Method Article
A Heterotopic Rat Heart Transplantation Model using Circulatory Death Donor Hearts
In This Article
Summary
Here, we describe the preparation and technical details of murine heterotopic heart transplantation utilizing a circulatory death donor heart.
Abstract
The objective of this protocol is to set up a rat heterotopic heart transplantation model with donation after circulatory death (DCD) donor hearts. There are two setups for this protocol: heart donor setup and recipient setup. In the heart donor setup, Sprague Dawley rats are anesthetized, endotracheally intubated, and ventilated. The right carotid artery is cannulated to deliver heparin and the paralytic agent vecuronium-bromide. The DCD process is initiated by terminating the ventilation. After 20 min, the heart is exposed and the aorta distal to the brachiocephalic branch is clamped. At 25 min from terminating the ventilator, ice-cold University of Wisconsin (UW) solution is perfused through the carotid catheter to flush the heart. The heart is procured by dividing the aorta, pulmonary artery, venae cavae, and pulmonary veins and stored in UW solution for implantation. In the recipient setup, the Lewis rat is anesthetized with isoflurane. Slow-release buprenorphine is administered subcutaneously to facilitate a smooth postoperative recovery. Through a midline abdominal incision, the infra-renal aorta and the inferior vena cava are isolated and clamped with an atraumatic vascular clamp. The donor heart aorta and pulmonary artery are sutured to the recipient abdominal aorta and vena cava, respectively, with a running 8-0 Prolene. The vascular clamp is removed to reperfuse the heart. The abdominal wall is closed and the rat is recovered. After a set interval (24 h to 2 weeks), the recipient rat is anesthetized, the transplanted heart is exposed, and a balloon-tip-catheter is inserted into the left ventricle via the apex to record developed pressure and dP/dt using a data acquisition system. The heart tissue is collected for histology, immunology, or molecular analysis. A successful DCD donor rat heart transplantation model will allow further studies on the cardioprotective approaches to improve heart transplantation outcomes from DCD donors.
Introduction
A small animal model of heart transplantation (HTx) is critical to conducting research studying the pathophysiological conditions that affect the transplanted heart. Heterotopic HTx in a murine model, as described by Oto and Lindsey, has allowed researchers to study the pathophysiological changes observed in the conditions of ischemia and reperfusion1. Traditionally, donor hearts for transplantation have been procured from beating heart donors, also known as donation after brain death (DBD) donors; however, there has been a disproportionate increase in the number of patients in need of HTx2. More recently, hearts from circulatory death donors, also known as donation after circulatory death (DCD) donors, have been used for transplantation in experimental settings3. The main distinction between DBD and DCD donor hearts is that, in the latter, hearts are subjected to varying durations of ischemia, precluding their use in routine HTx practice.
The previously described literature on murine heterotopic HTx has only utilized beating heart donor conditions4,5,6. Heterotopic DCD heart transplantation requires subtle modifications, without which the transplanted heart will not beat7. This protocol aims to share with the readers a refined technique of DCD HTx in rats. Global myocardial ischemia is innate to DCD organ donation. An experimental setup mimicking global myocardial ischemia has been studied only in the ex vivo setup5. Findings from ex vivo studies may not apply to the DCD HTx work since significant differences exist between in vivo (DCD) and ex vivo global ischemia models5. The results, or lack thereof, from interventions to mitigate reperfusion myocardial ischemia in ex vivo models may not be reproducible in the DCD HTx model. Hence, it is essential to simulate the human DCD HTx in an animal model, the findings from which can have a higher translational value. The DCD HTx model described here will allow the researcher to closely simulate the clinical DCD HTx and provide the opportunity to mitigate reperfusion injury through interventions in both the donor heart and the recipient. Upon recovery of the recipient rat, the transplanted heart's function, histopathology, and immunology can be studied at varying intervals from the time of transplantation.
Protocol
All animal experiments were conducted in accordance with institutional guidelines and the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publication No. 86-23, revised 2011)8. The following procedures were approved by the Virginia Commonwealth University's Institutional Animal Care and Use Committee. All procedures were performed following OSHA (occupational safety and health administration) guidelines and the recommended sterile techniques9. The Sprague Dawley rats were housed under controlled humidity at a temperature of 23 °C and 12 h dark/light cycles.
1. Setup of the lab
NOTE: Assign a dedicated space to conduct sterile rodent survival surgeries with an operating microscope. Maintain the ambient temperature of the operating room as warm; the use of warming pads both for surgery and the recovery process is essential to maintain the recipient rat's body temperature.
- Keep essential supplies (Table of Materials), including syringes, normal saline 0.9% (NaCl), anesthetic agents (isoflurane, ketamine/xylazine), heparin, vecuronium bromide, preservation solution, an ice bucket, and analgesic agent (buprenorphine slow release) stocked and readily available.
- Neatly lay down the microsurgical instruments (Figure 1A, B) on the surgical field. Keep a flash sterilization kit readily available to clean the contaminated essential instruments immediately.
2. In vivo rat DCD donor preparation
- Anesthetize the rat for tracheal intubation and carotid cannulation. Sedate the Sprague Dawley rat (8-12 weeks old) in a 3% isoflurane chamber, then anesthetize it with ketamine/xylazine (100/10 mg/kg, intramuscular).
- To expose the trachea and right carotid artery, place a fully anesthetized rat supine and cleanse the front of the neck and chest with alcohol and povidone solution. Make a V-shaped incision with the peak of the V close to the rat's jaw in the midline and each limb of the V pointing to the corresponding shoulder. Separate the skin from the subcutaneous tissue and flip over the skin onto the chest to expose the strap muscles of the neck (Figure 2A).
- Intubate the rat by separating the midline strap muscles (sternomastoid and sternohyoid) with forceps to expose the trachea (Figure 2B). Encircle the trachea with a 5-0 silk and open it by partially dividing the muscle tissue between the tracheal rings.
- Insert a 14 G angiocath into the trachea and secure it with the 5-0 silk (Figure 2C). Connect the angiocath to a ventilator (1 mL/kg at 90 breaths/min).
- For right carotid artery cannulation, identify the common carotid artery (it lies parallel to and immediately on the right side of the trachea), carefully isolate it to the full length of the neck, and tie off the distal (cranial) end with a 5-0 silk.
- Attach a hemostat to the free end of the tie for traction to facilitate cannulation of the carotid artery.
- Mobilize the proximal-most aspect of the carotid artery (toward the base of the neck) and clamp it using a vascular hemostat . Under an operating microscope, use micro-iris scissors to open the carotid artery by partially dividing it anteriorly on the distal-most end. Cannulate the carotid artery with a 22 G angiocath and secure it with a 5-0 silk tie (Figure 2D).
- Attach a three-way flow stopcock adapter to the angiocath for easy delivery of drugs or cardioplegia solution into the carotid artery, and connect to a pressure sensor to monitor heart rate and pressure during the DCD process (Figure 2D). A pulsatile backflow of blood should be noticed when the proximal vascular clamp is released.
- Once secured, connect the carotid artery catheter to the pressure sensor, and deliver heparin (1,000 U/Kg) and vecuronium bromide (4 mg/kg).
- Initiation of the DCD process: Let the vecuronium bromide circulate for 1 min. Watch for any signs of animal distress, and deliver additional anesthesia if required. Stop the ventilator (hypoxia/ischemia) to initiate the DCD process. Observe for the absence of respiratory activity.
NOTE: The DCD ischemic time begins from the time the ventilator support is withdrawn. In rats, 25 min of ischemia has been identified as the maximum length of time that results in significant but reversible injury7. Pressure tracings will demonstrate that, from the moment of interruption of the ventilatory support at ~3.5 min, the systemic pressure drops to below 50 mmHg, a pressure deemed insufficient to effectively perfuse the heart (Figure 3).
- Donor heart procurement: Allocate ~4-7 min to procure the heart and administer cold cardioplegia. To achieve the target ischemia time of 25 min, start the procurement of the heart after 18-21 min following the termination of ventilatory support.
NOTE: Modify the duration of procurement time based on the experience of the person performing the procurement.- Divide the abdominal wall along the costal margin starting from the level of the xiphoid, and then divide the ribcage parallel to the sternum on either side up to the clavicles to perform bilateral anterior thoracotomies (Figure 4A). Use an operating microscope under low magnification (5x) to facilitate this step.
- Flip the divided chest wall hinged on the clavicles toward the head and secure it with a hemostat (Figure 4B).
- Encircle the inferior vena cava (IVC) with a 5-0 silk, then partially divide it with micro scissors close to the dome of the liver (Figure 4C). The partially opened IVC allows for egress of cardioplegia as it distends the right side of the heart.
- Dissect the plane between the ascending aorta and the pulmonary artery (PA), then isolate the pulmonary trunk via transverse sinus with blunt-tip curved forceps to its bifurcation. Carefully divide the pulmonary artery close to its bifurcation point with micro scissors (Figure 4C).
- Encircle the aortic arch with blunt dissection. This is to allow access for a small right-angle vascular clamp to be placed across the aortic arch distal to the origin of the innominate artery.
- At the mark of 25 min from the termination of the ventilator, clamp the aortic arch and manually deliver cardioplegia (10 mL of University of Wisconsin solution at 4 °C, over 2-3 min) through the carotid catheter (Figure 4D).
- Using micro scissors, divide the ascending aorta distally before the aortic arch (Figure 4E).
- Tie off the IVC toward the heart with 5-0 silk and divide it distally.
- Ligate the pulmonary veins and superior vena cavae (SVCs) together with 5-0 silk (Figure 4F). Since these ties hold a large amount of tissue, a hand tie is preferred over an instrument tie. Gently pull the heart down toward the abdomen with a cotton-tip swab to facilitate pulmonary vein ligation without bunching the atrial appendages into the tie.
- Divide the pulmonary veins with micro iris scissors, collect the heart, and place it in ice-cool normal saline (Figure 4G).
3. In vivo rat DCD heterotopic heart transplantation
- Sedate the recipient in an isoflurane chamber (3% induction), clip the hair over the abdomen, and cleanse the region with povidone and alcohol. Do not anticoagulate the recipient; this will lead to excessive bleeding from the anastomoses and graft failure.
- Place the rat supine on a heating pad. Place a probe between the rat and the heating pad to monitor the temperature. Maintain the rat's body temperature at 38 °C. Place two 50 mL pre-sterilized centrifuge tubes filled with warm saline or glass beads on either side of the rat's abdomen to facilitate keeping the rat warm.
- Despite the above-mentioned steps, if the body temperature decreases to below 37.5 °C, increase the room temperature (RT), and cover all exposed areas of the body using autoclaved aluminum foil to leave just enough area to perform abdominal surgery.
- Induce general anesthesia with 3% isoflurane via a nose cone and gradually decrease to 2%.
- Open the abdomen along the linea-alba (Figure 5A) and expose the working space by placing abdominal wall retractors (Figure 5B).
- Using sterile cotton tip applicators, move the dilated or full colon and place it in a warm moist gauze to the operator's left, periodically irrigating with warm (38 °C) saline (Figure 5C). Moving the colon aside provides more space for the DCD hearts because they are very stiff and require extra room to accommodate in the abdomen.
- Open the retroperitoneum in the midline with blunt dissection using cotton-tip swabs. Expose the infrarenal aorta and IVC.
- Using an atraumatic pediatric multi-angle vascular clamp, isolate 3-5 mm of the infra-renal aorta and IVC for anastomosis (Figure 5D).
- Enter the aorta with a 30 G needle mounted on a 1 cm3 syringe filled with normal saline mixed with 100 units of heparin. Flush with 0.2-0.3 mL of the solution (Figure 5E). Dab the excess solution with the sterile cotton tip applicators, as heparin may be absorbed and cause bleeding from suture lines.
- Using micro scissors, open the aorta along the long axis to match the donor aorta size. The IVC is not opened at this point, and do not attempt to create a plane between the IVC and the aorta; this will lead to bleeding.
- Proper orientation of the donor's heart during anastomosis is critical (Figure 5F). Orient the donor heart for anastomosis such that the anterior surface of the right ventricle is facing the ceiling, the apex is pointing to the right of the operator, and the donor aorta is slightly lower than the pulmonary artery. This orientation results in less tension on the pulmonary artery anastomosis.
- Aortic anastomosis: Use a pointed tip needle driver and precision tweezers for microvascular anastomosis. Perform anastomoses with 8-0 monofilament suture on a tapered 4 mm needle loaded on a 0.3 mm tip needle holder with the needle-driver lock removed, to prevent accidental tissue trauma when locking and unlocking.
- Place a stay suture at the 6 o'clock position on the aortic anastomosis. This is done to provide a symmetrical and hemostatic suture line. Then, begin the anastomosis at the 12 o'clock position with outside-to-inside on the donor aorta and inside-to-outside on the recipient aorta (Figure 6A, B). Keep only 5-7 cm of working suture and truncate the remainder.
- Move counter-clockwise, travel in short distances toward the 6 o'clock position, and then complete the anastomosis in a counter-clock fashion up to the 12 o'clock position by flipping the heart to the left (Figure 6C).
- Check for any loose suture line before tying.
NOTE: A secure anastomosis should be symmetrical and must approximate the donor and recipient intima without gaps. - Pulmonary artery anastomosis: Free the pulmonary artery from the aorta and orient it for anastomosis to the IVC without twists. Prepare the recipient's blood volume to fill the donor's heart and avoid hypotension by injecting 3-5 mL of subcutaneous (nape of the neck) normal saline.
- Monitor the respiratory pattern of the rat and decrease the isoflurane from 2.0% to 1.5% as the pulmonary artery anastomosis is near completion.
- Open the IVC cephalad in relation to the aortic anastomosis with micro iris scissors (Figure 6C). Use 0.2-0.3 mL of saline to flush the IVC. There will be a small number of blood clots seen; flush them carefully.
- Unlike aortic anastomosis, start the pulmonary anastomosis without a stay suture; it will hinder exposure. Start at the 12 o'clock position with the needle moving from outside to inside on the donor pulmonary artery and from inside to outside on the recipient IVC. Tie the suture and first complete the back wall anastomosis clockwise.
- Once at the 6 o'clock position, continue to suture clockwise until the 12 o'clock position is reached, and then tie it to the short end of the suture from the previous tie. Take care not to cinch the anastomosis since any narrowing will limit venous drainage from the heart. On average, it takes 30 min or less to complete both anastomoses.
- Place small pieces of absorbable hemostat over the anastomosis to contain bleeding from the needle holes.
- Unclamp the vessels and add more absorbable hemostats over the needle holes as needed (Figure 6E). The transplanted heart starts beating with occasional fibrillations before it resumes rhythmic breathing. Leave the absorbable hemostat in place for 5 min as long as the heart is beating and there is no overt bleeding.
- Once satisfied with hemostasis (~3-5 min), use sterile cotton tip applicators to remove the excess absorbable hemostat, irrigate with saline, and return the bowel back into the abdominal cavity (Figure 6F). Place the omentum over the anastomosis to help with hemostasis.
- Close the abdominal wall in two layers with 5-0 monocryl on a 13 mm cutting needle by first closing the linea alba and then close the skin (Figure 6G, H).
4. Recovery and monitoring
- Upon completion of abdominal closure, turn the rat onto its belly on a warm pad for recovery. Continue isoflurane via a nose cone at 1% for 5 min, then stop.
- Once the spontaneous breathing is regular, move the rat into a clean recovery cage and place it on a warm pad to continue the recovery process. Sudden movements of the rat will result in bleeding risk or a twist in the anastomosis. A long-acting analgesic administered prior to the initiation of surgery greatly facilitates the smooth recovery process.
5. Procurement and transplantation of the control beating heart
- Procure control beating-heart donor (CBD) hearts as a control to assess the quality of the donor heart in the absence of ischemia.
NOTE: The CBD donor undergoes all the steps that were described for the DCD heart, except for the termination of the ventilatory support. CBD hearts are procured while beating and fully supported on a ventilator. Administration of cardioplegia arrests the heart, and the procurement and transplantation are completed in the same way as described for DCD hearts.
6. Transplanted heart function assessment:
- At a predetermined interval from the time of heart transplantation (24 h to 14 days), anesthetize the recipient rat (3% inhalation isoflurane), place it on a warm pad supine, and open the abdominal incision to expose the transplanted heart.
- Place a balloon-tip catheter via the apex of the left ventricle to measure the developed pressure (DP), the max +dP/dt, and the min −dP/dt.
NOTE: Here a PowerLab station was used as the data acquisition system for blood pressure recordings.
Results
24 h to 14 days after the heterotopic heart transplantation, the abdomen can be reopened, and the heart can be exposed to measure the pressure developed by the left ventricle. A balloon-tip catheter is inserted in the left ventricle of the DCD (or CBD) heart to measure the developed pressure (DP), the max +dP/dt, and the min −dP/dt. Figure 7 shows an example of the expected DP, +dP/dt, and −dP/dt of a DCD heart compared to a CBD heart 24 h after transplantation. Compared to the CBD hearts, the DP of the DCD hearts was significantly reduced, and the +dP/dt(max) and −dP/dt(min) worsened.
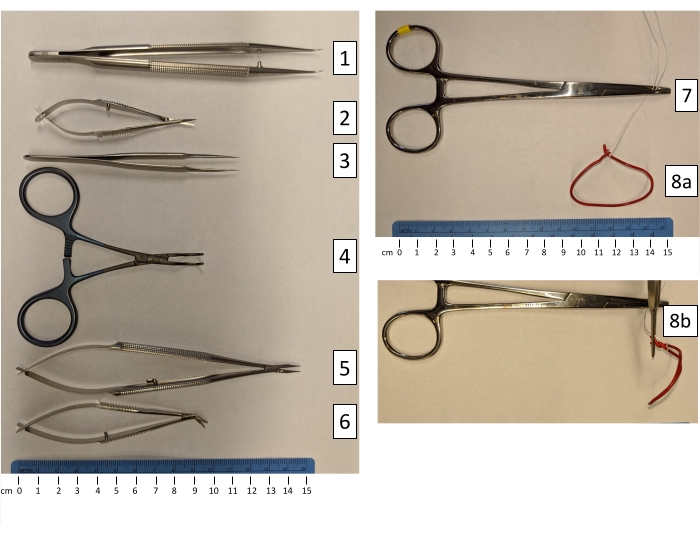
Figure 1: Microvascular instruments used for heterotopic rat heart transplantation. (1) Forceps with curved tips; (2) Iris micro scissors with straight tips; (3) Tweezers with a high precision point; (4) Ebakey atraumatic pediatric multi-angle vascular clamp; (5) Needle holder with the lock mechanism removed; (6) Micro scissors, right-angle and curved tips; (7) Heavy vascular clamp; (8a) Autoclavable curved hand-molded abdominal wall retractors, using large paperclips on suture (front view); (8b) Autoclavable curved hand-molded abdominal wall retractors (side view). Please click here to view a larger version of this figure.

Figure 2: Step-by-step description of the donor intubation and carotid cannulation. (A) With scissors, the skin above the neck region is cut, and the soft tissue is exposed. (B) Using a blunted dissection technique, the trachea and the right carotid artery are exposed. (C) A transversal incision is performed on the trachea and a tracheal tube is inserted to connect to a ventilator and secured. (D) A phlebotomy catheter is inserted into the right carotid artery, secured, and connected to a tubing line with a three-way stopcock. Please click here to view a larger version of this figure.

Figure 3: Example of systemic arterial pressure recordings during the DCD process. A carotid catheter was used to measure arterial pressure. Pressure and time are reported on the Y-axis and X-axis, respectively. Arrows indicate the moment of termination of the ventilatory support and the time at which the mean systemic pressure fell below 30 mmHg. Please click here to view a larger version of this figure.
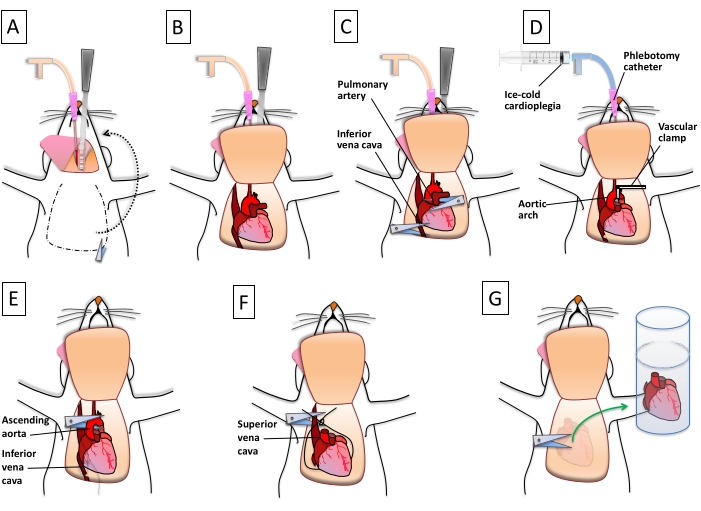
Figure 4: Step-by-step description of the procurement of the donor heart. (A) The carotid cannula is used to deliver a paralytic agent and heparin. Following the proper DCD or CBD protocol, a lateral thoracotomy is performed. (B) The anterior chest is flipped over the head to properly expose the aortic arch and the major thoracic vessels. (C) The pulmonary artery is cut as distally as possible. The inferior vena cava is nicked to decompress the right ventricle. (D) The aortic arch is clamped between the innominate artery and the common carotid. Ice-cold cardioplegia is delivered to the heart using the phlebotomy catheter/carotid access. (E) The ascending aorta is cut below the arch, and the inferior vena cava is ligated proximal to the right atrium. (F) A suture loop is created on the back of the heart to close the superior vena cava and the pulmonary veins. The thoracic vessels are cut. (G) The heart, separated from the thoracic vessels, and the connective tissues are stored in ice-cold saline. Please click here to view a larger version of this figure.
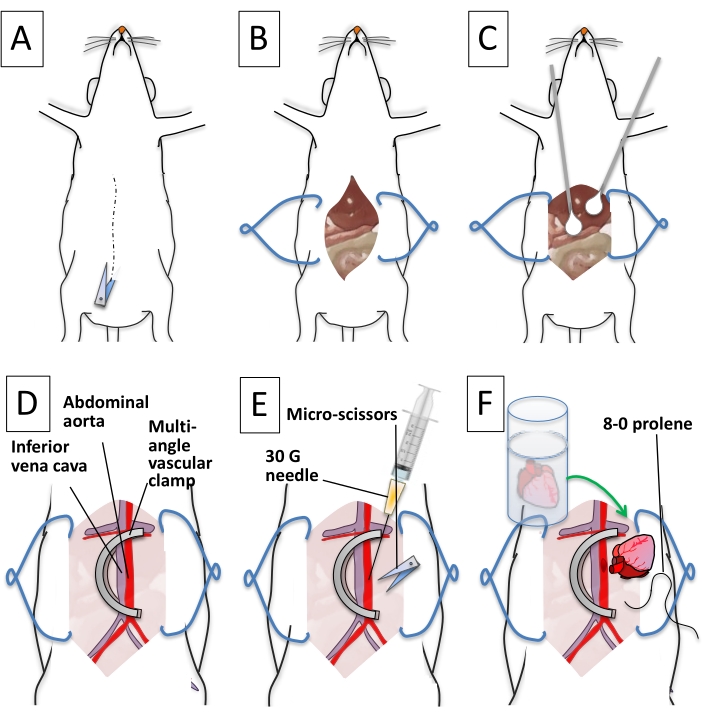
Figure 5: Step-by-step description of recipient preparation. (A) The abdominal skin and the muscle layer are cut longitudinally along the median line. (B) The abdominal organs are exposed. Tissue retractors widen the abdominal opening. (C) Q-tips are used to expose the back of the cavity and clean the inferior vena cava and abdominal aorta. (D) A U-shaped vascular clamp occludes the abdominal aorta and the inferior vena cava. (E) The abdominal aorta is punctured with a 30 G needle. Micro scissors are used to create a longitudinal aortotomy. (F) The donor heart aorta is aligned next to the opening on the recipient abdominal aorta. An 8-0 prolene suture is used to initiate the anastomosis. Please click here to view a larger version of this figure.

Figure 6: Step-by-step description of the heterotopic heart transplantation, heart reanimation, and completion of the surgical procedure. (A) A 12-o'clock stitch is placed to secure the donor aorta in position. (B) A continuous suture between the donor and the recipient aorta is initiated on the far side. (C) The aortic anastomosis is completed, and micro scissors are used to create a longitudinal venotomy. (D) The donor pulmonary artery is aligned next to the venotomy and secured to it with a 12-o'clock stitch. A continuous suture is used to complete the anastomosis. (E) The U-shaped vascular clamp is removed, and the heart is reperfused. (F) Q-tips are used to redistribute the intestine into the abdominal cavity. (G) A 5-0 nylon suture is used to close the abdominal wall and skin. (H) After completing the suturing process, the animal is recovered from surgery. Please click here to view a larger version of this figure.
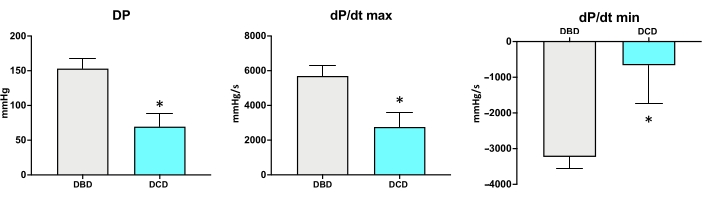
Figure 7: Examples of developed pressures (DP), +dP/dt(max), and -dP/dt(min) measured in rats 24 h after transplantation (N = 5/group). *p < 0.05. Compared to the CBD hearts, the DP, +dP/dt(max), and -dP/dt(min) of the DCD heart was significantly reduced. Please click here to view a larger version of this figure.
Discussion
For a successful DCD heterotopic rat HTx, it is critical that a meticulous and thoughtful setup of the experiment is established. The detailed setup takes into consideration several factors, including 1) selecting young rats as DCD donors, 2) using isoflurane as the anesthetic agent of choice, 3) effective delivery of cardioplegia to the donor heart, 4) storage of the donor's heart in ice-cold solution, 5) limiting the abdominal dissection without compromising the lumbar vessels to only expose the infrarenal aorta and vena cava, 6) maintaining the recipient body temperature during and after the surgery, 7) utilizing the avascular clamp that allows for isolation of the IVC and aorta for anastomosis, 8) allowing enough space in the recipient abdomen for the stiff DCD donor heart, 9) selection of microvascular instruments, 10) avoiding anticoagulation of the recipient, 11) meticulous hemostatic anastomosis, and 12) providing preemptive analgesia for a smooth recovery from surgery. The protocol described here provides the details of every step to successfully reproduce the outcomes.
The DCD rat HTx method described here has adopted the modifications to improve heterotopic HTx described in the literature since the first description of murine HTx by Abbott in 196410. The critical modifications in the protocol described above include the choice of anesthetic agent; isoflurane as an anesthetic agent is easy to monitor and regulate to the desired depth of anesthesia. The adaptation of side-to-side anastomosis of the donor heart aorta and pulmonary artery to the recipient abdominal aorta and inferior vena cava over the end-to-end anastomosis technique initially described by Abbott eliminated the high rate of postoperative paraplegia and mortality. To avoid circumferential dissection of the aorta, which can damage the lumbar vessels and risk paraplegia, the use of an atraumatic vascular clamp to isolate the abdominal aorta and vena cava is preferred. Besides decreasing the risk of paraplegia, the use of a vascular clamp is more time-efficient and more hemostatic. Since metabolic wastes and toxins invariably accumulate following ischemic injury in DCD hearts, an effective strategy to flush the heart at the time of procurement is necessary. By effectively cannulating the right carotid artery and clamping the aortic arch distal to the take-off of the brachiocephalic artery, effective delivery of preservation solution to the DCD heart and flushing out the metabolic wastes is accomplished. Routine administration of subcutaneous saline (1 mL/100 g) 10 min before the anticipated completion of vascular anastomosis preemptively repletes the anticipated blood loss from the needle holes made during anastomosis and the blood volume needed to fill the donor right atrium and ventricle at reperfusion. This avoids the potential hypotension that would otherwise ensue upon release of the vascular clamp, risking effective reperfusion of the transplanted heart and the recipient rat. Administering the long-acting analgesic buprenorphine before the initiation of surgery allows for smooth recovery of the recipient. Without adequate pain control, the recipient rat might move briskly, jeopardizing the hemostasis of the fragile vascular anastomosis. Paying meticulous attention to several factors described in the protocol brings success to the DCD heart transplantation process1,7,11,12,13.
The major limitation of the DCD rat HTx method, and that of all other murine HTx models described in the literature, is the non-working condition of the transplanted heart6,7,11,12,13. Modifications that are necessary to potentially convert the present model into a working heart model in the future are being studied. The other limitation is the immunologic response to the donor heart from the recipient. This limitation is easy to overcome with the use of syngeneic rats for transplantation. Several rat DCD HTx using syngeneic rats have been successfully done, the routine use of which is limited by the high cost of procuring syngeneic rats. Unlike the CBD hearts, DCD hearts do not resume beating upon reperfusion in the recipient's body. However, the coronary blood flow is established, a significant amount of which is pooled in the right side of the heart as it drains from the coronary sinus. If this blood remains stagnant, it will clot, and the graft fails; hence, mechanical emptying of the right-sided heart chambers with a cotton tip applicator rolled over the surface of the right ventricle toward the inferior vena cava is recommended. Keeping the coronary flowing in the donor heart and preventing it from distention allows it to resume beating in 1-2 min. During the anastomosis of the donor heart to the recipient's abdominal aorta/vena cava, the recipient's lower body remains ischemic (20-30 min) from occlusion of the infrarenal aorta. Unlike viscera, the limbs can withstand a longer duration of ischemia (upward of 2-3 h) without overt injury; nonetheless, it is very likely that the release of cytokines from reperfused limbs may influence the transplanted heart. Transplanted graft function, if assessed at a shorter duration from the time of reperfusion, may be affected by the release of cytokines. Since cytokine release is an acute phase response, the long-term influence of cytokines on the transplanted heart function would be minimal.
Establishing a rat in vivo DCD heart donor model that resembles the clinical DCD process significantly contributes to the research focused on expanding the donor heart pool for transplantation. Prior to this publication, the existing methods utilized global ischemia by first placing a beating heart on the Langendorff system (ex vivo) and then stopping the flow to initiate the DCD process6. Thus, the ex vivo ischemic model has several differences compared to the in vivo process, as observed in the clinical DCD protocol5. The DCD model described here closely resembles the clinical DCD process and has greater utility in testing the factors that are aimed at mitigating reperfusion injury to the DCD heart.
The existing clinical DCD protocol strictly follows the "death donor rule," where no physical or pharmacological interventions designed to facilitate organ procurement are allowed until the DCD donor is pronounced dead. As such, warm ischemia to various organs, especially the heart, is inevitable with the DCD donation. Keeping the clinical DCD process in mind, the rat DCD HTx model described here only allows perfusion of the donor heart after complete asystole (death) and following 25 min of warm global ischemia. The critical interventions that can be studied in a rat DCD model should be focused on targeting the reperfusion injury, which can contribute up to 50% of the total injury from ischemia and reperfusion14. To mitigate reperfusion, injury agents can be added to the reperfusion preservation solution delivered to the DCD heart at the time of procurement, targeting the electron transport chain in mitochondria, inflammasome modulators, and mitochondrial permeability transition pore stabilizers15. Other agents that have been studied on ex vivo global ischemia models, such as Na/H pump inhibitors, acidification of perfusates, NO generating agents, erythropoietin, and melatonin, all can be studied in vivo using the DCD HTx model that is described here. At present, in clinical practice, an ischemia duration of over 30 min is a contraindication to procuring DCD heart, even in the clinical study protocol. Perhaps with rigorous conduct of studies utilizing the DCD rat HTx model described here, the scientific community might propose interventions that one day could allow us to do clinical DCD HTx with longer ischemia durations.
Disclosures
The authors of this manuscript have no conflicts of interest to disclose.
Acknowledgements
This work was supported by a Merit Review Grant awarded to Dr. Mohammed Quader (1I01 BX003859) and funds from the Pauley Heart Center to Mohammed Quader and Dr. Stefano Toldo.
Materials
| Name | Company | Catalog Number | Comments |
| 5-0 nylon suture polyamide monofilament | Aros Surgical | SP17A05N-45 | |
| 5-0 silk suture | Surgical Specialties | SP116 | |
| 8-0 monofilament suture | Aros Surgical | T06A08N14-13 | |
| Autoclave | Steris | Amsco Lab 250 | |
| BD Insulin Syringe with Detachable Needle 1 mL Syringes | Fisher Scientific | 14-820-28 | |
| BD Syringe with Luer-Lok Tips (Without Needle) 10 mL Syringes | Fisher Scientific | 14-823-16E | |
| Belzer University of Wisconsin cold storage solution | Bridge to Life Northbrook IL USA | Adenosine 1.34 g/L, Allopurinol 0.136 g/L, Glutathione 0.922 g/L, Lactobionic Acid (as Lactone) 35.83 g/L, Magnesium Sulfate heptahydrate 1.23 g/L, Pentafraction 50 g/L, Potassium Hydroxide 5.61 g/L, Potassium Phosphate monobasic 3.4 g/L, Raffinose pentahydrate 17.83 g/L | |
| Buprenorfin SR Lab | Zoopharm LLC | ||
| Debakey atraumatic pediatric multi-angle vascular clamp | Aesculap | F341T | |
| Exel International Disposable Safelet I.V. Catheters 14 G | Fisher Scientific | 14-841-10 | |
| Exel International Disposable Safelet I.V. Catheters 22 G | Fisher Scientific | 14-841-20 | |
| Fogarty catheter size 4F | Edwands Lifesciences | 120404F | |
| Forceps with curved tips | Accurate Surgical & Scientific Instruments Corporation | ASSI.228 | |
| Gaymar Heating pump | Braintree Scientific, Braintree, MA, USA | TP700 | |
| Germinator-500 | Braintree Scientific | ||
| Heparin Sodium Injection, USP 1,000 U/mL | Pfizer | NDC 0069-0137-01 | |
| Iris micro-scissors with straight tips | Accurate Surgical & Scientific Instruments Corporation | ASSI.5253 | |
| Isoflurane USP | Patterson Veterinary | NDC 14043070406 | |
| Ketamine HCl 100 mg/mL | Henry Schein | NDC 6745710810 | |
| Lidocaine HCl 2% | Aspen Veterinary | 07-892-4325 | |
| McKesson General Medical 6IN Q-TIPS 2STER WOOD 100/PACK | Fisher Scientific | NC0650323 | sterile cotton tip applicators |
| Micro-scissors, right angle and curved tips | Braintree Scientific | SC-MS 154 | |
| Needle holder | Accurate Surgical & Scientific Instruments Corporation | ASSI.BSL158 | with the lock mechanism removed |
| Normal Saline | Baxter Infusion supplies | ||
| PowerLab station | AD Instruments, Denver, CO | data acquisition system | |
| Sodium Hydroxide/Hydrochloric Acid | adjust the solution to pH 7.4 | ||
| Sprague Dawley rats | male, 8–16 weeks of age, <400 g in weight | ||
| Surgical Microscope | Leika | Model M525 F40 | |
| Surgicel | Ethicon | absorbable hemostat | |
| Temperature probe Therma Waterproof Type T High Precision Thermocouple Meter | Thermoworks | THS-232-107 | |
| Tweezers with high precision point | Excelta | 17-456-109 | |
| Vecuronium Bromide | Sigma-Aldrich | PHR1627 | diluted in PBS for 100 mg/mL |
| Ventelite | Harvard Apparatus, Holliston, MA, USA | ||
| Xylazine 100 mg/mL | Pivetal Anased | NDC 04606675002 |
References
- Ono, K., Lindsey, E. S. Improved technique of heart transplantation in rats. Journal of Thoracic and Cardiovascular Surgery. 57 (2), 225-229 (1969).
- Colvin, M., et al. OPTN/SRTR 2019 annual data report: Heart. American Journal of Transplantation. 21, 356-440 (2021).
- Smith, D. E., et al. Early experience with donation after circulatory death heart transplantation using normothermic regional perfusion in the United States. Journal of Thoracic and Cardiovascular Surgery. (21), 01316-01317 (2021).
- Lesnefsky, E. J., Chen, Q., Tandler, B., Hoppel, C. L. Mitochondrial dysfunction and myocardial ischemia-reperfusion: Implications for novel therapies. Annual Review of Pharmacology and Toxicology. 57, 535-565 (2017).
- Quader, M., et al. The commonalities and differences in mitochondrial dysfunction between ex vivo and in vivo myocardial global ischemia rat heart models: Implications for donation after circulatory death research. Frontiers in Physiology. 11, 681 (2020).
- Wyss, R. K., et al. Mitochondrial integrity during early reperfusion in an isolated rat heart model of donation after circulatory death-consequences of ischemic duration. Journal of Heart and Lung Transplantation. 38 (6), 647-657 (2019).
- Quader, M., et al. Refining murine heterotopic heart transplantation: A model to study ischemia and reperfusion injury in donation after circulatory death hearts. Animal Models and Experimental Medicine. 4 (3), 283-296 (2021).
- National Research Council. . Guide for the Care and Use of Laboratory Animals. Eighth Edition. , 86 (2011).
- Jagger, J., Detmer, D. E., Cohen, M. L., Scarr, P. R., Pearson, R. D. Reducing blood and body fluid exposures among clinical laboratory workers. Meeting the OSHA standards. Clinical Laboratory Management Review. 6 (5), 415-417 (1992).
- Abbott, C. P., Lindsey, E. S., Creech, O., Dewitt, C. W. A technique for heart transplantation in the rat. Archives of Surgery. 89, 645-652 (1964).
- Ruzza, A., et al. Heterotopic heart transplantation in rats: improved anesthetic and surgical technique. Transplantation Proceedings. 42 (9), 3828-3832 (2010).
- Shan, J., et al. A modified technique for heterotopic heart transplantation in rats. Journal of Surgical Research. 164 (1), 155-161 (2010).
- Wang, D., Opelz, G., Terness, P. A simplified technique for heart transplantation in rats: Abdominal vessel branch-sparing and modified venotomy. Microsurgery. 26 (6), 470-472 (2006).
- Yellon, D. M., Hausenloy, D. J. Myocardial reperfusion injury. New England Journal of Medicine. 357 (11), 1121-1135 (2007).
- Quader, M., Mezzaroma, E., Wickramaratne, N., Toldo, S. Improving circulatory death donor heart function: A novel approach. Journal of Thoracic and Cardiovascular Surgery Techniques. 9, 89-92 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved