Method Article
A Fluorescent Intravital Imaging Approach to Study Load-Induced Calcium Signaling Dynamics in Mouse Osteocytes
In This Article
Summary
The current article describes performing an intravital imaging approach to observe mechanically induced calcium signaling of embedded osteocytes in vivo in real-time in response to tissue-level mechanical loading of the mouse third metatarsal.
Abstract
Bone tissue is exquisitely sensitive to differences in mechanical load magnitude. Osteocytes, dendritic cells that form a syncytium throughout the bone, are responsible for the mechanosensory function of bone tissue. Studies employing histology, mathematical modeling, cell culture, and ex vivo bone organ cultures have greatly advanced the understanding of osteocyte mechanobiology. However, the fundamental question of how osteocytes respond to and encode mechanical information at the molecular level in vivo is not well understood. Intracellular calcium concentration fluctuations in osteocytes offer a useful target for learning more about acute bone mechanotransduction mechanisms. Here, we report a method for studying osteocyte mechanobiology in vivo, combining a mouse strain with a fluorescently genetically encoded calcium indicator expressed in osteocytes with an in vivo loading and imaging system to directly detect osteocyte calcium levels during loading. This is achieved with a three-point bending device that can deliver well-defined mechanical loads to the third metatarsal of living mice while simultaneously monitoring fluorescently indicated calcium responses of osteocytes using two-photon microscopy. This technique allows for direct in vivo observation of osteocyte calcium signaling events in response to whole bone loading and is useful in the endeavor to reveal mechanisms in osteocyte mechanobiology.
Introduction
The bone matrix is organized according to mechanical demand1,2 and can change dynamically to account for shifting mechanical requirements3,4,5. Seminal work regarding the mechanosensory mechanism in bone was published as a modeling paper about 30 years ago6,7,8, where it was proposed that osteocytes embedded within the bone sense tissue level mechanical deformation via fluid movement in their local environment. This model was validated experimentally with in vitro and ex vivo experiments, where it was clearly shown that osteocytes are quite mechanosensitive1,2,3,4,5,6 and also express cytokines, which direct the behavior of bone building osteoblasts7 and osteoclasts8,9,10,11,12.
Calcium signaling is a ubiquitous second messenger that has been established as a central figure and dependable experimental target in osteocyte mechanobiology13,14,15,16. Calcium signaling has the advantage of being widely studied in cell biology17, which means there is a lot known about its downstream effects and the corresponding fluorescent tools available for experimental observation. In vitro analyses have used calcium signaling as a means of identifying osteocyte mechanical activation and characterizing dynamic signaling behavior5,18,19. Of note, observation of calcium signaling in an osteocyte cell line provided definitive evidence that fluid activation at the integrin attachments along the cell process is likely the main form of fluid flow activation20. This study is one of the many that showcase the utility of calcium signaling. It is reliably induced with the mechanical activation of osteocytes and thus serves as a potent target for interrogating osteocyte mechanobiology.
Osteocyte mechanotransduction in vivo is highly dependent on their immediate microenvironment. Matrix-binding proteins (e.g., proteoglycans, integrins) provide functional attachments between osteocyte cell membranes in an arrangement that is critical for fluid activation of the cell21,22. While two-dimensional (2D) in vitro analyses are helpful, they are limited in that they do not incorporate these critical three-dimensional (3D) features. Ex vivo preparations of osteocyte calcium signaling using confocal microscopy have shed some light on how osteocytes respond while keeping the surrounding matrix intact13,16. However, removing the blood supply from bone is thought to change the nutrients and fluid dynamics of the lacunocanalicular system. Interrogation of osteocyte mechanobiology requires in vivo investigation of load-induced osteocyte signaling.
In vivo investigation of embedded cells in bone has been largely hindered by the limitations of traditional imaging techniques, such as bright field and confocal microscopy. Osteocytes are positioned in a mineralized matrix23 composed of hydroxyapatite, collagen type 1, and other non-collagenous proteins that limit the optical accessibility24 and make the in vivo investigation technically challenging. However, recent advancements in non-linear fluorescent imaging and genetically encoded calcium reporters present the opportunity to overcome these challenges.
Here, we report a novel approach with the ability to image osteocytes in vivo to measure calcium signaling with cellular level resolution25. This is achieved by using a dynamic fluorescent marker using a genetically encoded calcium indicator for the first time in mouse osteocytes. Mice with DMP1-Cre driven osteocyte-targeted expression of GCaMP6f exhibit visible fluorescence in osteocytes throughout the diaphyseal cortex of mice metatarsals17,26. We use a three-point bending system in order to apply physiological levels of strain magnitude between 250-2,000 με27. This technique allows for in vivo visualization of osteocyte calcium signaling dynamics during mechanical loading of the whole bone and could be useful to anyone seeking to understand osteocyte mechanobiology. This method would be appropriate for researchers seeking to expand their work from in vitro analyses to in vivo applications, especially while retaining functional-based molecular analyses (i.e., investigating cellular activity via calcium signaling) as opposed to broad-based phenotyping studies.
Protocol
All methods have been approved by the Institutional Animal Care and Use Committee (IACUC) at Cornell University.
1. Preparation of materials and equipment
- Design and build the metatarsal loading device for use with an overhead multiphoton microscope.
NOTE: Details for component specifications and device operation are described in the supplementary documentation of a previous study25. Briefly, the major components consist of an actuator in series with a load cell and loading bracket, a water bath, and a fulcrum pin. The actuator and load cell can be obtained commercially and should be capable of accommodating loads up to 300 g. The water bath, fulcrum pin, and load bracket should be constructed of titanium or stainless steel. They can be fabricated in-house or commercially via 3D metal printing. - Calibrate the strain magnitude values to the actuator displacement in advance for individual mouse groups (i.e., sex, treatment, genotype). This is done using surface strain mapping in a procedure outlined in a previous study25.
- Use mice with C57BL/6 background, aged 16-18 weeks, that express fluorescent calcium indicator constructs in the osteocytes. Osteocyte targeted expression is achieved by crossing flox-activated GCaMP6f and Dmp1-Cre mice. The details of breeding strategies and vendors for founder mice are described in a previously published study25.
- Sterilize all surgical tools, load bracket, water bath and fulcrum pin using an autoclave (57.2 °C, 15 psi, 3-4 min). Disinfect microscope objectives with 100% ethanol.
- Turn on electronic components, including laser source, microscope, load cell, and actuator. Allow 15-20 min for each component to warm up prior to intravital imaging.
- Make sure the actuator and load cell components are connected and activated in the control device. Generate the control software with a variety of platforms, including Matlab, LabView, and Python, according to the preferences of the user. The main Matlab code used in this study is called Actuator_Control_Software_withLoadCell.m (inside the MT3 Loading Device Control Software.zip folder Supplementary File 1).
NOTE: Most commercially available two-photon microscope systems come with robust imaging software. The key features required for this method are the ability to tune a laser to the appropriate excitation for GCaMP6f (920 nm), the ability to adjust laser power intensity, and a time series collection with a frequency of at least 6 Hz. Prior to experimentation, laser connection to the imaging software and appropriate light path arrangement for two-photon imaging should be confirmed. This is confirmed in different ways depending on the imaging set up. Usually, there is a light indicator.
- Make sure the actuator and load cell components are connected and activated in the control device. Generate the control software with a variety of platforms, including Matlab, LabView, and Python, according to the preferences of the user. The main Matlab code used in this study is called Actuator_Control_Software_withLoadCell.m (inside the MT3 Loading Device Control Software.zip folder Supplementary File 1).
2. Surgery
- Prepare a surgical stage and sterilize the surface with 70% ethanol. Ensure that aseptic techniques are employed throughout the surgical procedures. Maintain the level of cleanliness required for this protocol, as it is a terminal procedure.
- Anesthetize the mouse with vaporized isoflurane mixed with medical-grade air or oxygen at a flowrate of 1 L/min. Perform initial anesthetization in a rodent induction chamber with 3%-5% isoflurane. Maintain anesthesia at 1%-2.5% isoflurane in a nose cone for surgical and intravital imaging procedures.
- Place a heating device underneath or beside the mouse to assist in maintaining homeostatic core body temperature. Monitor the mouse's body temperature, heart rate, and oxygen saturation throughout the surgery. Keep the breath rate between 60-80 breaths per minute and maintain it by adjusting the isoflurane dosage.
- Place the mouse in a supine position. Confirm the depth of anesthesia before the incision via a toe pinch. Identify the location between the second and third metatarsals. Make a scalpel incision through the dermis between the second and third metatarsals, starting at the distal end and progressing proximally along the length of the bones (~5 mm).
NOTE: The metatarsals are distal to the ankle bones on the paw and follow directly proximal to the distal phalanges. They consist of (medial to lateral) metatarsals I, II, III, IV, and V. The third metatarsal is centered in the middle of the volar aspect of the hind paw. - Using forceps, open the surgical site by pulling the skin toward the medial/lateral edges. Resect the extensor tendons using straight Vannas scissors to expose the third metatarsal.
- Insert the center fulcrum pin of the metatarsal loading device beneath the third metatarsal.
- Initially, insert the pin in the small space at the distal end where the third metatarsal meets the proximal phalange and then move the pin proximally until it rests at the mid-shaft of the third metatarsal.
- Take special care to guarantee a direct interface between the bone and the pin, excluding any muscle and facia. This is critical for stable imaging during loading protocols.
- Hold the ankle with forceps and place the foot into theDulbecco's phosphate-buffered saline (DPBS) filled water bath (8-10 mL). Secure the paw in place with the loading bracket and connect the bracket to the actuator/load cell arrangement. A completed surgical preparation and arrangement within the device is shown in Figure 1.
3. Imaging
NOTE: The complete two-photon microscopy setup used in this protocol has two software packages: Chameleon Discovery GUI V2.0.6 for the laser (Figure 2) and ThorImageLS4.0 for the microscope (Figure 3). To image on the two-photon, press Live (Figure 3, blue play button). When conducting an experiment to image and save data, press Capture (Figure 3, the tab at the top, next to Capture Setup). These are specific to the equipment and the vendors used in this experiment, but many alternatives with the necessary specifications found in this protocol (excitation wavelengths, viewable window size, image capture rate, etc.) would also work.
- Very carefully pick up the loading device setup with the mouse and place it onto the microscope platform.
- Start with a low magnification objective in epifluorescence mode (Figure 3, dropdown menu at the top with selections Multiphoton or Fluorescence Setup) to locate and center the mid-diaphysis of the bone.
- After a region of interest is located, switch to a higher water immersion objective magnification (20x-40x) and switch the optics to two-photon (Figure 3, dropdown menu at the top with selections Multiphoton or Fluorescence Setup).
NOTE: The recommended field of view size is at least 250 μm x 250 μm for cellular activity and can be achieved with either optical or digital zoom depending on the imaging system.- The suitable minimum pixel density is 512 pixels x 512 pixels. To set the pixel density, use the Galvo/Resonance Area Control settings (Figure 3).
- When finding the ROI, avoid photobleaching by reducing the power using the Power Control option (Figure 3,) and increasing the PMT amplification in the Galvo/Resonance Scanner Control settings (Figure 3).
- Identify the surface of the bone in two-photon mode by looking for the fibrous periosteum autofluorescence in the green channel that uses a bandpass filter centered at 525 nm.
NOTE: The autofluorescence is most visible with the excitation wavelength at 1,000 nm but is also available at 920 nm excitation (Figure 3, Multiphoton Laser Control). This landmark is important for determining the relative depth of the ROI into the metatarsal cortex when observing osteocytes. It should be noted that during time series experiments, the exact depth of the plane imaged is not recorded, although it tends to range between 15-30 µm. If exact depth measurements are desired, then a z-stack would have to be taken.
- Run a test loading bout to ensure the surgical preparation and positioning within the loading device were effective at achieving a stable ROI field (i.e., no z-plane displacement). Briefly, run a loading cycle to appraise the stability of the surgical preparation. Perform loading by running the section Switch on Servo and Move in the Matlab code.
- Apply a small tare load. Ensure that the appropriate haversine wave loading protocols are prepared ahead of time. Input appropriate loading parameters obtained during calibration in section 1.2. Perform loading by running the section Switch on Servo and Move in the Matlab code.
- If there is a visible movement of the cells on-screen, which comes from micromotion of the bone in response to loading or breathing, select a different ROI along y- or z- directions to ensure it is directly over the center fulcrum pin. If movement persists, adjust the surgical position of the pin manually underneath the third metatarsal.
NOTE: A good region of interest (ROI) is one where the cells do not move in and out of the plane/focus. Movement in the x- and y-directions can be adjusted for post-processing using the template matching plugin on ImageJ.
- Conduct z-stack or t-series imaging with loading according to the experimental design to record morphometric or dynamic load-induced signaling events, respectively.
- Set the excitation wavelength for the fluorescent indicator (e.g., 920 nm for GCaMP6f). For z-stack, use a low enough resolution to capture features of the osteocytes, such as 0.25-1 μm steps. For t-series, use settings to sample at least 6x the loading rate. For a 1 Hz loading rate, imaging at 6 frames per second (FPS) represents the minimally appropriate capture rate (Figure 3, Galvo/Resonance Scanner Control).
- For loading and live imaging, collect non-loaded baseline data with each bout. In this study, 60 s of non-loaded imaging followed by 60 s of loading and imaging is preferred. Design the loading and imaging protocols accordingly. To collect data, press Capture (Figure 3, tab at the top, next to Capture Setup).
- The refractory period for a full calcium signaling response in bone cells is estimated to be approximately 15 min28. Wait at least that long between sequential loading bouts when collecting dynamic signaling data.
- Throughout the entire experiment, do not leave the animal unattended to ensure that it does not unexpectedly wake from anesthesia or experience distress without the opportunity for intervention.
NOTE: Euthanasia at the end of the procedure should be performed via cervical dislocation or other appropriate measures in accordance with IACUC-approved methods. This is a non-survival protocol meaning that all animals are euthanized after imaging.
Results
Here we report a methodology for studying calcium signaling in embedded osteocytes in vivo using acute surgical preparation and multiphoton fluorescent imaging. Green fluorescent signal can be observed from genetically encoded calcium indicators in osteocytes via DMP1-Cre expression. Figure 4 is a single-plane image of embedded osteocytes. Note that all cells are labeled and express baseline green fluorescence, making it easy to find candidates for experimental observation. Cell bodies are clearly visible, and occasionally cell processes can be made out. In our experience, bone lining cells do not express the GCaMP6f construct driven by DMP1-Cre expression. Osteocytes are the only embedded cell in the bone matrix, which makes them easy to identify and differentiate from any other cells that might express DMP1 (e.g., muscle cells).
This system can also be used for the 3D analysis of embedded cell morphology. Z-stack imaging is readily available on multiphoton systems, and stacks of up to 100 μm can be achieved in fully calcified bone tissue with two-photon microscopy. Figure 5 shows a representative 40 μm volume, which can be routinely achieved.
Cyclic loading can be applied using a custom design loading apparatus, providing the opportunity to observe dynamic load-induced calcium signaling events in real-time. It should be noted that image capture frequency will vary depending on the capabilities of the two-photon microscope. Figure 6 is an example data set from a single mechanically activated osteocyte loaded in bone experiencing 1,000 μs cyclically at 1 Hz, representing physiological loading conditions. The y-axis shows the change in fluorescence intensity normalized to an individual cell's non-loaded baseline fluorescence. In responding osteocytes, fluorescence intensity increases in line with loading, with little variation in the peak intensity changes (Figure 6). Fluorescence intensity changes can be quantified for individual osteocytes for each frame in the t-series using the ROI manager on ImageJ (NIH). We recommend using Matlab (Metatarsal_Loading_Intensity_Data_Analysis.m within Intensity Data analysis.zip [Supplementary File 2]) or other computing software to analyze data. We use a threshold of 3x standard deviations from the mean value of background intensity to validate cell ROIs as the real signal. Intensity data is linearly detrended to account for photobleaching during imaging, and a 2.5 Hz lowpass filter is applied. Cell ROIs are classified as "responsive" or "nonresponsive" based on whether their fluorescence intensity changes more than 25% over the baseline intensity computed by the cell. Responsive cell mean intensity and maximum intensity during loading are then evaluated. Linear regression analysis is performed for both the number of responding cells and the fluorescent intensity against strain magnitude, assuming that data was taken for multiple strain magnitudes from 250-2,000 με (p < 0.05 for significant differences in regression parameters when comparing two or more mouse groups, n = 6 mice/group, m = 15-50 cells/mouse). Regression analysis can be done in R, Microsoft Excel, or any other preferred statistical software. One-way and two-way ANOVAs can also be used to determine intragroup and complex intergroup statistics.

Figure 1: A surgically exposed metatarsal positioned in the loading device apparatus. The third metatarsal is placed between the center fulcrum pin at the mid-diaphysis and the actuating loading bracket at the proximal and distal ends. A DPBS water bath maintains homeostatic osmotic conditions. Please click here to view a larger version of this figure.
Figure 2: Screenshot of the software to control the Coherent laser. When both lights are green, the laser is ready to use. This protocol uses the tunable laser, so make sure to set the box "Tunable AOM Output" to external control "ext" so the ThorImage LS can control it. Please click here to view a larger version of this figure.

Figure 3: Imaging software. Screenshot of the capture setup for the software to control the two-photon microscope. Please click here to view a larger version of this figure.
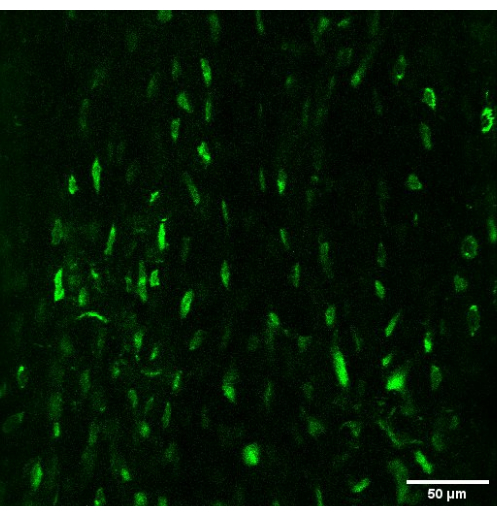
Figure 4: Fluorescent GCaMP6f in osteocytes in vivo. A single plane two-photon image of metatarsal osteocytes expressing GCaMP6f in vivo (20x magnification, 2x digital zoom). Endogenous expression of calcium reporter constructs provides a fluorescent target in vivo without the need for incubation. Please click here to view a larger version of this figure.
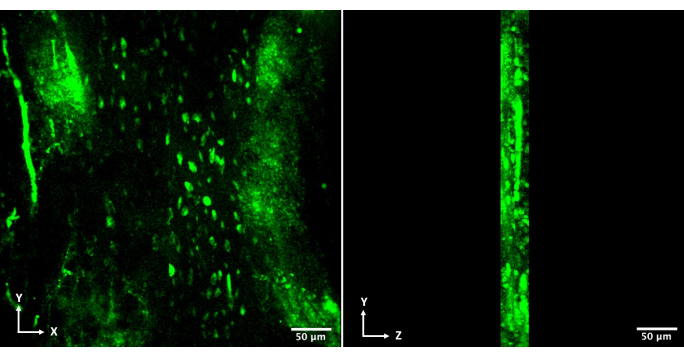
Figure 5: 3D reconstruction of volumetric acquisition with fluorescent GCaMP6f osteocytes. 3D z-projection of osteocytes in vivo, captured with two-photon microscopy (20x magnification in water-immersion objective, 920 nm excitation, 520 ± 20 nm bandpass emission). The left pane shows the transverse cut in the XY plane, and the right pane shows a sagittal cut to show the depth of the Z-stack. Please click here to view a larger version of this figure.
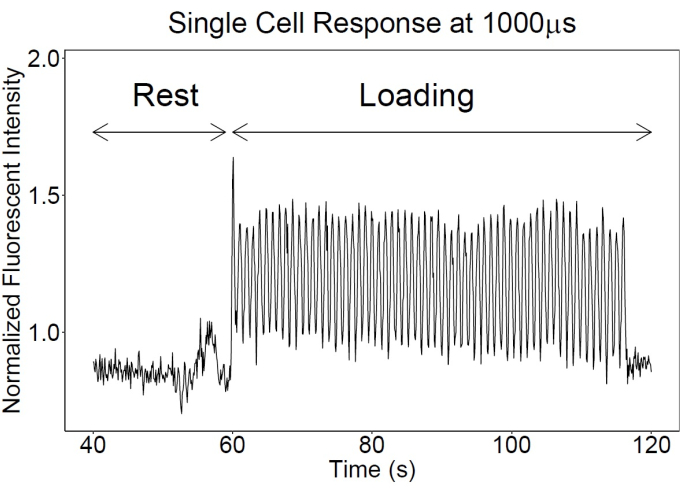
Figure 6: Osteocyte fluorescent calcium signaling responses. An example time course of GCaMP6f fluorescent signal from an embedded osteocyte in vivo. Note that, at rest, low-level noisy fluctuations are exhibited. With loading, the calcium fluctuations increase in magnitude and regularity, often occurring in step with applied loading. Please click here to view a larger version of this figure.
Supplementary File 1: MT3 Loading Device Control Software.zip. This zip folder contains the Matlab code to control the load cell and actuator for the loading device. The main code that runs everything is named Actuator_Control_Software_withLoadCell.m. Please click here to download this File.
Supplementary File 2: Intensity Data Analysis.zip. This zip folder contains the Matlab code and practice example to run the intensity data analysis. Please click here to download this File.
Discussion
It is difficult to study osteocytes experimentally because of their embedded position in the hard tissue matrix, to which they rapidly deteriorate or dedifferentiate upon removal of this niche. Researching osteocytes has required immense ingenuity over the last 3 decades, such as the creation of osteocyte-like lines29,30,31, the establishment of protocols to isolate primary osteocytes32,33, and even the efforts to load dissected bones shortly after euthanasia to visualize the fluorescent responses from potentially waning osteocytes16. Numerous works have already established osteocytes' mechanosensitive behavior via calcium signaling13,14,15,16.
The method described here has achieved the goal of imaging real-time live osteocyte calcium signaling dynamics in a living mouse during mechanical loading.This protocol is capable of imaging osteocytes intravitally and during active loading and movement of the bone.
The use of targeted DMP1-Cre static fluorescent markers has been used in osteocytes before34. However, it was only recently used to drive the expression of a genetically encoded calcium indicator25. GCaMP (e.g., GCaMP3, GCaMP6) is commonly used in other fields, such as neuroscience, to study calcium signaling in nerves17. Given that it is well established that calcium signaling is the key to osteocytes' mechanosensitive behavior, translating this technology to bone is vital. GCaMP is a complex of green fluorescent protein (GFP), calmodulin, and M13 skeletal muscle myosin light chain kinase. The binding of calcium causes calmodulin to phosphorylate M13, releasing energy in the form of fluorescence. This single fluorophore genetically encoded calcium indicator offers faster kinetics and easier imaging protocols compared to FRET sensors35,36. One limitation of using GCaMP in vivo is the inability to calibrate fluorescence intensity to absolute calcium concentration. In in vitro preparations, calibration of sensors is performed using tightly controlled calcium concentrations in culture media or patch clamping to correlate calcium concentration with changes in fluorescence intensity. These sort of approaches are not possible for in vivo studies. Our lab has also noticed differences in homozygous versus heterozygous GCaMP mice, and for that reason, we recommend consistency within a single study.
There are several considerations to executing this protocol properly. The surgical placement of loading apparatuses is critical for obtaining a stable viewing plane and eliminating motion artifacts. Moreover, the surgery must be done with special attention to keeping the periosteum intact. Disruption of the periosteum may induce acute inflammatory events, which may lead to changes in load-induced osteocyte calcium signaling behavior, creating a confound in functional experiments. Occasionally, ruptured blood vessels can occlude the objective's field of view. Redirecting blood vessels with fine-pointed forceps into the water bath is a reliable solution for such an issue. Finally, this is a terminal procedure limited to about 4-5 h. As such, experiments attempting to image over multiple days or weeks to track bone remodeling events are currently not possible.
Ongoing development of this approach includes the use of multiple markers with different fluorescence emission properties to label both structural and functional activity. This protocol can be combined with any number of fluorescent markers, limited only by the present state of the art in fluorescent markers. This would drastically expand the ability to characterize load-induced responses among osteocytes in a single ROI of the bone. Future developments will also include acquiring volumetric data of dynamic responses, a capability presently limited to single-plane acquisition. The option to better understand 3D signaling patterns among neighboring osteocytes will be available with capability.
Overall, this is an incredibly important milestone in the field that serves to interrogate osteocytes in their native environment with their chief role as resident tissue mechanosensors, turning tissue-level strains into a diverse range of biological signals.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| #3 Handle scalpel | Electron Microscopy Sciences | 72040-03 | Surgical supplies |
| 20x Water immersion objective | Olympus | XLUMPLFLN20XW | This is a 20x objective, but 40x can also be used for this protocol. We recommend water immersions because it needs to be dipped in the bath during imaging, so open-air objective may cause abberrations |
| A.M. Bickford Omnicon F/AIR | AM Bickford | 80120 | A sensible answer to anesthesia gas problems in the operating room, the F/AIR anesthesia gas filter was specifically designed to remove waste anesthesia gases such as Isoflurane, Halothane, Enflurane, etc. from the operating room environment. |
| A.M. Bickford Omnicon F/AIR Kit | AM Bickford | 80000 | An entire kit with tube and adaptors to connect F/AIR to setup |
| B6J.Cg-Gt(ROSA)26Sortm95.1(CAG-GCaMP6f)Hze/MwarJ | The Jackson Laboratory | 28865 | GCaMP6f Mice for cross-breeding with Dmp1-Cre mice |
| B6N.FVB-Tg(Dmp1-cre)1Jqfe/BwdJ | The Jackson Laboratory | 23047 | Dmp1-Cre Mice for cross-breeding with GCaMP6f mice |
| Compression load cell | FUTEK Advanced Sensor Technology, Inc. | 905898 | LCM100 , 1000 g , Sub-miniature tension & compression load cell (Miniature/Inline Threaded) , RoHS lead free, Material - 17-4 PH S.S. , M3x0.5-thread , 34 Awg 4 conductor braided polyester cable , 5 ft Long |
| Corning 500 mL DPBS (Dulbecco's phosphate buffered saline), 1x [+] calcium, magnesium | VWR International | 21-030-CV | Ionically balanced bath that contains calcium submerges the metatarsal during imaging |
| Dental pick tool | Electron Microscopy Sciences | Surgical supplies | |
| Ethyl alcohol pure 200 proof ACS reagent >99.5% | Sigma Aldrich | SIAL-459844-500ML | Sterilization purposes |
| Fulcrum pin | Fathom | N/A | Fabricated by direct 3D laser sintering of stainless steel (PH1 alloy) from 3D SolidWorks STL files. Original vendor was GPI Prototype & Manufacturing Services, Inc, now acquired by FATHOM, specifications are provided in the previously published document |
| High resolution and speed USB220 output kit | FUTEK Advanced Sensor Technology, Inc. | 717435 | Used to connect load cell to laptop |
| ImageJ 1.53t with Java 1.8.0_172 (for Windows 64-bit) | NIH | N/A | Install here https://imagej.nih.gov/ij/ |
| Isoflurane | Piramal Critical Care | 66794-013-25 | 100% inhalation vapour liquid |
| Loading bracket | Fathom | N/A | Fabricated by direct 3D laser sintering of stainless steel (PH1 alloy) from 3D SolidWorks STL files. Original vendor was GPI Prototype & Manufacturing Services, Inc, now acquired by FATHOM, specifications are provided in the previously published document |
| MATLAB R2019a | MathWorks | For running the loading device | |
| Matrx VIP 3000 vaporizer well fill isoflurane | Butler Schein Animal Health | 14309 | Vaporizer for anesthetic |
| Nonin pulse oximeter Model 2500A Vet | 2500A Vet | ||
| Piezo servo controller | PI-USA | E-625 | Electronic component recommended by the company to be used with the actuator |
| PiezoMove high-stiffness linear piezo actuator | PI-USA | P-602.5SL | Actuator for the loading device |
| Scalpel blades, No. 10 for handle No. 3, pack of 100 | Electron Microscopy Sciences | 72044-10 | Surgical supplies |
| Stainless steel tweezers with sharp, fine tips. Length: 120 mm | Electron Microscopy Sciences | 78326-42 | Surgical supplies |
| Thorlabs Bergamo multiphoton microscope | ThorLabs | N/A | This is only the imaging system and does not have the laser included, although ThorLabs has laser options if desired |
| Titanium-Saphire Chameleon Discovery NX with Total Power Control (TPC) | Coherent | N/A | This system technically has two lasers, both a tunable and a fixed laser. However, for the protocol, only the tunable is needed. |
| Vannas spring scissors - 4 mm cutting edge | Fine Science Tools | 15018-10 | Surgical supplies |
| Water bath | Fathom | N/A | Fabricated by direct 3D laser sintering of stainless steel (PH1 alloy) from 3D SolidWorks STL files. Original vendor was GPI Prototype & Manufacturing Services, Inc, now acquired by FATHOM, specifications are provided in the previously published document |
References
- Lu, X. L., Huo, B., Park, M., Guo, X. E. Calcium response in osteocytic networks under steady and oscillatory fluid. Bone. 51 (3), 466-473 (2012).
- Wu, D., Schaffler, M. B., Weinbaum, S., Spray, D. C. Matrix-dependent adhesion mediates network responses to physiological stimulation of the osteocyte cell process. Proceedings of the National Academy of Sciences of the United States of America. 110 (29), 12096-12101 (2013).
- Verbruggen, S. W., Vaughan, T. J., McNamara, L. M. Fluid flow in the osteocyte mechanical environment: A fluid-structure interaction approach. Biomechanics and Modeling in Mechanobiology. 13 (1), 85-97 (2013).
- Genetos, D. C., Kephart, C. J., Zhang, Y., Yellowley, C. E., Donahue, H. J. Oscillating fluid flow activation of gap junction hemichannels induces atp release from MLO-Y4 osteocytes. Journal of Cellular Physiology. 212 (1), 207-214 (2007).
- Lu, X. L., Huo, B., Chiang, V., Guo, X. E. Osteocytic network is more responsive in calcium signaling than osteoblastic network under fluid flow. Journal of Bone and Mineral Research. 27 (3), 563-574 (2012).
- Schaffler, M. B., Kennedy, O. D. Osteocyte signaling in bone. Current Osteoporosis Reports. 10 (2), 118-125 (2012).
- Kramer, I., et al. Osteocyte Wnt/ -Catenin signaling is required for normal bone homeostasis. Molecular and Cellular Biology. 30 (12), 3071-3085 (2010).
- Nakashima, T., et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nature Medicine. 17 (10), 1231-1234 (2011).
- Kennedy, O. D., et al. Activation of resorption in fatigue-loaded bone involves both apoptosis and active pro-osteoclastogenic signaling by distinct osteocyte populations. Bone. 50 (5), 1115-1122 (2012).
- Rawlinson, S. C. F., Wheeler-Jones, C. P. D., Lanyon, L. E. Arachidonic acid for loading induced prostacyclin and prostaglandin E2 release from osteoblasts and osteocytes is derived from the activities of different forms of phospholipase A2. Bone. 27 (2), 241-247 (2000).
- David, V., et al. Ex vivo bone formation in bovine trabecular bone cultured in a dynamic 3D bioreactor is enhanced by compressive mechanical strain. Tissue Engineering Part A. 14 (1), 117-126 (2008).
- Davies, C. M., et al. Mechanically loaded ex vivo bone culture system "Zetos": systems and culture preparation. European Cell & Materials. 11, 57-75 (2006).
- Adachi, T., et al. Osteocyte calcium signaling response to bone matrix deformation. Journal of Biomechanics. 42 (15), 2507-2512 (2009).
- Huo, B., Lu, X. L., Guo, X. E. Intercellular calcium wave propagation in linear and circuit-like bone cell networks. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences. 368 (1912), 617-633 (2010).
- Morrell, A. E., et al. Mechanically induced Ca2+ oscillations in osteocytes release extracellular vesicles and enhance bone formation. Bone Research. 6, 6 (2018).
- Jing, D., et al. In situ intracellular calcium oscillations in osteocytes in intact mouse long bones under dynamic mechanical loading. FASEB Journal. 28 (4), 1582-1592 (2014).
- Chen, T. -. W., Wardill, T. J., Sun, Y., Pulver, S. R., Renninger, S. L. Ultra-sensitive fluorescent proteins for imaging neuronal activity. Nature. 499 (7458), 295-300 (2013).
- Jacobs, C. R., Temiyasathit, S., Castillo, A. B. Osteocyte mechanobiology and pericellular mechanics. Annual Review of Biomedical Engineering. 12, 369-400 (2010).
- Jing, D., et al. Spatiotemporal properties of intracellular calcium signaling in osteocytic and osteoblastic cell networks under fluid. Bone. 53 (2), 531-540 (2013).
- Thi, M. M., Suadicani, S. O., Schaffler, M. B., Weinbaum, S., Spray, D. C. Mechanosensory responses of osteocytes to physiological forces occur along processes and not cell body and require αVβ3 integrin. Proceedings of the National Academy of Sciences of the United States of America. 110 (52), 21012-21017 (2013).
- Wang, Y., McNamara, L. M., Schaffler, M. B., Weinbaum, S. A model for the role of integrins in flow induced mechanotransduction in osteyocytes. Proceedings of the National Academy of Sciences of the United States of America. 104 (40), 15941-15946 (2007).
- McNamara, L. M., Majeska, R. J., Weinbaum, S., Friedrich, V., Schaffler, M. B. Attachment of osteocyte cell processes to the bone matrix. The Anatomical Record. 292 (3), 355-363 (2009).
- Franz-Odendaal, T. A., Hall, B. K., Witten, P. E. Buried alive: How osteoblasts become osteocytes. Developmental Dynamics. 235 (1), 176-190 (2005).
- Jing, D., et al. Tissue clearing and its application to bone and dental tissues. Journal of Dental Research. 98 (6), 621-631 (2019).
- Lewis, K. J., et al. Osteocyte calcium signals encode strain magnitude and loading frequency in vivo. Proceedings of the National Academy of Sciences of the United States of America. 114 (44), 11775-11780 (2017).
- Lu, Y., et al. DMP1-targeted Cre expression in odontoblasts and osteocytes. Journal of Dental Research. 86 (4), 320-325 (2007).
- Rubin, C. T., Lanyon, L. E. Dynamic strain similarity in vertebrates; an alternative to allometric limb bone scaling. Journal of Theoretical Biology. 107 (2), 321-327 (1984).
- Jacobs, C. R., et al. Differential effect of steady versus oscillating flow on bone cells. Journal of Biomechanics. 31 (11), 969-976 (1998).
- Kato, Y., Windle, J. J., Koop, B. A., Mundy, G. R., Bonewald, L. F. Establishment of an osteocyte-like cell line, MLO-Y4. Journal of Bone and Mineral Research. 12 (12), 2014-2023 (1997).
- Kato, Y., et al. Establishment of an osteoid preosteocyte-like cell MLO-A5 that spontaneously mineralizes in culture. Journal of Bone and Mineral Research. 16 (9), 1622-1633 (2001).
- Woo, S. M., Rosser, J., Dusevich, V., Kalajzic, I., Bonewald, L. F. Cell line IDG-SW3 replicates osteoblast-to-late-osteocyte differentiation in vitro and accelerates bone formation in vivo. Journal of Bone and Mineral Research. 26 (11), 2634-2646 (2011).
- vander Plas, A., Nijweide, P. J. Isolation and purification of osteocytes. Journal of Bone and Mineral Research. 7 (4), 389-396 (1992).
- Gu, G., Nars, M., Hentunen, T. A., Metsikkö, K., Väänänen, H. K. Isolated primary osteocytes express functional gap junctions in vitro. Cell and Tissue Research. 323 (2), 263-271 (2006).
- Paic, F., et al. Identification of differentially expressed genes between osteoblasts and osteocytes. Bone. 45 (4), 682-692 (2009).
- Tian, L., et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nature Methods. 6 (12), 875-881 (2009).
- Grienberger, C., Konnerth, A. Imaging calcium in neurons. Neuron. 73 (5), 862-885 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved