Method Article
Gelatin Zymography to Detect Gelatinase Activity in Melanoma Cells
In This Article
Summary
Metalloproteases (MMPs) are secreted by many cells, including malignant melanoma. MMP-mediated cleavage of extracellular matrix components leads to the increased invasive potential of these cells. Gelatin zymography, presented here, is a quantifying method for studying gelatinase activity manifested as a digested gelatin area on a polyacrylamide gel.
Abstract
Melanoma cells, having highly invasive properties, exhibit the formation of invadopodia—structures formed by tumor cells and responsible for the digestion of the surrounding extracellular matrix (ECM). Several metalloproteases (MMPs) are secreted by cells to hydrolyze ECM proteins. They are mainly secreted through structures known as invadopodia. ECM degradation is crucial for tumor cells while forming metastases as the cells heading towards blood vessels must loosen dense tissue.
One group of metalloproteases secreted by melanoma cells comprises the gelatinases, i.e., metalloproteases 2 and 9. Gelatinases cleave gelatin (denatured collagen), a few types of collagen (including type IV), and fibronectin, all structural components of ECM. This paper describes a gelatin zymography assay to analyze the gelatinase activity of melanoma cells. This approach is based on analyzing the extent of digestion of a substrate (gelatin) added to a polyacrylamide gel. Several advantages, such as simplicity, sensitivity, low cost, and semiquantitative analysis by densitometry, as well as the detection of both active and inactive forms of MMPs, make this assay valuable and widely used.
This protocol describes how to concentrate medium devoid of intact floating cells, cell debris, and apoptotic bodies. Next, it focuses on preparing polyacrylamide gel with gelatin addition, performing sodium dodecylsulfate-polyacrylamide gel electrophoresis (SDS-PAGE), removing SDS, and staining of the gel to detect gelatin-free bands corresponding to the activity of gelatinases secreted by melanoma cells. Finally, the paper describes how to quantitatively analyze data from this assay. This method is a good alternative for estimating the gelatinase activity of melanoma cells to a fluorescent gelatin degradation assay, western blot, or enzyme-linked immunosorbent assays (ELISAs).
Introduction
Matrix metalloproteinases (MMPs) are a family of Zn2+-containing endopeptidases that cleave various ECM proteins and non-ECM proteins, such as growth factors, cell receptors, proteinases, and their inhibitors1,2,3,4. ECM-substrate specificity of MMPs is dependent on the peptide domains and motifs, as well as similarities in their sequences, thus defining their subgroups. There are, for example, collagenases digesting various types of collagens, as well as gelatin and aggrecan; gelatinases cleaving gelatins and collagens; and matrilysins or MT-MMPs that digest various ECM proteins5.
This paper focuses on two gelatinases: MMP-2 and MMP-9, as they can digest denatured collagen (gelatin) proteolytically, allowing the detection of their activity using a gelatin zymography assay3,6. Although MMP-2 and MMP-9 bear a strong structural resemblance, they do not have identical substrate specificity7. A C-terminal hemopexin-like domain of MMPs is responsible for recognizing substrate sequence8. Slight differences in their catalytic domains are responsible for the differences in the substrate selectivity of MMP-2 and MMP-9, e.g., MMP-2, unlike MMP-9, can cleave native type I collagen9. Nevertheless, their proteolytic activities can be unquestionably determined with gelatin zymography as they both can cleave gelatin3,6.
MMPs are already known to be involved in both physiological and pathological conditions. They were found to impact cell migration, invasion, spreading, and adhesion, thus impairing angiogenesis, inflammation, tumor progression, and metastasis10,11,12,13. As they take part in various important processes, they are extensively studied due to their high therapeutic or diagnostic potential14,15,16. MMP-2 (gelatinase A) occurs as a 72 kDa proenzyme whose prodomain binds Zn2+ in the catalytic site, leading to the inhibition of enzymatic activity9. MMP-2 can be activated through the cleavage of its zymogen's prodomain by MMP-14 (MT1-MMP), thrombin, and activated protein C17,18,19,20. Therefore, the mass of active MMP-2 is lower (~64 kDa). In contrast, MMP-9 (gelatinase B) is expressed as a ~92 kDa proenzyme and is activated by the cleavage of the N-terminal domain to obtain the 83 kDa protein. MMP-9 maturation results from a prodomain cleavage by serine proteases, other MMPs, and as a response to the oxidative stress21.
The progression and malignancy of melanoma are highly dependent on the ability of the tumor cells to digest ECM, as it is "a barrier" limiting the cells from progression and metastasis formation22. Cells need to first penetrate the basal membrane (BM) to enter the dermis, migrate toward blood vessels, adhere to vascular endothelium, and reach the blood. It has been shown that gelatinase expression was increased in different cancers and was correlated with increased invasion and migration and a worse prognosis for patients23,24. MMP-2 was highly expressed in melanoma cells, with its activation state correlated with progression25,26. MMP-9 was also found to accumulate in human skin tumors and melanoma cell lines27,28.
Due to the high correlation between the properties of MMPs with the invasiveness of melanoma cells, the availability of a simple, sensitive, low-cost, functional assay to determine their presence and activity is crucial for better understanding the biology of melanoma and designing new diagnostic techniques for their detection. This paper describes the gelatin zymography technique in detail, as it can be considered the best candidate for this purpose. This approach is based on SDS-electrophoresis under denaturing but nonreducing conditions, using polyacrylamide gels prepared with the addition of gelatin29,30. Although proteins, including MMP-2 and MMP-9, are denatured in the presence of SDS during electrophoresis, washing in buffer containing Triton X-100 causes their renaturation as the result of an SDS:Triton X-100 exchange31.
These renatured MMPs digest gelatin during gel incubation in an incubation buffer, which can be finally observed as clear zymolytic bands in the Coomassie Blue-stained gel5. The amount and the area of digested gelatin presented as transparent bands corresponding to the gelatinolytic activity of MMPs can be determined using both commonly used and open-source applications—ImageLab and ImageJ29,32. Although this method has many advantages, it also possesses some limitations mentioned in the discussion. This "step by step" protocol with notes and comments performed on the different melanoma cell lines should be sufficient for reproducibility and optimization to obtain representative results. Figure 1 presents the steps of the described procedure.
Protocol
1. Cell culture medium collection and concentration
- Seed the melanoma cells (here A375, SK-MEL-28, Hs 294T, WM9, WM1341D cell lines) into tissue-culture 75 cm2 flasks in complete medium [Dulbecco's modified Eagle's medium-high glucose with reduced concentration (1.5 g/L) of NaHCO3, supplemented with 10% (v/v) Fetal Bovine Serum (FBS), 1% (v/v) L-Glutamine, and 1% (v/v) Antibiotic-Antimycotic].
- Culture the cells under standard conditions (5% CO2, 37 °C).
- After the cells reach 80-90% confluence, aspirate and discard the supernatant and wash the flasks 3 times with serum-free medium or PBS warmed to 37 °C to remove residual medium in the culture flask.
NOTE: The supernatant (complete medium) should be removed completely before adding the serum-free medium. FBS contains various MMPs, which may lead to false-positive results and wrong data analysis and interpretation29. - Add 10 mL of warm serum-free medium and incubate the cells at 37 °C in a humidified atmosphere of 5% CO2 for 48-72 h.
NOTE: The duration of cell incubation in the serum-free medium may affect cell viability, as well as the amount of secreted proteins. Therefore, the duration of cell starvation must be optimized before performing zymography depending on the type of cell line. The trick is to choose when cells are most viable and secrete the highest amount of MMPs. All melanoma cell lines used here were cultured in a serum-free medium for 48 h without affecting viability. The condition of the cells was verified using a phase-contrast microscope, although an XTT or MTT cell viability assay can be performed. - Collect the media from the cell cultures after 48-72 h and transfer them to 15 mL tubes. Keep the media constantly on ice to avoid protein degradation.
- Centrifuge the media for 20 min at 7,000 × g at 4 °C33.
NOTE: Centrifugation is crucial for removing floating intact cells, cell debris, and apoptotic bodies, as they may be sources of active MMPs. Skipping this step could lead to false-positive results and wrong data analysis and interpretation. Optionally, media can be filtered through 0.22-µm filters to remove apoptotic bodies, large microvesicles, and cell debris34. It is important to centrifuge the media before transferring to -20 °C for storage, as freezing and thawing of cells may lead to their damage, thus releasing intracellular components35. - Store the media at -20 °C if required before further analyses (STOP POINT 1).
- Thaw the media on ice and concentrate them to achieve the desired final concentrate volume using ultracentrifugal filter units with a 10-kDa cutoff (see the Table of Materials), according to the manufacturer's recommendation.
NOTE: Here, the obtained concentration factor was approximately 20 times. As the molecular weight of the detected MMPs is 43-215 kDa, it is recommended to use ultracentrifugal filter units with 10 kDa Nominal Molecular Weight Limit (NMWL). Using higher NMWL (30 kDa) may result in losing the MMP molecules with lower molecular weight (e.g., 43 kDa collagenase MMP-1). - Transfer the concentrated medium immediately to -80 °C and store at -80 °C (STOP POINT 2).
NOTE: Fresh or thawed samples should be kept on ice during the next steps of the protocol to avoid protein degradation. - Measure the concentration of proteins in collected and concentrated media using, e.g., the Bradford or BCA method, according to the manufacturer's recommendation (STOP POINT 3).
2. Preparation of SDS-PAGE separating and stacking gels with gelatin (1 mg/mL) for gelatin zymography
- Place the spacer plate with a short plate into a casting frame to form a cassette and place the frame on the casting stand.
NOTE: The spacer plate with 0.75-1.5 mm integrated spacers can be used (here 1 mm). Ensure that the working surface is stable and perfectly leveled. - Prepare 2.65 mg/mL of gelatin by weighing the appropriate amount of gelatin and dissolving it in ultrapure or sterile deionized H2O by warming the solution to 65 °C. Sterilize the solution using a 0.22-µm syringe filter. Cool the gelatin solution down to room temperature before preparing the separating gel.
NOTE: The gelatin solution can be stored for 1 week at 4 °C. Long-term storage may cause bacterial contamination and the presence of proteolytic enzymes secreted by them36. - Prepare 10 mL of 10% separating polyacrylamide gel by mixing 3.95 mL of 2.65 mg/mL gelatin, 3.3 mL of 30% acrylamide/bis-acrylamide, 2.5 mL of 1.5 M Tris-HCl (pH 8.8), 48 µL of 10% SDS, 80 µL of 50% glycerol, 70 µL of H2O, 48 µL of 10% APS, and 4.8 µL of TEMED.
NOTE: CAUTION! Wear gloves and goggles when handling acrylamide/bis-acrylamide. APS (an activator) and TEMED (catalyst of gel polymerization) must be added at the end of gel preparation, after which it is important to work quickly and efficiently. CAUTION! TEMED must be added under a fume hood. This volume of the separating gel is sufficient to prepare two separating gels with a 1 mm spacer suited to the referenced electrophoresis apparatus (see the Table of Materials). If a lower percentage of the gel is required, recalculate the volumes of the components to obtain the desired percentage of acrylamide in the gel. - Mix the 10% separating gel solution by inversion six to eight times, avoiding air bubble formation, and pour the solution into the gel cassette sandwich (step 2.1) up to 75% of the height of the short plate.
NOTE: The thickness of the gel, the amount of loaded protein, and incubation time in the incubation buffer (see step 5.1.3 for composition) should be optimized to obtain areas of the gel with the fully digested gelatin bands separated for individual MMPs. Ultimately, this depends on the amount of MMPs secreted by a given cell type. - Layer the top of the gel with 70% ethanol.
NOTE: It is essential to remove bubbles and prevent the gel from drying out and forming contact with air, which is unsuitable for polymerization. - Leave the gel for approximately 30 min at room temperature. When the polymerization is complete and a clear separation line between the gel mix and ethanol layers is visible, remove the ethanol layer carefully.
- Prepare 4% stacking gel (5 mL) by mixing 3.05 mL of H2O, 0.67 mL of 30% acrylamide/bis-acrylamide, 1.25 mL of 0.5 M Tris-HCl (pH 6.8), 50 µL of 10% SDS, 50 µL of 10% APS, and 5 µL of TEMED. Mix the 4% stacking gel solution by inversion six to eight times.
NOTE: This volume is sufficient to prepare two stacking gels with a 1 mm spacer. - Insert a comb on the top of the gel sandwich cassette immediately and fill it with the stacking gel solution.
- Incubate the stacking gel for approximately 30 min at room temperature to allow it to polymerize completely.
- Wrap the gels with moist paper towels, place them in a plastic bag, and keep the gels at 4°C for at least overnight but no longer than 1 week.
- Load the gel sandwich cassette into an electrode assembly, transfer it to the tank, and fill it with 1x SDS-PAGE buffer [250 mM Tris-HCl, 1.92 M glycine, 1% (w/v) SDS] before running the SDS-PAGE electrophoresis.
NOTE: SDS-PAGE buffer can be stored at 4 °C for extended periods. - Remove the comb from the gel.
3. Preparation of SDS-PAGE separating and stacking gels for total protein content determination
NOTE: The steps in section 3 are similar to the steps in section 2 describing gel preparation for gelatin zymography. Note that the composition of the gels is different. A gel for total protein content determination must not contain gelatin, as it will also be stained by Coomassie Brilliant Blue solution.
- Prepare 10 mL of 10% separating gel by mixing 3.34 mL of 30% acrylamide/bis-acrylamide, 2.5 mL of 1.5 M Tris-HCl (pH 8.8), 100 µL of 10% SDS, 3.99 mL of H2O, 50 µL of 10% APS, and 20 µL of TEMED.
- Mix the solution by inversion, avoiding air bubble formation, and transfer the solution to the gel cassette sandwich up to 75% of the height of the short plate.
- Layer the top of the gel with ethanol and leave the gel for approximately 30 min at room temperature. Remove the ethanol.
- Prepare 5 mL of 4% stacking gel by mixing 650 µL of 30% acrylamide/bis-acrylamide, 1.25 mL of 1.5 M Tris-HCl (pH 8.8), 50 µL of 10% SDS, 2.99 mL of H2O, 25 µL of 10% APS, and 12.5 µL of TEMED.
- Place a comb on the top of the gel sandwich cassette and fill it with stacking gel solution.
- Leave the gel for approximately 30 min at room temperature.
NOTE: The gel can be prepared just before SDS-PAGE electrophoresis or stored for a few days at 4 °C. For gel storage, wrap the gel in a wet paper towel and place it in a plastic bag to keep it moist. - Put the cassettes with the gels in the tank, load the gel sandwich cassette into an electrode assembly, and transfer the assembly to a tank with 1x SDS-PAGE buffer [250 mM Tris-HCl, 1.92 M glycine, 1% (w/v) SDS] before running the SDS-PAGE electrophoresis.
- Remove the comb from the gel.
4. Sample loading and electrophoresis running
- Prepare each SDS-PAGE sample for zymography by mixing a 1:1 ratio of 3-20 µg (here 10 µg) of concentrated medium with 2x nonreducing loading buffer [10 mM Tris pH 6.8, 1% (w/v) SDS, 10% (v/v) glycerol, 0.03% (w/v) bromophenol blue] based on the estimated concentration of protein in the medium.
NOTE: Loading buffer should be stored at 4°C. Using β-mercaptoethanol and dithiothreitol (DTT) is not recommended due to their ability to destroy disulfide bonds between cysteine residues, which results in the inability of the MMP to refold after electrophoresis. - Prepare the gel for the determination of total protein content by mixing samples in a 1:3 ratio with 4x reducing buffer [40% (v/v) glycerol, 240 mM Tris (pH 6.8), 8% (w/v) SDS, 0.04% (w/v) bromophenol blue, 5% (v/v) β-mercaptoethanol].
NOTE: CAUTION! Wear gloves and goggles, and work under the fume hood. Loading buffer should be stored at -20 °C. The same amount of protein should be loaded into each lane. Samples can be optionally stored at -80 °C for a few days if required (STOP POINT 4). - Incubate the samples for gelatin zymography for 20 min at 37 °C before running the electrophoresis.
NOTE: Do not heat the samples at a temperature higher than 37 °C. Thermal denaturation of proteins may lead to the inactivation of protease activity or prevent the refolding of the enzymes causing false-negative results and wrong data interpretation31. - Incubate the samples for total protein content determination for 10 min at 95 °C.
- Spin the samples for a few seconds in a small table centrifuge and load them into the wells of the gels.
- Optionally, load a molecular weight ladder and negative (samples that have been boiled for 10 min at 95 °C) and positive control samples (e.g., recombinant MMP-2 and MMP-9 or a cell line known to secrete MMP-2 and MMP-936) into the wells.
- Run electrophoresis, keeping it on ice until the dye flows out of the gel to provide good MMP-9 and MMP-2 band separation. Use the following SDS-PAGE electrophoresis conditions: initially, 20 mA per gel, increasing the power to 40 mA per gel when the samples enter the separating gel.
NOTE: The maximum electric current should not exceed 130 mA for four gels. Otherwise, the gel will get overheated during electrophoresis. Electrophoresis can be performed in a box containing ice or in a cold room (4 °C), if available.
5. Activation, staining, and destaining of the polyacrylamide gel
- Gel washing and activation
- Remove the zymography gel from between the short and spacer plates and transfer it to a plastic container with washing buffer [50 mM Tris pH 7.5, 150 mM NaCl, 10 mM CaCl2, 2.5% (v/v) Triton X-100]. Prepare washing buffer on the day of gelatin zymography.
NOTE: As Triton X-100 is very viscous, pipet it according to the rules for pipetting viscous liquids to add the same volume of Triton X-100 each time the buffer is prepared37. - Incubate the gel twice in the washing buffer, each time for 30 min with gentle agitation to remove SDS from the gel and replace it with Triton X-100.
- Transfer the gel to a plastic tank containing incubation buffer (50 mM Tris pH 7.5, 150 mM NaCl, 10 mM CaCl2).
NOTE: For long-term storage, keep the incubation buffer at 4 °C. Prepare fresh buffer if there are any signs of microbial contamination or precipitation. - Incubate the gel in the incubation buffer for 12-20 h (here 16 h) at 37 °C.
NOTE: The thickness of the gel and amount of loaded protein influence the incubation period in the incubation buffer, which should be optimized to obtain the areas of well-digested gelatin in the gel.
- Remove the zymography gel from between the short and spacer plates and transfer it to a plastic container with washing buffer [50 mM Tris pH 7.5, 150 mM NaCl, 10 mM CaCl2, 2.5% (v/v) Triton X-100]. Prepare washing buffer on the day of gelatin zymography.
- Gel staining and destaining
- Prepare the staining solution containing an aqueous solution of 0.5% (w/v) Coomassie Brilliant Blue R-250, 30% (v/v) of ethanol, and 10% (v/v) of acetic acid.
NOTE: CAUTION! Work under the fume hood and wear gloves when using acetic acid. Dissolve the weighed amount of Coomassie Blue in water, add ethanol, and mix the solution. In the end, slowly add acetic acid to the ethanol-water solution to avoid an exothermic reaction. - Prepare the destaining solution containing 30% (v/v) of ethanol and 10% (v/v) of the acetic solution in H2O.
NOTE: Mix ethanol with H2O. Add acetic acid slowly to the ethanol-water solution at the end to avoid an exothermic reaction. - Transfer the gel to a plastic container with Coomassie Brilliant Blue solution and stain the gel for 30 min at room temperature with gentle agitation.
NOTE: FastGene Q-Stain can also be used for staining the gel. The advantage of this dye is that it is ready to use and that the stained gel does not require any destaining step. - Incubate the gel in the destaining buffer at room temperature with gentle agitation to destain. Continue the destaining until clear bands representing the gelatinase activity are visible.
NOTE: If the staining with Coomassie Brilliant Blue solution is very intense, change the destaining buffer a few times during incubation. - Keep the gel moisturized and do not allow it to dry out until its visualization.
NOTE: Replace the destaining buffer with water to slow down the destaining process until visualization.
- Prepare the staining solution containing an aqueous solution of 0.5% (w/v) Coomassie Brilliant Blue R-250, 30% (v/v) of ethanol, and 10% (v/v) of acetic acid.
6. Visualization of proteins in polyacrylamide gels
NOTE: See the Table of Materials for details on the imaging system and software used to visualize proteins in polyacrylamide gels (Figure 2A-C). Other readily available gel visualization systems (such as iBright Imaging Systems, UVP PhotoDoc-It Imaging System, E-Gel Imager, and Azure Imagers) can also be used for this purpose.
- Switch imaging equipment on and open the software.
- Click File | New project to open a new project.
- Choose the application type by clicking Select... | Protein gels | Coomassie blue.
- Transfer the gels, one by one, into the imaging system and check the gel position using the Position Gel button.
- Visualize the gel by clicking Run protocol.
- Save the image as .scn to allow further analysis using the referenced software.
- Additionally, export file as .tif by clicking File | Export | Export for Analysis, which will be useful for analysis using ImageJ (STOP POINT 5).
7. Data analysis
- Gel analysis using the imaging software
- Open the imaging software and images of the zymogram and the gel representing the total protein content.
- Invert the image to obtain black bands on a white background. Under Go to the Image transform setting, click on the Invert image display.
- Select lines and bands on zymogram representing MMP-2 and MMP-9 manually or automatically using the Analysis Tool Box | Lane and Bands section. Create one band per line and adjust its size to include the whole line area for total protein content determination on the gel representing the total protein content.
- Adjust the lines and bands using the tools available in the Lane and Band section (Figure 2A'-C').
- Go to the Analysis Table and copy the Volume (intensity) and molecular weight of each lane and band (Figure 2A"-C").
- Divide the Volume of each lane per Volume of the selected control lane (here A375) to compare the total protein content between lanes (Figure 2A").
- Divide the Volume of each band per the value of the corresponding lane (from step 7.6) to normalize the Volume of MMP activity to the total protein content (Figure 2B",C").
- To compare the activity of gelatinases between lines, divide the normalized Volume of the studied line/studied condition per normalized Volume of the control line/standard condition (here A375) (Figure 2B",C").
- Alternative gel analysis using ImageJ software
- Open the ImageJ software and open the inverted gel image (black bands on a white background).
- Outline the first lane with gelatin-digested bands using the rectangle tool.
- Choose Analyze | Gels | Select First Lane in the ImageJ toolbar to select and mark the first lane.
- Transfer the first outlined selection (yellow rectangle) into the next lane and click Analyze | Gels | Select Next Lane to select and mark the next lane.
- Select all lanes presented on the gel in the same way (Figure 3A-C).
- Go to Select Analyze | Gels | Plot Lanes to make the lane profile plots (Figure 3A'-C').
- Using the Straight tool, separate bands with vertical lines at the edges of each peak and then draw horizontal lines to "close" their area (Figure 3A'-C'; yellow lines).
- Measure the size of each peak using the Tracking tool. Click in the area of each peak to select it and wait for the peaks to be outlined in yellow.
- Look for the size of each selected peak in the Results table (Figure 3A"-C").
- Normaize the data to the total protein content as described in step 7.1.
- Divide the "area" of the studied line/condition per "area" of the control line/standard condition (Figure 3A"-C").
- Data presentation
- Show the results obtained with the imaging software as the MMP activity normalized to total protein content and control line (Figure 4A,B).
- Present the results obtained with ImageJ as the MMP activity normalized to total protein content and control line (Figure 4C,D).
Results
In this protocol, we describe the procedure for gelatin zymography (Figure 1) using media obtained from five melanoma cell lines (A375, SK-MEL-28, Hs-294T, WM9, WM1341D) as samples. The zymography approach shown here includes two separate SDS-PAGE electrophoreses. One SDS-PAGE electrophoresis results in the Coomassie Blue-stained gel, representing the total protein content loaded in each line (Figure 2A). It is used for the normalization of gelatinase activity data. The second SDS-PAGE electrophoresis results in the gel with loaded MMPs that digest gelatin added to the gel. The result of gelatin zymography is the zymogram which, when stained with Coomassie Blue solution, should exhibit transparent bands at the proper molecular weight position, depending on the types of secreted MMPs (Figure 2B,C). These gelatin-digested areas are mostly identified as the gelatinolytic activity of MMP-9 and MMP-2.
The zymograms in this paper show the presence of both latent (pro) and active forms of MMP-9 and MMP-2 in all tested melanoma cell lines (Figure 2B,C). Two forms of both MMPs are observed owing to the different activation mechanisms of pro-MMPs and active MMPs. Active MMPs are activated proteolytically in vitro, whereby their N-terminal domains are cleaved, decreasing their molecular mass7. Pro-MMPs are denatured during SDS-PAGE electrophoresis, leading to the opening of their active sites and their autocatalytic activation but without proteolytic cleavage38. Subsequent renaturation causes protein refolding to make MMP-mediated gelatin digestion possible. Hence, pro-MMPs also exhibit gelatinolytic activity in gelatin zymography; however, they can be distinguished from the active MMPs because of their higher, zymogenic molecular mass39.
In the case of MMP-9, these data show that the molecular weight for pro- and active forms are approximately 93 and 80 kDa, respectively (Figure 2B',B''). Pro-MMP-2 is represented as a 68 kDa band and the active form of MMP-2 as 60 kDa (Figure 2C',C''). The molecular weight of MMP-2 as a proenzyme is 72 kDa in the literature. Nevertheless, the cleavage of MMP-2's prodomain results in its activation and changes the mass to 64 kDa9. MMP-9 is present in cells in the 92 kDa proenzyme form, and its activation by cleavage leads to the decrease in its protein mass to 83 kDa. The reason for these differences in the molecular masses of MMPs presented here compared to the literature is that the samples and the protein ladder migrate differently because the protein ladder, unlike the samples, is reduced31. Although gelatin zymography is commonly used to detect MMP-2 and MMP-9 activity, the activity of other MMPs, such as MMP-1 (collagenase 1, 43 kDa), may also be visible after gelatin zymography40. These bands, if visible, have much less intensity because gelatin is not their main substrate5. MMP-9 complexes with α2-microglobulin-related protein (125, 215 kDa) can also be detected after gelatin zymography, as they are based on disulfide bridges that are not dissociated in gelatin zymography31,41.
Densitometric analysis using ImageLab or ImageJ applications helps determine the total protein content in each lane (Figure 2A', 3A') and the level of gelatinase activity in melanoma cell lines (Figure 2B',C' and Figure 3B',C'). The result of densitometric analyses using ImageLab is the volume (the sum of all the intensities within the band boundaries) of the band as well as molecular weight (Figure 2A"-C"). ImageJ gives information about the peak area representing each band (Figure 3A"-C"). The more intense and the bigger the digested areas are, the higher the MMP activity that the test cell lines exhibit.
Here, we observed the highest pro-MMP-9 activity in Hs 294T cells and the highest activity of active MMP-9 in SK-MEL-28 cell line (Figure 2B", Figure 3B", Figure 4A, and Figure 4C). Although data analysis using both applications showed that MMP-9 activity is higher in SK-MEL-28 cells, there were differences in the observed values. In the case of the ImageJ data, the MMP-9 activity is more than 5 times higher than that of A375 cells but only approximately 3 times higher in the ImageLab data (Figure 2B'', Figure 3B'', Figure 4A, and Figure 4C). In the case of MMP-2 gelatinase, we observed a dramatically lower level of pro-MMP2 activity in the SK-MEL-28 cell line (Figure 4B and Figure 4D). However, the level of active MMP-2 was comparable in its activity among different melanoma cell lines when the ImageLab software was used for analysis (Figure 2C" and Figure 4B). Nevertheless, the data obtained from the ImageJ application indicated approximately 1.5 times higher MMP-2 activity in Hs 294T and 1.7 times in WM9 and WM1341D cells compared to A375 cells (Figure 3C" and Figure 4D). The slight differences in the results between the ImageLab and ImageJ analyses arise from the different calculations used by these applications.
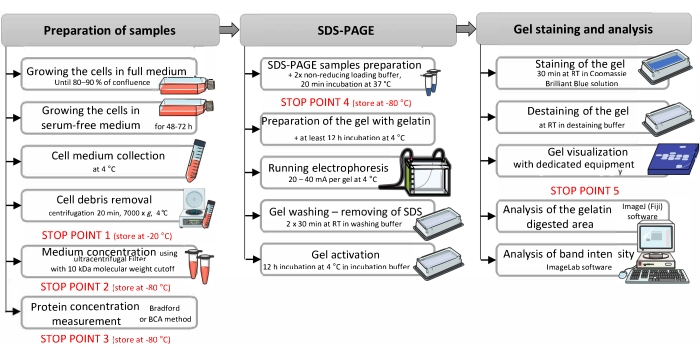
Figure 1: Schematic presentation of gelatin zymography protocol. Zymography can be divided into three main parts: i) Sample preparation, ii) SDS-PAGE electrophoresis, and iii) gel staining and analysis. The scheme shows the main steps of each part of this method with an extra annotation, which is important for the proper performance of the gelatin zymography. Stop points are indicated in red. The figure was assembled with the Servier Medical Art by Servier, licensed under a Creative Commons Attribution 3.0 Unported License42. Abbreviations: SDS-PAGE = sodium dodecyl sulfate-polyacrylamide gel electrophoresis; RT = room temperature. Please click here to view a larger version of this figure.

Figure 2: Representative results of gelatin zymography with bioinformatic analysis of gelatinase activity of MMPs in different melanoma cell lines using the ImageLab application. (A) After SDS-PAGE electrophoresis under denaturing but nonreducing conditions, the gel was stained with Coomassie Blue R-250 solution and visualized. (A', A") Analysis of total protein content in each lane. Every lane at its full height was selected and adjusted as one band (A'). The intensity of the signal of the total protein content for every lane was then automatically measured and presented in a table (A"). Additionally, the total protein content was normalized to the second lane (here, A375 cells). (B,C) Zymograms were obtained in gelatin zymography, representing the gelatin-free area digested by pro- and active-MMP-9 (B) and pro- and active-MMP-2 (C). Before analysis, the images representing white bands on the dark background were reversed to obtain the image showing black bands on a white background (shown as lower panels of B and C). (B',C') Densitometric analysis of the gelatinolytic activity of MMPs. Bands representative for pro- and active forms of (B') MMP-9 and (C') MMP-2 were selected and adjusted. (B",C") Tables showing densitometric data for gelatinolytic activity of (B") MMP-9 and (C") MMP-2 were obtained. The molecular weight and volume (intensity) of bands were measured automatically. The tables present the intensity of specific bands, normalized to the total protein content and then to the volume of the band from the second lane (A375). Please click here to view a larger version of this figure.
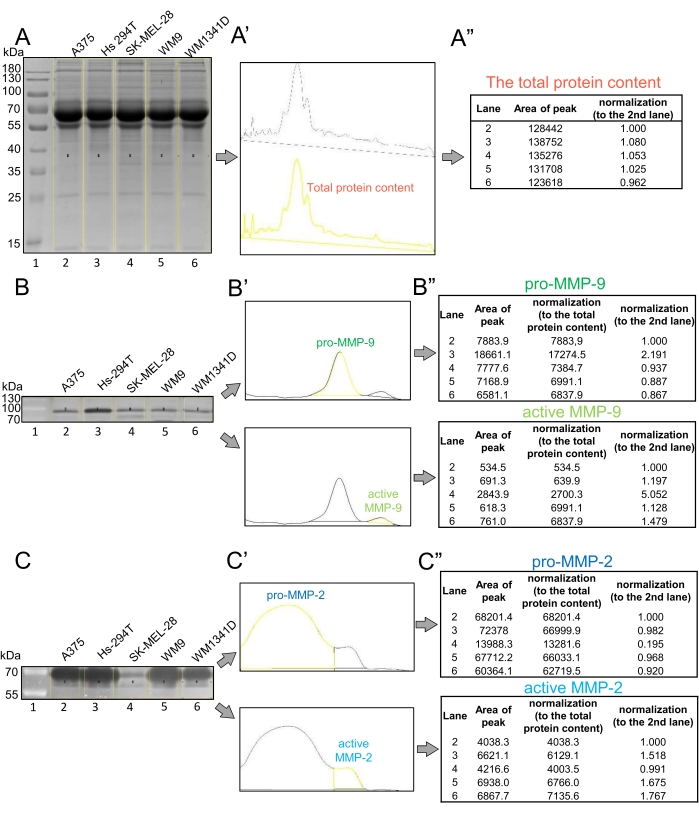
Figure 3: Representative results of gelatin zymography and bioinformatic analysis of the gelatinolytic activity of MMPs in different melanoma cell lines using the ImageJ application. Gels were visualized with ImageLab, but MMP activity was analyzed using the ImageJ application. (A-A") Analysis of the total protein content. (A) Every lane at its full height was selected and then adjusted as one band (A'), and the line profile plots for each lane were created. (A") Next, the area of peaks was automatically measured and presented in a table. The peak area values normalized to the second lane (A375 cell line) are also shown. (B,C) Zymograms with selected lines were obtained after the gelatin zymography. (B',C') Plot profiles showing gelatin-free areas digested by pro- and active forms of (B') MMP-9 and (C') MMP-2. (B",C") Densitometric analysis of the gelatinolytic activity of MMP-2 and MMP-9. Tables showing peak areas determined using ImageJ data for (B") MMP-9 and (C") MMP-2 activity. The peak areas were measured automatically. Tables also show the peak areas for specific bands normalized to the total protein content and the volume of the band from the second lane (here, A375 cells). Please click here to view a larger version of this figure.
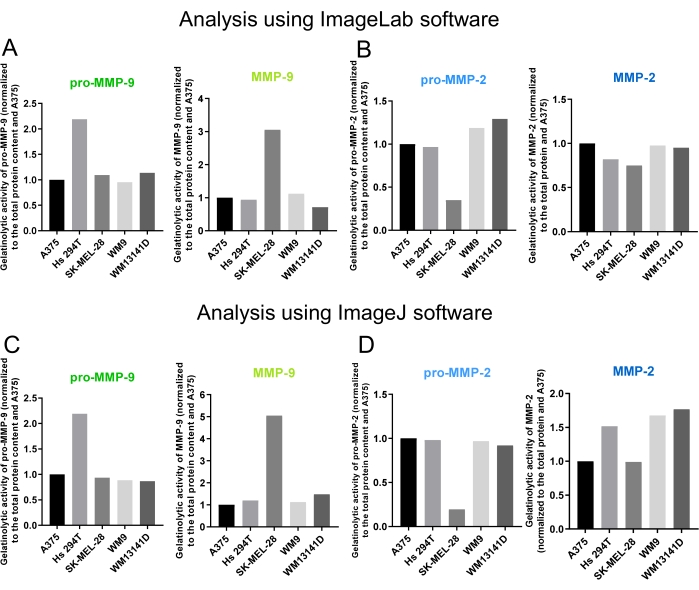
Figure 4: Final results showing the gelatinolytic activity of MMP-2 and MMP-9, determined using gelatin zymography, presented as graphs. The values presented in Figure 2A",B",C" and Figure 3A",B",C" were used to create graphs representing the gelatinase activity of the MMPs. Data are presented as MMP activity, normalized to the total protein content and A375 cell line. (A, B) Results obtained using ImageLab software, showing the activity of pro- and active forms of (A) MMP-9 and (B) MMP-2 gelatinases. (C, D) The ImageJ software results show the activity of pro- and active forms of (C) MMP-9 and (D) MMP-2 gelatinases. Please click here to view a larger version of this figure.
Discussion
Despite the "step by step" protocol elaborated here, gelatin zymography requires optimization depending on the samples/cell lines being analyzed. Different cell types and cell lines (melanoma cell lines shown here) may secrete both forms (pro- and active) of MMP-2 and MMP-9 but with different gelatinase activity. The optimization of the procedure includes mainly the duration of cell starvation, the thickness of the polyacrylamide gel, the amount of loaded protein, and the duration of gel incubation in the incubation buffer. Extended cell culture times may result in higher amounts of secreted MMPs, requiring less protein to be loaded on the gel. Furthermore, the gelatin copolymerized with a thinner gel will be digested more efficiently and rapidly by MMPs, leading to a reduction in gel incubation time to obtain sufficiently destained bands and reliable results. Despite optimization, repeating the protocol may still yield different-looking zymograms because of incompletely mixing gelatin within the gel during gel preparation, variation in temperature during SDS-PAGE electrophoresis or gel incubation in the incubation buffer, and possible partial degradation of samples during freeze-thaw cycles.
It is important to optimize the percentage of a gel. Here, we presented zymograms performed with 8% gels for MMP-2 and 10% gels for MMP-9. However, the percentage of the gel depends on the form of the MMP to be detected and the distribution pattern of the activity of the pro- and active forms of MMPs. For example, if the studied cells secrete a high level of MMP-2 with high gelatinase activity, this may obscure latent MMP-2 activity. In such a situation, the gel percentage must be decreased to separate the bands or reduce the incubation time in the incubation buffer to visualize less intense smaller bands.
The next critical step during zymography is maintaining a constant temperature of 4°C during SDS-PAGE. This will help prevent the active forms of the MMPs from digesting the gelatin during SDS-PAGE. Conducting SDS-PAGE at high temperatures will be manifested as blurred bands and smears. It is recommended to show the gelatin-digested areas due to all forms of MMPs secreted by the test cells on one zymogram. However, as presented here, it is necessary to run more than one SDS-PAGE experiment with different gel percentages to distinguish between different forms of MMPs or calculate their ratios. Alternatively, a gradient gel can be prepared. Its advantages include an increased range of molecular weights that can be separated in a single gel and better separation of proteins with close molecular weight43.
Additionally, to detect the weak activity of MMPs, the duration of incubation in the incubation buffer should be prolonged, as this period affects the size of the gelatin-digested area. However, prolonged gelatin digestion may reduce the reliability of the densitometric analysis of the more intense bands as all the gelatin will be over-digested. Positive controls will help estimate the molecular mass of MMPs, e.g., recombinant MMP-2 and MMP-9 or a cell line with known MMP activity. Negative controls can be reduced samples (boiled for 10 min at 95°C) or samples without gelatinase activity of MMPs. The negative control can also be the medium used for cell starvation to check whether the buffers used in the experiment are free from contamination, e.g., bacterial proteases36. Treatment of cells with tissue inhibitors of metalloproteinases (TIMPs) cannot be considered a negative control for gelatin zymography as they dissociate from MMPs during gelatin zymography5. It is crucial to culture the cells in a serum-free medium before sample collection and remove cell debris by centrifugation as FBS and cells contain various MMPs44. This could lead to false-positive results.
The absence of visible bands could result from reduced samples, no or very low MMP activity in samples, too short incubation of a gel in the activation buffer, too high gelatin concentration in the gel, or insufficient loaded protein. First, the amount of protein can be standardized by loading a broad range of protein amounts into the gel45. The absence of latent forms of the MMPs could be due to problems with the SDS:Triton X-100 exchange not leading to renaturation of pro-MMPs and subsequent gelatinase activation. As mentioned earlier, smears with blurred bands may be caused by gelatin digestion by MMPs activated during electrophoresis. A zymogram should exhibit sharp bands (areas with digested gelatin) against a dark background. Suppose the digested areas of the gelatin in the gel are not completely transparent compared to the dark blue background. In that case, it indicates insufficient gel destaining or incomplete gel digestion by MMPs, probably due to excessive gel thickness. Prolonged decolorization of the gel can result in less contrast between the background and the gelatin-digested areas. This may lead to problems with the analysis and unreliable results.
Although gelatin zymography is considered a quantitative assay because of densitometry used for data analysis, data interpretation must be done carefully. Additionally, data analysis is strongly dependent on the quality of zymograms, e.g., separation of bands and their size and shape. As gelatin-digested areas are manually selected and adjusted during analysis, the results may vary between data obtained from ImageJ and ImageLab. Moreover, although pro-MMPs — activated by SDS during SDS-PAGE (opening of active sites without proteolytic cleavage) and refolded — exhibit activity in gelatin zymography, they may not be physiologically active inside cells46. Further, if the active form of an MMP exhibits high gelatinolytic activity on a gelatin zymogram, its activity may not be high inside a cell. MMPs are bound to their inhibitors, which block their activity in vitro47 but dissociate from the MMPs during gelatin zymography, allowing them to be active and digest gelatin5. Thus, the results of gelatin zymography may not reflect the state of MMP activity inside a cell. Hence, we recommend normalizing MMP activity to the total protein content detected for every cell line. This will avoid misinterpretation of data in the case of unequal amounts of the loaded protein.
The main advantage of gelatin zymography is its sensitivity, which is much higher than that of western blot analysis. Even picograms of MMPs can be detected with gelatin zymography39. Additionally, the MMP activity measured by gelatin zymography may not strictly correlate with the amounts of MMPs secreted by the cells. This assay allows the detection of two types of MMPs that share gelatin specificity. An alternative or supplement for gelatin zymography is a fluorescent gelatin degradation assay48. This may help confirm the presence of gelatinase activity in melanoma cell lines. The basis of the gelatin degradation assay is an estimation of the activity of invadopodia based on their ability to digest a fluorescent gelatin-coated surface. The final data are usually presented as the size of the gelatin-fluorescein layer digested per cell or per area of a single cell. Although fluorescent gelatin degradation assay facilitates the correlation of gelatin degradation with the number and activity of invadopodia, it cannot, unlike gelatin zymography, distinguish the types of MMPs and estimate their ratios49,50,51. Additionally, the invadopodia formed by cells may not always colocalize with the gelatin-degradation area, and their number may not correlate with the size of the gelatin-digested area in melanoma cells52. As invadopodia may contain proteases other than MMPs, such as seprase53, the fluorescent gelatin degradation assay is not specific for MMPs. The gelatin zymography assay identifies and determines MMP activity based exclusively on their molecular weight. Additionally, MMP activity can be determined in concentration units in gelatin zymography. Another method that detects gelatinase activity is the ELISA targeting specific MMPs54. Although the method is highly sensitive and quantitative, it is definitely more expensive than gelatin zymography and cannot detect pro- and active forms of MMPs.
Gelatin zymography may be useful as a diagnostic technique for detecting MMP activity in biopsies from patients with melanoma, as it has been found that melanoma cells are rich in MMPs, which correlates with a poor prognosis for patients55. Thus, MMPs could be promising biomarkers for estimating melanoma occurrence, invasiveness, or progression. Gelatin zymography has been described as a method for detecting disease progression and metastasis of breast, pancreatic, bladder, ovarian, prostate, and brain tumors56. By correlating the activity of MMPs in the sera of breast cancer patients with their clinicopathological parameters, Minafra and colleagues showed that the determination of MMP activity may be a putative diagnostic tool not only to confirm the presence of breast cancer in patients but also to distinguish breast cancer subgroups57.
The literature shows that gelatin zymography has been successfully used to determine MMP activity in tumor cell lines, such as breast cancer with different metastatic potential (e.g., MCF-7, ZR-75-1, MDA-MB-435, MDA-MB-231, Hs578T)58,59, ovarian cancer (SKOV3 and COV504)60, and other different gynecological cancers61. Moreover, this technique is also used for tissue samples obtained from patients with colon61, rectal62, breast58,63, oral64, prostate65, and gastrointestinal66 cancers or in clinical research on MMP activity in the sera of normal individuals and breast cancer patients57. The use of gelatin zymography is also noted in noncancer cells crossing through or remodeling tissue barriers such as monocytes, macrophages, or osteoclasts67,68. The activity of MMPs in these cells arises from podosomes — structures similar to invadopodia69. Altogether, these reports demonstrate that a wide range of samples can be analyzed using gelatin zymography.
Disclosures
The authors declare no competing financial interests.
Acknowledgements
This work was supported by the National Center for Science, Poland (project #2016/22/E/NZ3/00654, granted to AJM).
Materials
| Name | Company | Catalog Number | Comments |
| 22 µm syringe filters | Nest | 331011 | |
| Acetic acid, 80% solution | Chempur | 115687330 | |
| Acrylamide/bis-acrylamide, 30% Solution, 37.5:1 | Bioshop | ACR010.500 | |
| Amicon Ultra-15 Centrifugal Filter Units | Millipore | UFC901008 | ultracentrifugal filter units with 10 kDa cutoff |
| Ammonium Persulfate (APS) | Sigma-Aldrich | A-3678 | |
| Antibiotic-Antimycotic | Gibco | 15240062 | |
| Bradford reagent | Sigma | B6916 | |
| Bromophenol blue | Polskie Odczynniki Chemiczne | 184070219 | |
| Calcium chloride dihydrate - CaCl2 · 2H2O | Sigma-Aldrich | C-5080 | |
| ChemiDoc System | Bio-rad | imaging system | |
| Coomasie Brilliant Blue R-250 | Merck | 1.12553.0025 | |
| Ethanol | Chempur | 1139641800 | |
| FastGene Q-Stain | NIPPON Genetics EUROPE | FG-QS1 | |
| Fetal Bovine Serum - FBS | Gibco | 10270-106 | |
| Gelatin from porcine skin | Sigma-Aldrich | G-8150 | |
| Glycerol | Sigma-Aldrich | L-4909 | |
| glycine | BioShop | CAS #56-40-6 | |
| high glucose Dulbecco’s modified Eagle’s medium with reduced concentration (1.5 g/l) of NaHCO3 | Pracownia Chemii Ogólnej IITD PAN | 11-500 | |
| ImageJ software (Fiji) | https://imagej.nih.gov/ij/ | version 1.52p | |
| ImageLab software | Bio-rad | ||
| L-Glutamine | Gibco | 25030-024 | |
| Mini-PROTEAN Tetra Cell | Bio-Rad | #1658001EDU | |
| N,N,N′,N′-Tetramethylethylenediamine (TEMED) | Sigma-Aldrich | T9281 | |
| PageRuler Prestained Protein Ladder | Thermo Fisher Scientific | 26616 | |
| Pierce BCA Protein Assay Kit | Thermo Fisher Scientific | 23225 | |
| PowerPac Basic Power Supply | Bio-rad | 1645050EDU | |
| Sodium chloride - NaCl | Chempur | 7647-14-5 | |
| Sodium dodecyl sulfate (SDS) | Sigma-Aldrich | L4509 | |
| Tissue-culture 75 cm2 flask | VWR | 10062-872 | |
| Trisma base | Sigma-Aldrich | T1503 | |
| Triton X-100 | Sigma-Aldrich | X100 |
References
- Giannelli, G. Induction of cell migration by matrix metalloprotease-2 cleavage of laminin-5. Science. 277 (5323), 225-228 (1997).
- Fowlkes, J. L., Enghild, J. J., Suzuki, K., Nagase, H. Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. Journal of Biological Chemistry. 269 (41), 25742-25746 (1994).
- Morodomi, T., Ogata, Y., Sasaguri, Y., Morimatsu, M., Nagase, H. Purification and characterization of matrix metalloproteinase 9 from U937 monocytic leukaemia and HT1080 fibrosarcoma cells. Biochemical Journal. 285 (2), 603-611 (1992).
- Crabbe, T., Ioannou, C., Docherty, A. J. P. Human progelatinase A can be activated by autolysis at a rate that is concentration-dependent and enhanced by heparin bound to the C-terminal domain. European Journal of Biochemistry. 218 (2), 431-438 (1993).
- Snoek-van Beurden, P. A. M., Vonden Hoff, J. W. Zymographic techniques for the analysis of matrix metalloproteinases and their inhibitors. BioTechniques. 38 (1), 73-83 (2005).
- Okada, Y., et al. Matrix metalloproteinase 2 from human rheumatoid synovial fibroblasts. Purification and activation of the precursor and enzymic properties. European Journal of Biochemistry. 194 (3), 721-730 (1990).
- Birkedal-Hansen, H., et al. Matrix metalloproteinases: a review. Critical Reviews in Oral Biology & Medicine. 4 (2), 197-250 (1993).
- Murphy, G., Knäuper, V. Relating matrix metalloproteinase structure to function: Why the "hemopexin" domain. Matrix Biology. 15 (8-9), 511-518 (1997).
- Björklund, M., Koivunen, E. Gelatinase-mediated migration and invasion of cancer cells. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 1755 (1), 37-69 (2005).
- Abécassis, I., Olofsson, B., Schmid, M., Zalcman, G., Karniguian, A. RhoA induces MMP-9 expression at CD44 lamellipodial focal complexes and promotes HMEC-1 cell invasion. Experimental Cell Research. 291 (2), 363-376 (2003).
- Legrand, C., et al. Airway epithelial cell migration dynamics: MMP-9 role in cell- extracellular matrix remodeling. Journal of Cell Biology. 146 (2), 517-529 (1999).
- Iida, J., et al. Cell surface chondroitin sulfate glycosaminoglycan in melanoma: Role in the activation of pro-MMP-2 (pro-gelatinase A). Biochemical Journal. 403 (3), 553-563 (2007).
- Noë, V., et al. Release of an invasion promoter E-cadherin fragment by matrilysin and stromelysin-1. Journal of Cell Science. 114, 111-118 (2001).
- Radisky, E. S., Raeeszadeh-Sarmazdeh, M., Radisky, D. C. Therapeutic potential of matrix metalloproteinase inhibition in breast cancer. Journal of Cellular Biochemistry. 118 (11), 3531-3548 (2017).
- Raeeszadeh-Sarmazdeh, M., Do, L., Hritz, B. Metalloproteinases and their inhibitors: potential for the development of new therapeutics. Cells. 9 (5), 1313 (2020).
- Hadler-Olsen, E., Winberg, J. -. O., Uhlin-Hansen, L. Matrix metalloproteinases in cancer: their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biology. 34 (4), 2041-2051 (2013).
- Ricci, S., D'Esposito, V., Oriente, F., Formisano, P., Di Carlo, A. Substrate-zymography: a still worthwhile method for gelatinases analysis in biological samples. Clinical Chemistry and Laboratory Medicine. 54 (8), 1281-1290 (2016).
- Deryugina, E. I., et al. MT1-MMP initiates activation of pro-MMP-2 and integrin αvβ3 promotes maturation of MMP-2 in breast carcinoma cells. Experimental Cell Research. 263 (2), 209-223 (2001).
- Nguyen, M., Arkell, J., Jackson, C. J. Activated protein C directly activates human endothelial gelatinase A. Journal of Biological Chemistry. 275 (13), 9095-9098 (2000).
- Nguyen, M., Arkell, J., Jackson, C. J. Thrombin rapidly and efficiently activates gelatinase A in human microvascular endothelial cells via a mechanism independent of active MT1 matrix metalloproteinase. Laboratory Investigation. 79 (4), 467-475 (1999).
- Klein, T., Bischoff, R. Physiology and pathophysiology of matrix metalloproteases. Amino Acids. 41 (2), 271-290 (2011).
- Nakahara, H., et al. A Mechanism for regulation of melanoma invasion. Journal of Biological Chemistry. 271 (44), 27221-27224 (1996).
- Egeblad, M., Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer. 2 (3), 161-174 (2002).
- Van't Veer, L. J., et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 415 (6871), 530-536 (2002).
- Hofmann, U. B., et al. Expression and activation of matrix metalloproteinase-2 (MMP-2) and its co-localization with membrane-type I matrix metalloproteinase (MTI-MMP) correlate with melanoma progression. Journal of Pathology. 191 (3), 245-256 (2000).
- Ohnishi, Y., Tajima, S., Ishibashi, A. Coordinate expression of membrane type-matrix metalloproteinases-2 and 3 (MT2-MMP and MT3-MMP) and matrix metalloproteinase-2 (MMP-2) in primary and metastatic melanoma cells. European Journal of Dermatology EJD. 11 (5), 420-423 (2001).
- Karelina, T. V., Hruza, G. J., Goldberg, G. I., Eisen, A. Z. Localization of 92-kDa type IV collagenase in human skin tumors: comparison with normal human fetal and adult skin. Journal of Investigative Dermatology. 100 (2), 159-165 (1993).
- Houde, M. Differential regulation of gelatinase b and tissue-type plasminogen activator expression in human bowes melanoma cells. International Journal of Cancer. 53 (3), 395-400 (1993).
- Hu, X., Beeton, C. Detection of functional matrix metalloproteinases by zymography. Journal of Visualized Experiments: JoVE. (45), e2445 (2010).
- Heussen, C., Dowdle, E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Analytical Biochemistry. 102 (1), 196-202 (1980).
- Woessner, F. J. Quantification of matrix metalloproteinases in tissue samples. Methods in Enzymology. 248, 510-528 (1995).
- Schindelin, J., et al. Fiji: an open platform for biological image analysis. Nature Methods. 9 (7), 676-682 (2012).
- Drożdż, A., et al. Low-vacuum filtration as an alternative extracellular vesicle concentration method: A comparison with ultracentrifugation and differential centrifugation. Pharmaceutics. 12 (9), 872 (2020).
- Lobb, R. J., et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. Journal of Extracellular Vesicles. 4 (1), 27031 (2015).
- McGann, L. E., Yang, H., Walterson, M. Manifestations of cell damage after freezing and thawing. Cryobiology. 25 (3), 178-185 (1988).
- Toth, M., Fridman, R., Sohail, A., Fridman, R. Assessment of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Metastasis Research Protocols. 878, 121-135 (2012).
- Marx, V. Pouring over liquid handling. Nature Methods. 11 (1), 33-38 (2014).
- Oliver, G. W., Leferson, J. D., Stetler-Stevenson, W. G., Kleiner, D. E. Quantitative reverse zymography: analysis of picogram amounts of metalloproteinase inhibitors using gelatinase A and B reverse zymograms. Analytical Biochemistry. 244 (1), 161-166 (1997).
- Kleiner, D. E., Stetlerstevenson, W. G. Quantitative zymography: detection of picogram quantities of gelatinases. Analytical Biochemistry. 218 (2), 325-329 (1994).
- Troeberg, L., Nagase, H. Measurement of matrix metalloproteinase activities in the medium of cultured synoviocytes using zymography. Inflammation Protocols. 225, 77-88 (2003).
- Triebel, S., Bläser, J., Reinke, H., Tschesche, H. A 25 kDa α2-microglobulin-related protein is a component of the 125 kDa form of human gelatinase. FEBS Letters. 314 (3), 386-388 (1992).
- . Servier Medical Art Available from: https://smart.servier.com/ (2021)
- Walker, J. M. Gradient SDS polyacrylamide gel electrophoresis. Methods in Molecular Biology. 1, 57-61 (1984).
- Jobin, P. G., Butler, G. S., Overall, C. M. New intracellular activities of matrix metalloproteinases shine in the moonlight. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 1864 (11), 2043-2055 (2017).
- Gogly, B., Groult, N., Hornebeck, W., Godeau, G., Pellat, B. Collagen zymography as a sensitive and specific technique for the determination of subpicogram levels of interstitial collagenase. Analytical Biochemistry. 255 (2), 211-216 (1998).
- Murphy, G., Crabbe, T. Gelatinases A and B. Methods in Enzymology. 248, 470-484 (1995).
- Baker, A. H., Edwards, D. R., Murphy, G. Metalloproteinase inhibitors: biological actions and therapeutic opportunities. Journal of Cell Science. 115 (19), 3719-3727 (2002).
- Mazurkiewicz, E., Mrówczyńska, E., Simiczyjew, A., Nowak, D., Mazur, A. J. A fluorescent gelatin degradation assay to study melanoma breakdown of extracellular matrix. Melanoma. Methods in Molecular Biology. 2265, 47-63 (2021).
- Malek, N., et al. The origin of the expressed retrotransposed gene ACTBL2 and its influence on human melanoma cells' motility and focal adhesion formation. Scientific Reports. 11 (1), 3329 (2021).
- Malek, N., et al. Knockout of ACTB and ACTG1 with CRISPR/Cas9(D10A) technique shows that non-muscle β and γ actin are not equal in relation to human melanoma cells' motility and focal adhesion formation. International Journal of Molecular Sciences. 21 (8), 2746 (2020).
- Makowiecka, A., et al. Thymosin β4 regulates focal adhesion formation in human melanoma cells and affects their migration and invasion. Frontiers in Cell and Developmental Biology. 7, 304 (2019).
- Mazurkiewicz, E., et al. Gelsolin contributes to the motility of A375 melanoma cells and this activity is mediated by the fibrous extracellular matrix protein profile. Cells. 10 (8), 1848 (2021).
- Monsky, W. L., et al. A potential marker protease of invasiveness, seprase, is localized on invadopodia of human malignant melanoma cells. Cancer Research. 54 (21), 5702-5710 (1994).
- Barascuk, N., et al. A novel assay for extracellular matrix remodeling associated with liver fibrosis: An enzyme-linked immunosorbent assay (ELISA) for a MMP-9 proteolytically revealed neo-epitope of type III collagen. Clinical Biochemistry. 43 (10-11), 899-904 (2010).
- Nikkola, J., et al. High serum levels of matrix metalloproteinase-9 and matrix metalloproteinase-1 are associated with rapid progression in patients with metastatic melanoma. Clinical Cancer Research. 11 (14), 5158-5166 (2005).
- Roy, R., Yang, J., Moses, M. A. Matrix metalloproteinases as novel biomarker s and potential therapeutic targets in human cancer. Journal of Clinical Oncology. 27 (31), 5287-5297 (2009).
- La Rocca, G., Pucci-Minafra, I., Marrazzo, A., Taormina, P., Minafra, S. Zymographic detection and clinical correlations of MMP-2 and MMP-9 in breast cancer sera. British Journal of Cancer. 90 (7), 1414-1421 (2004).
- Figueira, R. C., et al. Correlation between MMPs and their inhibitors in breast cancer tumor tissue specimens and in cell lines with different metastatic potential. BMC Cancer. 9 (1), 20 (2009).
- Das, A., Monteiro, M., Barai, A., Kumar, S., Sen, S. MMP proteolytic activity regulates cancer invasiveness by modulating integrins. Scientific Reports. 7 (1), 14219 (2017).
- Cui, Y., et al. Knockdown of EPHA1 using CRISPR/CAS9 suppresses aggressive properties of ovarian cancer cells. Anticancer Research. 37 (8), 4415-4424 (2017).
- Schröpfer, A., et al. Expression pattern of matrix metalloproteinases in human gynecological cancer cell lines. BMC Cancer. 10 (1), 553 (2010).
- Kim, T. -. D., et al. Activity and expression of urokinase-type plasminogen activator and matrix metalloproteinases in human colorectal cancer. BMC Cancer. 6 (1), 211 (2006).
- Di Cara, G., et al. New insights into the occurrence of matrix metalloproteases -2 and -9 in a cohort of breast cancer patients and proteomic correlations. Cells. 7 (8), 89 (2018).
- Ikebe, T., et al. Gelatinolytic activity of matrix metalloproteinase in tumor tissues correlates with the invasiveness of oral cancer. Clinical & Experimental Metastasis. 17, 315-322 (1999).
- Di Carlo, A., et al. Matrix metalloproteinase-2 and -9 in the urine of prostate cancer patients. Oncology Reports. 24 (1), 3-8 (2010).
- Augoff, K., et al. Upregulated expression and activation of membrane-associated proteases in esophageal squamous cell carcinoma. Oncology Reports. 31 (6), 2820-2826 (2014).
- Yang, Y., Lu, N., Zhou, J., Chen, Z. -. N., Zhu, P. Cyclophilin A up-regulates MMP-9 expression and adhesion of monocytes/macrophages via CD147 signalling pathway in rheumatoid arthritis. Rheumatology. 47 (9), 1299-1310 (2008).
- Wittrant, Y., et al. Regulation of osteoclast protease expression by RANKL. Biochemical and Biophysical Research Communications. 310 (3), 774-778 (2003).
- Murphy, D. A., Courtneidge, S. A. The "ins" and "outs" of podosomes and invadopodia: characteristics, formation and function. Nature Reviews Molecular Cell Biology. 12 (7), 413-426 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved