7,8,9. Recent advancements in the development of collagen-based biomaterials have focused on the refinement of biophysical properties to better guide the mechanical and biochemical cues that are essential for specific tissue regeneration. Creating collagen biomaterials with a fibrillar structure in vitro is relatively simple, as acid-soluble collagen molecules are capable of self-assembling into nanofibrils when exposed to warm temperatures and neutral pH10,11. However, without guided manipulation, these fibrils will form into random networks, rendering them unsuitable for anisotropic tissue engineering applications. Techniques capable of producing aligned fibrils include electrospinning and wet-spinning, along with other solution extrusion techniques12,13, freeze drying14, electrochemical or magnetic fabrication15,16, shear flow deposition17, flow-induced crystallization18, gel-extrusion19, strain-induced alignment20, and force-guided alignment through the manipulation of fluid flow21,22. However, many of these methods are either not highly compatible with collagen, produce alignment on the micro-scale but not on a nanoscale, necessitate additional post-processing steps to induce alignment and stability or require highly specialized equipment and reagents, making their widespread adoption challenging.
The current study presents a facile shear-based method for the fabrication of nanofibrillar collagen, with aligned or randomly oriented fibril patterning, without the need for specialized equipment such as syringe pumps or electrospinners. This technique capitalizes on the pH dependency of collagen fibrillogenesis. Application of shear force to acidic monomeric collagen within a neutralizing buffer promotes aligned fibrillogenesis along the direction of shear. Similarly, collagen can be induced to undergo fibrillogenesis in the absence of shear, which produces fibrils with random patterns. The resulting 3D collagen strips, comprised of either aligned or randomly patterned nanofibrillar collagen, can serve as tools to study the effects of nanoscale patterning on cell behavior. Additionally, they offer diverse possibilities for both anisotropic and isotropic tissue engineering applications23,24,25,26,27. The patterning may be customized to match the structure of the native tissue of interest. The collagen strips may also be combined into various-sized bundles to serve as a transplanted engineered therapeutic in diverse sizes and shapes of injuries.
Protocol
1. Preparation of dialyzed collagen ( Figure 1)
- Cut dialysis tubing to a length of approximately 3 inches. Use tubing with the following specifications: 32 mm flat width, 20 mm inflated diameter, and 4.8 nm pore size.
- Rehydrate dialysis tubing in ultra-pure water.
- Clip one end of the tubing with a dialysis tubing clip.
- Transfer 5-6 mL of rat tail collagen-type I (concentration: 9-10 mg/mL in 0.02 N acetic acid) into the tubing using a 10 mL syringe equipped with an 18 G needle. Clip the other end of the tubing to close.
- Place a 0.5-1 cm thick layer of polyethylene glycol (PEG) flakes on the bottom of a glass dish, and then place the collagen-filled dialysis tubing on top of the PEG layer.
NOTE: A 13 inch x 9 inch x 2 inch glass dish can typically accommodate a row of 4-5 collagen filled tubes. Dish size can be adjusted based on the volume of collagen needed. - Add more PEG flakes to completely cover the tubing and place the tray into a 4 °C fridge.
- Every 10-15 min, remove the wet PEG flakes from the surface of the dialysis tubing and re-cover the tubing with a fresh layer of dry PEG. Return the dish to the 4 °C fridge.
- Repeat step 1.7 two more times, and after 30-35 min, rinse off the wet PEG flakes using tap water.
- Rinse the tubing once more using ultra-pure water and pat the tubing dry with a tissue.
- Unclip one end of the tubing and transfer the dialyzed collagen (final concentration: ~30 mg/mL) into microcentrifuge tubes. Briefly spin down air bubbles in the collagen using a mini-centrifuge (2,000 x g, ≤30 s, room temperature [RT]) and store at 4° C for later use.
- One day prior to use, load the collagen into syringes.
- Using a 16-gauge needle, draw up ~1 mL of dialyzed collagen into a 1 mL syringe.
NOTE: Collagen is a viscous substance. The needle head may need to be pre-loaded with collagen prior to drawing up more collagen. Remove the needle head and expel large air pockets as necessary - Remove the needle and wrap the syringe head with parafilm.
- Store the syringe filled with collagen upright at 4° C overnight to allow small air bubbles to pop.
- Using a 16-gauge needle, draw up ~1 mL of dialyzed collagen into a 1 mL syringe.
2. Preparation of glass chips ( Figure 1)
CAUTION: Utilize eye protection for this portion of the protocol.
NOTE: Specially coated hydrophobic glass slides are used for cell culture experiments. The slides must be handled by the edges to prevent the removal of the surface coating. Perform the glass-cutting procedure on a suitable cutting surface.
- Measure and mark out the desired dimensions of the glass chip using a permanent marker
- If creating 8-strip scaffolds, mark out approximately 1 cm increments along the long edge of the slide. Adjust dimensions as necessary based on the desired scaffold size.
- Using a straight edge (i.e., a 6 well plate edge), glide the glass cutter down the length of the first measurement to score the glass.
- The slide will either break along the scored line on its own or flip the slide over onto a tissue (hydrophobic side down) and manually apply pressure to the scored region.
- Mark the coated side of the hydrophobic glass slide using an ethanol marker pen.
- Rinse the glass chip in ultra-pure water to remove residual glass dust.
- Dry the glass chips either using a benchtop nozzle for pressurized air or by stacking them at an angle in the fume hood.
- Repeat as needed for the required number of chips. Store the chips at RT. For best cell culture results, use prepared chips within 7 days.
3. Preparation of 10x phosphate buffered saline (PBS)
- Fill a glass bottle with 500 mL of ultra-pure water.
- Add 10 PBS tablets to the bottle.
NOTE: This protocol assumes that the tablet ratio for 1x PBS is 1 tablet per 500 mL. Adjust the number of tablets as needed if using a different tablet size or brand. - Add a stir bar to the bottle and place on a stir plate until the tablets are dissolved.
- On the day of hydrogel fabrication, transfer the needed volume of 10x PBS to 50 mL conical tubes.
- Place the PBS-filled tubes in a 37 °C water bath for at least 15 min prior to collagen fabrication.
4. Fabrication of aligned collagen nanofibrils (Figure 2)
- Remove a syringe of collagen from the fridge and attach a blunt 22 G needle.
- Place a glass slide in the lid of a 4-well plate.
- Cover the slide with enough warmed 37 °C 10x PBS to submerge it (30-50 mL).
- Holding the syringe at approximately a 30-45° angle, manually extrude thin strips of collagen onto the glass chip using a velocity of ~340 mm/s and a flow rate of ~3.2 mL/min.
NOTE: Complete the collagen extrusion within 30-60 s of adding the warmed PBS to ensure the PBS does not cool excessively. - Wait for 1-2 min to allow the collagen to complete fibrillogenesis or until the collagen turns an opaque white color.
- Use forceps to cut the collagen strips to length. Ensure the strips are long enough so 3-5 mm can be folded under either end of the chip.
- One at a time, gently drape the strips of collagen lengthwise (parallel to scored region) over a prepared glass chip. Tuck the edges under the chip.
- Continue placing collagen strips until the desired dimensions are achieved.
- Position the newly formed collagen hydrogel across two wells of a 12-well plate. This enables airflow during the drying process and prevents the tail ends of the collagen from adhering to undesired surfaces.
- Leave hydrogels on the benchtop to dry for 1-3 h or until PBS salt crystals cover 50%-90% of the hydrogel surface.
NOTE: Drying time will vary depending on hydrogel size and the presence of excess liquid. - Submerge each hydrogel in 1x PBS for about 30-60 s or until salt crystals are dissolved.
- Gently dab off excess PBS with a tissue.
- Place hydrogels back on the well plate and leave to dry in a fume hood overnight.
- Store the dried hydrogels at RT for about 1 week.
5. Fabrication of random collagen nanofibrils (Figure 3)
- Remove a syringe of collagen from the fridge and attach a blunt 22 G needle.
- Extrude collagen at ~3.2 mL/min onto a dry glass chip. Extrude enough collagen to obtain similar dimensions to the aligned collagen hydrogels. Prevent air bubble accumulation as necessary.
- Extrude additional collagen to wrap around the edges of the glass chip. This minimizes the risk of accidental detachment.
- Use forceps to submerge the extruded collagen into a 50 mL tube of warmed 10x PBS.
- Wait for 45-60 s for the collagen to complete fibrillogenesis or until the collagen turns an opaque white color.
- Gently dab off excess PBS with a tissue.
- Complete steps 4.9-4.14 to rinse and dry the hydrogels
6. Sterilization and rehydration
NOTE: Complete all the following steps in a biosafety cabinet
- Sterilize the collagen hydrogels and surrounding well plate in 70% ethanol for 15 min.
- Briefly elevate the hydrogels halfway through sterilization using forceps to ensure that both the underside of the glass chip and the hydrogel are in contact with ethanol.
- Remove ethanol and allow hydrogels to air-dry for 10 min.
- Wash hydrogels 3 times in sterile 1x PBS, for 10 min each, to remove residual ethanol and to rehydrate the hydrogels. Hydrogels are now ready for use (cell culture, etc.). Hydrogels may be briefly left in PBS after the final wash step if not immediately ready for use.
Results
This protocol describes a straightforward shear-based extrusion technique for the fabrication of collagen hydrogels composed of either aligned (Figure 2) or randomly (Figure 3) oriented nanofibrils. Nanofibril patterning relies on the precise control of shear forces, which is achieved through the combined modulation of syringe speed and collagen extrusion rate. Optimal values for inducing fibril alignment have been previously determined to be a syringe velocity of 340 mm/s and a collagen extrusion rate of 3.2 mL/min28,29. This balance is critical not only for aligned nanofibril formation but also for ensuring the functionality of the hydrogels on the macroscale. If this ratio is misaligned, there is a risk of fabricating hydrogels that are not patterned as expected, as well hydrogels containing collagen strips that are too thin (<0.5 mm diameter), thick (>1.5 mm diameter), or wavy for use. For example, too fast of a syringe movement, coupled with too slow of an extrusion rate, results in thin strips, whereas the opposite ratio yields thick or wavy strips (Figure 4A-D), depending on the degree of imbalance in the ratio. These differences in macroscopic properties can create enough strip-to-strip variability to influence cell deposition and local adhesion.
Following collagen extrusion and assembly, hydrogel fabrication proceeds through an initial drying phase, often 1 to 3 h, before undergoing a wash step in 1x PBS (Figure 2 and Figure 3). Achieving optimal topographic outcomes relies on a delicate balance between the timing and intensity of the wash step. Insufficient or delayed washing results in disrupted fiber topography, denoted by the imprints of PBS salt crystals on the fibrils. In contrast, excessive or premature washing of collagen post-fabrication results in a dissolved layer of collagen that covers the topographical features (Figure 4E,F).
Scanning electron microscopy (SEM) confirms the presence of aligned or random nanofibril orientation within the collagen hydrogels. In both the aligned and random hydrogels, the nanofibrils exhibit fibril diameters between 30-50 nm29. Qualitatively, it is evident that aligned hydrogels contain uniaxially aligned nanofibrils (Figure 5). This alignment has been previously quantified using 2D fast Fourier transform analysis, in which the alignment plot displays two distinct peaks spaced 180° apart for aligned hydrogels, contrasting with randomly oriented hydrogels that only exhibit low-frequency peaks28.
Brightfield images of primary mouse skeletal muscle myoblasts illustrate that anisotropic nano-topographical guidance of collagen fibrils promotes cellular alignment (Figure 6). These nanopatterned collagen hydrogels have been shown to induce cellular alignment not only in myoblasts26 but also in other cell types, such as endothelial cells23,24.

Figure 1: Schematic illustration of ultra-high-density collagen and cut-glass slide preparation. (A-F) Process of collagen dialysis: (A) preparation of dialysis tubing into 3-inch strips and securing of the first clip, (B) loading of high-density stock collagen into tubing and securing the second clip, (C) encasing of the loaded dialysis tubing with polyethylene glycol (PEG) flakes, (D) removal of saturated PEG flakes, (E) rinsing off of PEG flakes with water, and (F) storage of ultra-high-density collagen into microcentrifuge tubes. (G-L) Process of cutting glass slides: (G) acquisition of glass slides for study, (H,I) scoring of glass slide with diamond tip glass cutter, (J) breaking of glass slide from unscored side, (K) rinsing of cut glass with water, and (L) drying of glass slide. Made using BioRender. Please click here to view a larger version of this figure.
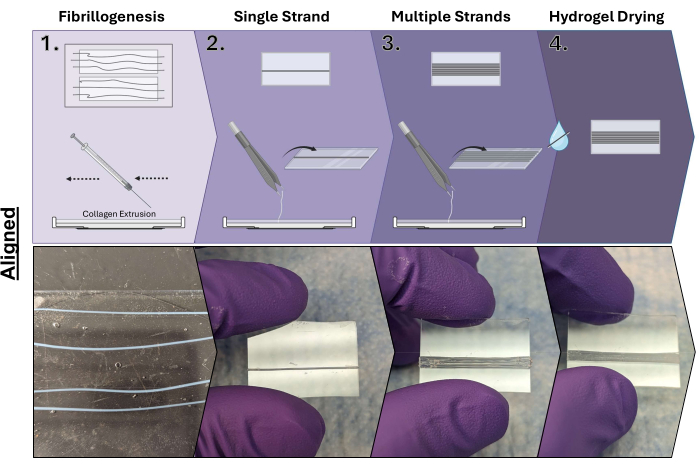
Figure 2: Aligned collagen hydrogel fabrication process. Step 1: Collagen extrusion under shear into the 10x PBS warmed buffer and induction of fibrillogenesis; Step 2: Mounting of extruded collagen on cut glass slides; Step 3: Mounting of additional extruded collagen to increase the size of the hydrogel; and Step 4: Drying of collagen hydrogels. Made using BioRender. Please click here to view a larger version of this figure.
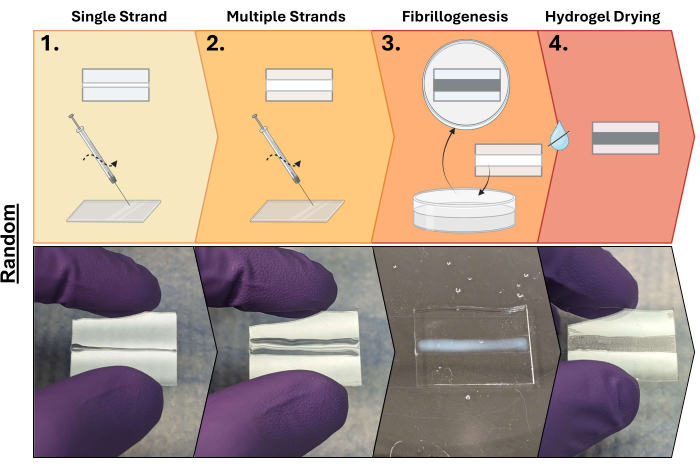
Figure 3: Random collagen hydrogel fabrication process. Step 1: Collagen extrusion onto cut glass slides (single strip); Step 2: Continued extrusion of collagen increases the size (multiple strips); Step 3: Submersion of extruded collagen in a 10X PBS buffer to initiate fibrillogenesis; and Step 4: Drying of hydrogels. Made using BioRender. Please click here to view a larger version of this figure.
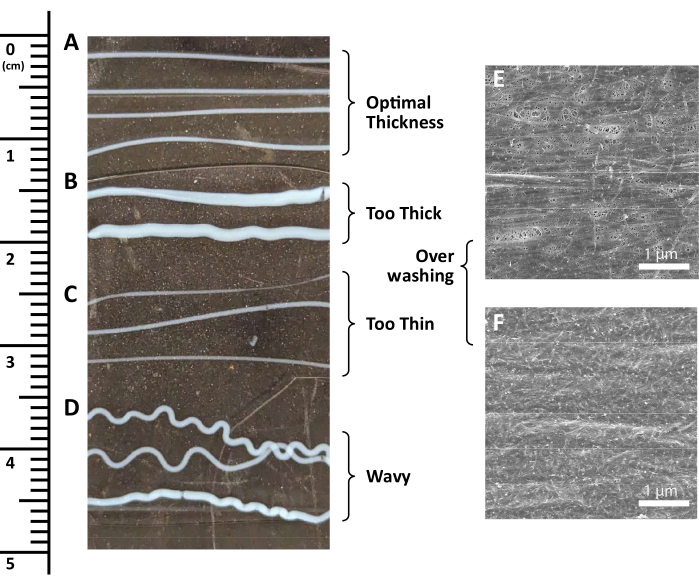
Figure 4: Possible error steps in the collagen hydrogel fabrication process. (A-F) Examples of (A) optimally thick collagen strips, (B) excessively thick collagen strips, (C) insufficiently thick collagen strips, (D) squiggly collagen strips, and (E,F) over-washed collagen surfaces shown using scanning electron microscopy. Scale bar = 1 µm. Please click here to view a larger version of this figure.
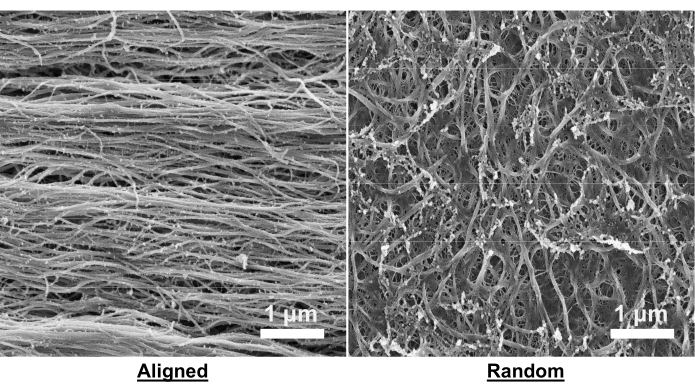
Figure 5: Scanning electron microscopy images of aligned and random collagen hydrogels. Images were taken using an electron microscope. The beam conditions were set to 2 keV, with a current range of 25-100 pA, and a working distance of 4-5 mm. Images were consistently saved at a resolution of 6144 x 4096 pixels with a dwell time of 1 µs. Scale bar = 1 µm. Please click here to view a larger version of this figure.
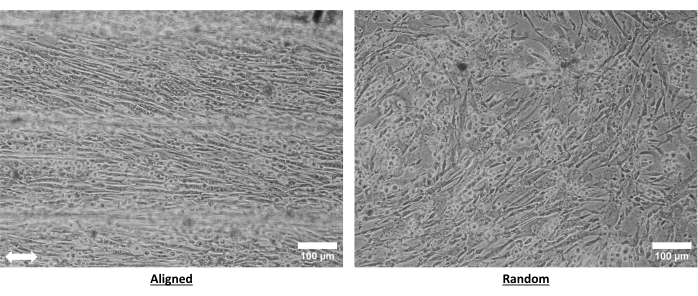
Figure 6: Primary mouse myoblasts cultured on aligned and random collagen hydrogels. Myoblasts were cultured on the hydrogels for 3 days prior to imaging. Bright field images were taken at 10x magnification using an inverted microscope. The arrow denotes aligned nanofibril orientation. Scale bar = 100 µm. Please click here to view a larger version of this figure.
Discussion
The critical steps of the protocol can be distilled into three main parts: 1) collagen fibrillogenesis, 2) hydrogel mounting, and 3) washing. The process of collagen fibrillogenesis occurs spontaneously under neutralizing conditions and relies on the self-assembly of individual collagen molecules into larger stabilized fibrillar structures10,11. This is achieved through the application of a neutralizing buffered medium like 10x PBS that is warmed to 37 °C and is critical for guiding the electrostatic interactions that ensure the complete development of collagen into fibrils. Nanoscale fibrillar alignment is achieved by the application of shear forces simultaneously with the occurrence of fibrillogenesis. The presence or absence of shear as well as the shear rate, will determine the patterning of the fibrils. Second, hydrogel mounting is a key step in reinforcing the stability of the material by providing physical anchors during the drying process, during which contraction and dehydration of the collagen take place. This step is especially critical if the intended use is for in vitro cell seeding, as it secures the collagen to a stiff substrate that prevents uncontrolled contraction. The mounting process is slightly different between the random and aligned patterned hydrogels. Proper adherence for random hydrogels requires additional collagen deposition along the edges of the mounting surface (typically glass) prior to fibrillogenesis to secure it to the surface. The hydrogels with aligned patterning are wrapped around the edges of the mounting surface to serve the same anchoring purpose. An important additional consideration for aligned hydrogels is that the initial placement of the collagen strip determines the hydrogel's shape and uniformity. Subsequent strip placement is guided by the surface tension of the deposited collagen, with care taken to avoid touching the hydrogel after placement. Lastly, proper hydrogel drying and washing post-mounting is vital to maintain the hydrogel's topographical features. Inadequate drying followed by washing with 1x PBS or water may lead to a dissolved or cake-like layer of collagen. To prevent this, the complete drying of the hydrogels is essential and can be visually confirmed by the formation of PBS crystals and an increase in collagen opacity.
The collagen hydrogels have been used for both in vitro studies and regenerative engineering applications in vivo23,24,25,26,27. It is important to note that the hydrogels are fabricated under non-sterile conditions and must be sterilized prior to any form of cell culture or transplantation. The recommended form of sterilization is 70% ethanol, as other forms of sterilization, like ultraviolet light, will disrupt the nanofibril patterning through crosslinking and degradation of the collagen molecules30. However, the removal of ethanol through evaporation and washing steps is important for the minimization of residual ethanol, which can be detrimental to cell compatibility. Batch-to-batch variability of the stock collagen may lead to variability in the working concentration of the dialyzed, high-density collagen, which may impact the fibrillogenesis process. Depending on the application, random isotropic patterning may be preferred over aligned anisotropic patterning; therefore, in the current protocol, we included both approaches to modulate the fibril organization.
A limitation of the shear-based extrusion method described in this protocol is that the process is highly manual and user-dependent. Significant training and practice are needed to calibrate each operator to yield reproducible results. This means that throughput can be low and time-consuming to ensure precision. Some of these limitations could be resolved using highly automated, machine-based fabrication methods such as 3D printing, electrospinning, and soft - and surface lithography. However, unlike these other methods, this entirely manual nanofibril fabrication procedure does not require specialized equipment and is accessible and affordable. As advancements in 3D printing methodologies and cost-effectiveness continue to improve, there is potential to adapt and integrate this protocol with 3D printing technology. Such integration, while not yet possible, would stand to enhance this protocol's consistency and throughput.
The fabrication of two distinct hydrogel topographies offers a unique opportunity to delve into the nano-topographical guidance of collagen fibrils on cellular morphology, behavior, and tissue regeneration. By observing the response to different fibril topographies, we can gain insights into how substrate nanoscale patterning influences cell phenotype. For example, previous work indicates that when endothelial cells adopt an elongated morphology atop aligned nanofibrils, they exhibit an anti-inflammatory phenotype and increased resistance to disturbed flow conditions24. These findings not only further our understanding of the development of atherosclerotic lesions but also hold promise for the design of future atheroprotective bypass grafts. These collagen hydrogels can be used for tissue engineering applications and enriched with growth factors or cells prior to implantation to enhance therapeutic efficacy25,26,27. Comparative analysis reveals that hydrogels with aligned topographies exhibit superior muscle regeneration and vascularization compared to hydrogels with random topographies26 and that these hydrogels can be used in an acellular "off the shelf" form if laden with myogenic growth factors to enhance tissue regeneration in a mouse injury model27. While the primary focus thus far has been using these hydrogels as potential therapeutics for muscle injuries, they could be applied to a broad spectrum of regenerative and tissue engineering applications.
Disclosures
There are no conflicts of interest to declare.
Acknowledgements
This research was supported in part by funding from the Alliance for Regenerative Rehabilitation Research & Training, MTF Biologics, the Oregon Health & Science University Foundation, and the Collins Medical Trust. K.M.H. was supported by the National Science Foundation Graduate Research Fellowship (DGE-1937961) and the Oregon Students Learn and Experience Research (OSLER) Fellowship (5TL1TR2371-8). K.H.N. was supported by grants from the NIH/NHLBI (R00HL136701) and NIH/NIAMS (R01AR080150).
Materials
| Name | Company | Catalog Number | Comments |
| Collagen I, High Concentration, Rat Tail | Corning | CB354249 | |
| Eclipse TE-2000-U microscope | Nikon | TE-2000-U | Inverted microscope |
| Extra Thick Microscope Slides | Fisher Scientific | 22-267-005 | Works well for collagen extrusion surface |
| FEI Helios G3 NanoLab DualBeam | Thermo Fisher Scientific | N/A | Scanning electron microscope |
| Glass Cutter Tool Set 2mm-20mm Pencil Style Oil Feed Carbide Tip | Amazon/MOARMOR | B07Y1D243H | Option for a glass cutter |
| Hamilton Kel-F Hub Blunt Point Needles (Luer Lock, 22 G) | Fisher Scientific | 14-815-574 | Needles for collagen extrusion |
| Nexterion Slide H 3-D | Schott | NC0782819 | Hydrophobic slides |
| PBS Tablets | Thermo Fisher Scientific | 18912014 | |
| Polyethylene Glycol 8000 | Fisher BioReagents | BP233-1 | |
| Seamless Cellulose Dialysis Tubing | Fisher Scientific | S25645G | |
| SnakeSkin Dialysis Clips | Thermo Fisher Scientific | PI68011 | |
| Synthware Glass Cutter | Synthware | 31-501-927 | Option for a glass cutter |
References
- Padhi, A., Nain, A. S. ECM in differentiation: A review of matrix structure, composition and mechanical properties. Ann Biomed Eng. 48 (3), 1071-1089 (2020).
- Schlie-Wolter, S., Ngezahayo, A., Chichkov, B. N. The selective role of ECM components on cell adhesion, morphology, proliferation and communication in vitro. Exp Cell Res. 319 (10), 1553-1561 (2013).
- Ricard-Blum, S. The collagen family. Cold Spring Harb Perspect Biol. 3 (1), a004978 (2011).
- Shoulders, M. D., Raines, R. T. Collagen structure and stability. Annu Rev Biochem. 78, 929-958 (2009).
- Gillies, A. R., Lieber, R. L. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 44 (3), 318-331 (2011).
- Silver, F. H., Freeman, J. W., Seehra, G. P. Collagen self-assembly and the development of tendon mechanical properties. J Biomech. 36 (10), 1529-1553 (2003).
- Friess, W. Collagen-biomaterial for drug delivery. Eur J Pharm Biopharm. 45 (2), 113-136 (1998).
- Lee, C. H., Singla, A., Lee, Y. Biomedical applications of collagen. Int J Pharm. 221 (1-2), 1-22 (2001).
- Liu, X., Zheng, C., Luo, X., Wang, X., Jiang, H. Recent advances of collagen-based biomaterials: Multi-hierarchical structure, modification and biomedical applications. Mater Sci Eng C. 99, 1509-1522 (2019).
- Harris, J. R., Reiber, A. Influence of saline and pH on collagen type I fibrillogenesis in vitro: Fibril polymorphism and colloidal gold labelling. Micron. 38 (5), 513-521 (2007).
- Williams, B. R., Gelman, R. A., Poppke, D. C., Piez, K. A. Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J Biol Chem. 253 (18), 6578-6585 (1978).
- Zhong, S., et al. An aligned nanofibrous collagen scaffold by electrospinning and its effects on in vitro fibroblast culture. J Biomed Mater Res A. 79A (3), 456-463 (2006).
- Malladi, S., et al. Continuous formation of ultrathin, strong collagen sheets with tunable anisotropy and compaction. ACS Biomater Sci Eng. 6 (7), 4236-4246 (2020).
- Lowe, C. J., Reucroft, I. M., Grota, M. C., Shreiber, D. I. Production of highly aligned collagen scaffolds by freeze-drying of self-assembled, fibrillar collagen gels. ACS Biomater Sci Eng. 2 (4), 643-651 (2016).
- Torbet, J., Ronzière, M. C. Magnetic alignment of collagen during self-assembly. Biochem J. 219 (3), 1057-1059 (1984).
- Provenzano, P. P., Eliceiri, K. W., Inman, D. R., Keely, P. J. Engineering three-dimensional collagen matrices to provide contact guidance during 3d cell migration. Curr Protoc Cell Biol. Chpater 10 (Unit 10.17), (2010).
- Saeidi, N., Sander, E. A., Zareian, R., Ruberti, J. W. Production of highly aligned collagen lamellae by combining shear force and thin film confinement. Acta Biomater. 7 (6), 2437-2447 (2011).
- Paten, J. A., et al. Flow-induced crystallization of collagen: A potentially critical mechanism in early tissue formation. ACS Nano. 10 (5), 5027-5040 (2016).
- Yunoki, S., Hatayama, H., Ebisawa, M., Kondo, E., Yasuda, K. A novel method for continuous formation of cord-like collagen gels to fabricate durable fibers in which collagen fibrils are longitudinally aligned. J Biomed Mater Res B Appl Biomater. 107 (4), 1011-1023 (2019).
- Vader, D., Kabla, A., Weitz, D., Mahadevan, L. Strain-induced alignment in collagen gels. PLoS One. 4 (6), e5902 (2009).
- Ahmed, A., et al. Local extensional flows promote long-range fiber alignment in 3d collagen hydrogels. Biofabrication. 14 (3), (2022).
- Su, C. Y., et al. Engineering a 3D collective cancer invasion model with control over collagen fiber alignment. Biomaterials. 275, 120922 (2021).
- Nakayama, K. H., et al. Bilayered vascular graft derived from human induced pluripotent stem cells with biomimetic structure and function. Regen Med. 10 (6), 745-755 (2015).
- Nakayama, K. H., et al. Nanoscale patterning of extracellular matrix alters endothelial function under shear stress. Nano Lett. 16 (1), 410-419 (2016).
- Nakayama, K. H., et al. Rehabilitative exercise and spatially patterned nanofibrillar scaffolds enhance vascularization and innervation following volumetric muscle loss. NPJ Regen Med. 3, 16 (2018).
- Nakayama, K. H., et al. Treatment of volumetric muscle loss in mice using nanofibrillar scaffolds enhances vascular organization and integration. Commun Biol. 2 (1), 170 (2019).
- Alcazar, C. A., Hu, C., Rando, T. A., Huang, N. F., Nakayama, K. H. Transplantation of insulin-like growth factor-1 laden scaffolds combined with exercise promotes neuroregeneration and angiogenesis in a preclinical muscle injury model. Biomater Sci. 8 (19), 5376-5389 (2020).
- Lai, E. S., Huang, N. F., Cooke, J. P., Fuller, G. G. Aligned nanofibrillar collagen regulates endothelial organization and migration. Regen Med. 7 (5), 649-661 (2012).
- Huang, N. F., et al. Spatial patterning of endothelium modulates cell morphology, adhesiveness and transcriptional signature. Biomaterials. 34 (12), 2928-2937 (2013).
- Weadock, K. S., Miller, E. J., Bellincampi, L. D., Zawadsky, J. P., Dunn, M. G. Physical crosslinking of collagen fibers: Comparison of ultraviolet irradiation and dehydrothermal treatment. J Biomed Mater Res. 29 (11), 1373-1379 (1995).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved