Method Article
Organ Culture System for Assessing the Toxicity of Intraocular Treatment Excipients and Pharmaceuticals
In This Article
Summary
The goal of this protocol is to evaluate changes in metabolic activity and refractive function of the lens in response to experimental treatment.
Abstract
As the leading cause of blindness, cataracts are a significant burden for the tens of millions of people affected globally by this condition. Chemical exposures, among other environmental factors, are an established cause of cataracts. Ocular toxicity testing can assess whether pharmaceuticals and their components may contribute to lens damage that may lead to cataracts or aid the treatment of cataracts.
In vitro studies and in vivo animal testing can be used for assessing the safety of chemicals prior to clinical studies. The Draize test-the current in vivo standard for ocular toxicity and irritancy testing-has been criticized for lack of sensitivity and objective measurements of determining ocular toxicity. In vitro cell-based assays are limited as cell cultures cannot appropriately model an intact functional lens.
The method described here is a sensitive in vitro alternative to animal testing, designed to evaluate the response of the intact bovine lens to treatment at both the cellular activity level and for overall refractive performance. The non-toxic reagent resazurin is metabolized in proportion to the level of cell activity. The lens laser-scanner assay measures the ability of the lens to refract incident beams of light to a single point with minimal error, directly relevant to its natural function. The method may be used to determine both acute and delayed changes in the lens, as well as the recovery of the lens from chemical or environmental exposures.
Introduction
Affecting over 20 million people, cataracts are the most prevalent cause of blindness worldwide1,2. Cataracts are most commonly due to age-related changes in the lens but are also induced from trauma, genetic conditions, disease, or toxic exposures2. Currently, treatment involves surgical intervention to replace the lens, an expensive and invasive procedure accessible mainly to those in developed countries. The extensive burden of cataract has directed decades of research towards cataract prevention and the development of non-surgical treatment. In both cases, the importance of preclinical testing for toxicity, efficacy, and pharmacokinetics of ophthalmic drugs is paramount. This process of drug development relies heavily on the information provided by studies performed in animals.
The current standard for ocular toxicity testing in vivo is the Draize test, involving the delivery of a test compound to the conjunctival sac of a live animal. The test has been significantly criticized, particularly concerning animal ethics, subjectivity, poor repeatability, and variability3. Additionally, there is no component of the Draize test that directly monitors the effects of test substances on the lens. Considerable effort has been invested in developing alternative in vitro models4. However, none have been sufficiently validated to replace the Draize test5. Similarly, many of these models face limitations with respect to the direct application to cataracts and other complex pathologies6. For example, methods grading lens transparency when placed over a grid are inherently subjective7. Cell culture studies are reliable and highly utilized, though cell monolayer characteristics may diverge from primary tissue culture8.
Whole lenses can be dissected from the eyes of animals and cultured to maintain their original structure and function. One assay that is useful for assessing lens function while maintaining the organ's condition is the lens laser-scanner assay involving a scanner developed at the University of Waterloo in Canada. The assay is a scanning system that uses a series of laser projections to measure the optical quality or refractive performance of the lens. Lenses are scanned in their custom two-segment culture chambers, allowing beams to pass from below through the lens (Figure 1A). A camera fixed inside the scanner captures the image of the laser passing through the lens at numerous points. The scanner software computes the distance behind the lens at which it intersects with a central axis (back vertex distance, BVD), producing a series of measurements that indicate how consistently the lens focuses light to a single point (Figure 1).
The cellular properties of the lens, such as the tight and ordered arrangement of its cells, help maintain transparency and minimize scatter so that the lens can functionally focus light9. This measure can be used to interpret how significantly a chemical disrupts the essential structure of the lens, such as the gradient refractive index, and how much function is compromised because of the induced opacities. Other studies that have followed the response of cultured lenses and lens vesicles suggest that light scatter is a product of structural changes, as compared to metabolic changes, and that disruptions to lens lipids and proteins may affect the refractive index and consequently increase scatter10,11.
The lens laser-scanner can be used in conjunction with metabolic reagents in assays to determine biochemical measures of cell toxicity. Resazurin is a non-toxic chemical reagent metabolized by active cells, producing a reduced product (resorufin) with a measurable fluorescence12. The lens is largely devoid of organelles, except the metabolically active mitochondria concentrated within the anterior epithelium and superficial cortical fiber cells, fulfilling lens energy requirements13,14. Damage to the lens at the cellular level may disrupt metabolism and often precedes the onset of pathogenic structural changes and cataract15.
The purpose of this method is to evaluate the effect of xenobiotic and environmental exposures on the lens, which may contribute to cataract development. The protocol involves two assays to evaluate the effect of a treatment using the cultured bovine lens. The advantage of this approach is that it provides both a cellular and functional evaluation of how the lens as a primary tissue responds to treatment. It is a sensitive and objective evaluation of the lens as compared to other common methods16,17,18.
The model has been successfully used to evaluate the effects of various exposures, including surfactants, consumer products, alcohols, and ultraviolet radiation17,19,20. Changes in optical quality are consistently present in cultured lenses as a response to toxic exposure21. The ability of this method to maintain long-term lens culture is well-suited for monitoring the potentially delayed effect of a compound, and the recovery of the lens from induced damage or cataract22,23. Results produced from the application of this protocol can be used to reduce dependence on animal testing in the development of ophthalmic products.
Protocol
All experimental protocols were carried out in compliance with the University of Waterloo ethics policies for research using animal tissue. The bovine eyes for the current study were abattoir-provided, obtained from non-dairy cows within a few hours of death, and were dissected immediately, a process that takes up to 8 h from obtaining the eyes. Eyes should be dissected immediately to preserve sterility and dissection quality. The culture medium is prepared to a pH of 7.4 and sterile-filtered prior to supplementation with FBS21. All procedures are carried out under sterile conditions, with material and equipment sources listed in the Table of Materials.
1. Bovine lens culture
- Dissect lenses by removing extraocular muscles and tissue from the sclera, removing the posterior half of the globe and vitreous, removing the iris and ciliary body together with the lens, then cutting away the zonular attachments, with the final cut positioned such that the lens will drop into the medium-filled culture chamber.
- Culture the dissected lenses in custom chambers with a base adapted to fit the laser-scanning system, with 21 mL of sterile-filtered medium, at 37 °C and 5% CO2. Use 3% fetal bovine serum (FBS)-supplemented culture medium with 1% penicillin-streptomycin, 9.4 g/L M-199, 0.1 g/L L-glutamine, 5.96 g/L HEPES, 2.2 g/L sodium bicarbonate, and 7 mL/L NaOH. Store unused culture medium in the refrigerator for up to 7 days.
- Replace the culture medium every 1-2 days. Warm the culture medium for an hour at 37 °C prior to use. Use a suction-connected sterile Pasteur pipette to aspirate the medium from an individual culture chamber and replenish immediately with the supplemented medium.
- Culture the lenses for 48 h after dissection to allow time for physical damage to manifest. Inspect and scan the lenses for optical quality according to section 4 prior to inclusion in an experiment.
NOTE: Lenses are oriented face-down in preparation for the laser-scanner assay.
2. Control procedure
- Prepare a solution with the desired concentration of a solubilizing agent that is compatible with the chemical compound and culture medium.
- Prepare a vehicle control solution by adding the solubilizing agent to supplemented medium. Warm the control solution for an hour at 37 °C prior to experimentation.
- Use a Pasteur pipette connected to suction to aspirate the culture medium from control lens chambers. Replenish the chambers with the control solution. If required, perform this step with samples in triplicate.
NOTE: A minimum volume of 7 mL is required to completely cover the lens in the anterior face-up position. The conditions for controls in the current study were 21 mL of untreated medium control, 21 mL of a vehicle control medium, and 7 mL of phosphate-buffered saline (PBS). - Culture the lenses in control medium for 2 days, then replace with new control medium according to section 1. For short-term exposures, aspirate the control solution from the chambers and perform a rinse at least three times with unsupplemented culture medium before resuming the lens culture protocol as described in section 1.0.
- Allow the lenses to acclimate in the incubator at 37 °C and 5% CO2 for at least 3.5 h prior to the assessment of optical quality.
3. Exposure procedure
- Prepare a solution with the desired concentration of a solubilizing agent that is compatible with the chemical compound and culture medium. Combine the chemical with the solubilizing agent, allowing the appropriate time for interaction if required.
NOTE: 2-Hydroxypropyl-β-cyclodextrin was used in the current study to enhance the solubility of lanosterol (0.033 g/L) in medium. Benzalkonium chloride (BAK 0.0075%) solution was prepared using PBS. - Incorporate the solubilized chemical into the culture medium. Prepare a total volume sufficient to provide 21 mL of medium to all lens samples. Warm the experimental medium for an hour at 37 °C prior to experimentation.
- Use a Pasteur pipette connected to suction to aspirate the culture medium from the lens chambers. Immediately replenish the chambers with the test solution. After the exposure interval, replace with supplemented culture medium, performing a rinse first if needed. If required, perform this step with samples in triplicate.
NOTE: In the current study, test conditions were 21 mL of lanosterol-supplemented medium and 7 mL of a BAK 0.0075% solution. A minimum volume of 7 mL is required to completely cover the lens. In this case, lenses must first be oriented anterior face-up. A Pasteur pipette can be used to create a current in the culture chamber with some of the surrounding culture medium to repositions the lens. - Return the lenses to the incubator for at least 3.5 h to acclimate prior to the assessment of optical quality.
NOTE: Lenses are oriented face-down in preparation for the laser-scanner assay.
4. Optical quality assay (lens laser-scanner)
- Ensure that the lenses are oriented with the anterior face down and visually level in the culture chamber, using the technique from step 3.3 to adjust the position of the lens if needed.
NOTE: This position ensures the incident beam will be reflected up through the chamber bottom and pass through the lens from the anterior to the posterior surface. - Position a lens culture chamber into the laser-scanner such that the chamber carriage pin fits into the slot in the chamber base.
- Select the lens and scanpoint for which to perform a scan, right-click on scan lens, and wait for the scanner prompts for the beam to be found and aligned. Select well-distanced beginning and endpoints for the beam behind the lens. Ensure the central beam is aligned using the gross and fine adjustment buttons in the scanner software and adjustment knob on the scanner. When the beam is aligned, click calibrate and input an appropriate selection of radial steps and beam separation.
NOTE: For this study using the bovine lens, the settings for radial steps and beam separation were set to 10 steps and 0.5 mm, respectively. The first scan that the scanner performs serves to calibrate the laser-scanner. This is required once, and all additional lens scans may be performed directly after alignment. - Once the scanner has been calibrated, click scan. Wait for values to be generated for BVD mean, standard deviation, and error along one axis after completion of the lens scan, and the scope of the beam has been set. Set the scope from where beams exit the lens to the desired endpoint, selecting the maximum distance behind the lens while excluding any apparent interference.
- Use the generated chart to view the individual back vertex distances for each beam.
NOTE: Exclude beams passing through lens sutures. To exclude a beam, use the on-screen legend to right-click on an individual beam and select exclude. - Manually pivot the position of the culture chamber base inside the scanner by 90°, such that the second scan along the anterior lens surface will be perpendicular to the previous scan. Ensure the central beam is aligned as detailed in step 4.3, and then scan the lens.
5. Metabolic activity assay (resazurin)
- Prepare medium without FBS. Warm the medium for an hour at 37 °C. Prepare 50 mL of 8% resazurin reagent in unsupplemented medium.
NOTE: It is sufficient to prepare 50 mL to fill all wells in a 12-well plate. The solution should be prepared sterile under dim conditions in an opaque container, as resazurin is light-sensitive. - Add 3.8 mL of 8% resazurin solution to each well within a clear-bottom, sterile 12-well plate to cover the lens.
- Use sterile metal scoops to carefully lift lenses from under the posterior surface and perform a rinse using a Pasteur pipette to wash the lens with a small volume of unsupplemented culture medium. Place the lenses individually in each well.
- Incubate the well plate at 37 °C and 5% CO2 for 5 h. After the incubation, remove the lenses with metal scoops from the wells and place them in an animal waste disposal container. Transfer a 100 µL sample from each well into a sterile, clear-bottom, 96-well plate.
NOTE: Lenses may be assessed multiple times with the metabolic activity assay. In this case, return the lenses to the culture chambers with fresh medium for incubation. The metabolic activity assay is best performed once all scans are completed, as the dye may affect the BVD measurements. - Measure the endpoint fluorescence of the 100 µL samples using a fluorescent plate reader, with the excitation and emission wavelengths set at 560 nm and 590 nm, respectively.
6. Data analysis
- For the optical quality assay, calculate the average of the software-calculated values for BVD error24 from the two scans performed at each time point. Perform this calculation for all lenses and all scanpoints.
- For the metabolic activity assay, collect the endpoint relative fluorescence values generated by the plate reader. Normalize the data by setting the average of all control values at a scanpoint to 100%. Calculate the activity of each lens as a percentage of the average control value for that scanpoint.
- Perform a normality test for all experiment groups. Assuming the data are normal, analyze the BVD error differences between control and experimental lenses using the two-way ANOVA. Additionally, perform Tukey's post-hoc multiple comparisons test. Perform a one-way ANOVA and Dunnett's post-hoc test for the metabolic activity data.
Results
Figure 2 and Figure 3 (n = 6) demonstrate the results of a study testing the effect of chemical treatment (lanosterol) on the bovine lens. Lanosterol is a naturally occurring sterol in the lens that once showed promising results as a potential pharmaceutical intervention for cataracts25, although this has yet to be proven26. The study design included a medium and vehicle control for the compound. There was no significant difference between the vehicle (2-hydroxypropyl-β-cyclodextrin) and medium control (p > 0.05), indicating that any potential effect in the experimental group is not likely to be due to the vehicle. There was no significant difference in BVD variability between the treatment and the control groups (p > 0.05). These results were consistent with the metabolic activity assay (Figure 3). Therefore, the treatment did not introduce significant toxicity to the cells or significantly affect the refractive performance of the lens (p > 0.05).
Figure 4 and Figure 5 (n = 3) show the results of treatment with BAK on the lens. BAK is a surfactant and the most commonly used preservative in ophthalmic formulations27. A 10 min exposure resulted in significantly greater BVD variability in the treated lenses compared to the control at 4 days postexposure (p < 0.05). The treatment also produced a significant difference in lens metabolic activity (p < 0.05).

Figure 1: Determination of back vertex distance as a measure of optical quality using laser-scanner. (A) A series of beams are passed through the lens while it is seated in its culture chamber along one axis. (B) The beams pass through the lens at specified intervals. The back vertex distance is determined for each beam, and BVD mean (in mm) and BVD error values are generated as quantitative measures of lens refractive function. This information is displayed graphically, with BVD shown on the x-axis and beam position on the y-axis. The more sharply that beams are focused to a consistent point behind the lens (C), the lesser the calculated BVD error value compared to lenses of poorer optical quality (D). Abbreviation: BVD = back vertex distance. Please click here to view a larger version of this figure.
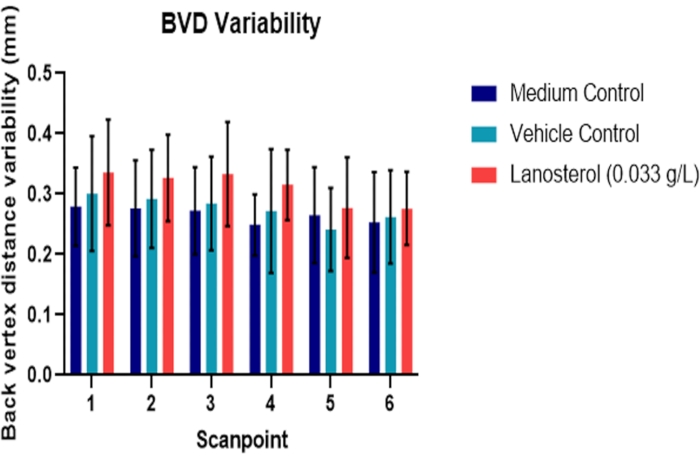
Figure 2: Effect of lanosterol suspension on bovine lens optical quality. Back vertex distance variability reflects the ability of the lens to refract light to a single point. The optical quality of lanosterol-treated lenses was similar to that of untreated medium and vehicle control lenses (p > 0.05) (n = 6). The data are represented as mean ± standard deviation. Abbreviation: BVD = back vertex distance. Please click here to view a larger version of this figure.
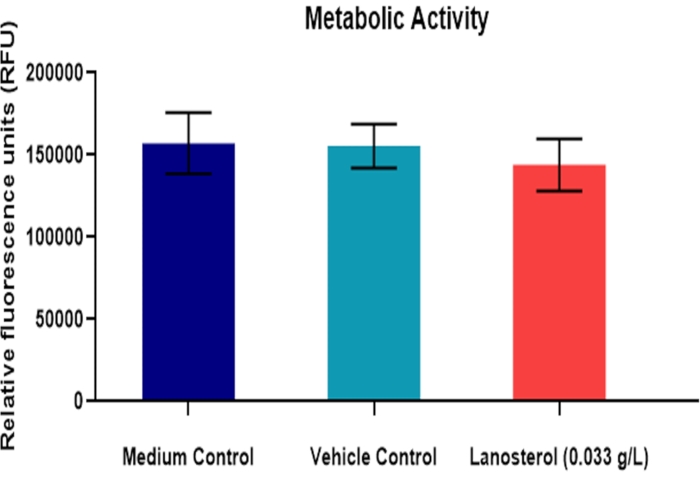
Figure 3: Effect of lanosterol suspension on the metabolic activity of the bovine lens. Mean metabolic activity of bovine lenses, quantified by the relative fluorescence of a metabolized indicator after exposure to a vehicle-suspended lanosterol treatment (n = 6). The data are represented as mean ± standard deviation. Please click here to view a larger version of this figure.

Figure 4: Effect of benzalkonium chloride on bovine lens optical quality. An exposure to BAK 0.0075% for 10 min produced gradually increasing back vertex distance variability within the treatment lenses (n = 3). Differences were significant between treated and medium control lenses 4 days postexposure, as well as for the treated lenses between their preexposure and postexposure scanpoints (p < 0.05). The data are represented as mean ± standard deviation. Abbreviations: BVD = back vertex distance; BAK = benzalkonium chloride. Please click here to view a larger version of this figure.
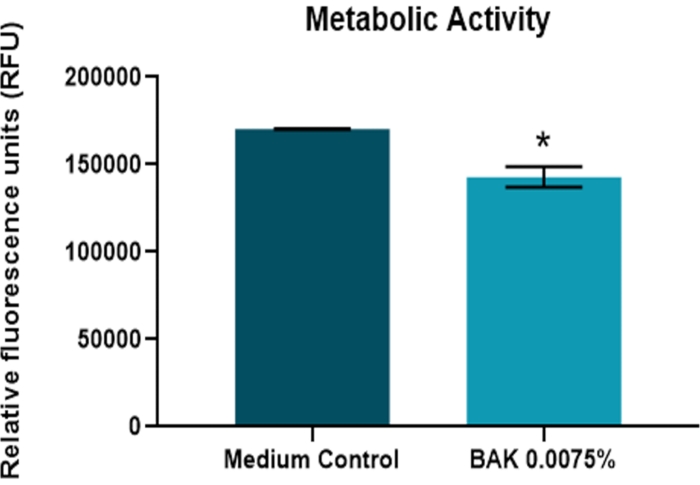
Figure 5: Effect of benzalkonium chloride on the metabolic activity of the bovine lens. The endpoint of metabolic activity was measured 4 days after a 10 min exposure to BAK 0.0075% (n = 3). Changes in metabolic activity were significantly different from the control (p > 0.05). The data are represented as mean ± standard deviation. Abbreviation: BAK = benzalkonium chloride. Please click here to view a larger version of this figure.
Discussion
The purpose of this protocol is to directly evaluate the effects of chemicals or environmental exposures on the lens in primary tissue culture. First, lenses are dissected and scanned for optical quality. Prevention of contamination and ensuring dissection quality are critical. Lenses are scanned at periodic intervals to continuously monitor changes in refractive function with respect to the control group or preexposure condition. The metabolic activity assay represents an endpoint to determine whether the exposures have impacted cellular metabolism. These are the critical steps to determine whether a xenobiotic substance or environmental condition causes significant toxicity, potentially leading to cataracts and whether the lens may recover from this treatment.
Lenses are scanned for optical quality within their respective culture chambers. Although lenses can also be exposed to a test substance within their chambers, one of the limitations of this protocol is that lenses are sensitive to changes in osmolarity and must be continually nourished with serum and maintained within an appropriate medium. This presents a challenge for treatments with long exposure intervals or poor solubility within culture medium28. As the assay uses video imaging, suspensions with large amounts of insoluble particles may introduce scatter, which is not an indication of lens performance. The conditions that produced the representative data using a lanosterol suspension indicate that the protocol can tolerate certain low-level concentration suspensions. While it has been suggested previously that the cultured bovine lens can correlate with responses in the human lens21, key differences, including but not limited to UV filtration, age-related compaction, and phospholipid content, limit the range of substances for which this protocol is appropriately used in preclinical testing29,30.
Ocular toxicity testing necessarily involves a large battery of tests to determine a broad picture of the safety and tolerance of a compound, beginning with in vitro and animal testing before proceeding to clinical trials. The bovine lens assay has high throughput for the number of times a lens can be scanned as the method is non-destructive and can be performed easily within a few minutes. However, testing large numbers of lenses can have a low throughput, as dissecting a lens from an eye can be time-consuming. Use of the laser-scanner has been more broadly used to study guinea pig, fish, pig, rat, and chick lenses31,32,33,34,35. Ideally, the results of preclinical testing provide insight into the safety and potential risk in humans. While human lenses would be most useful in this respect, as human and animal lenses will inevitably differ in some cases36, abattoir-provided lenses are useful in the balancing of available resources and ethics. This protocol represents a sensitive, reproducible, non-toxic, and objective in vitro method for testing both the cellular and functional conditions of the lens in response to treatment.
In comparison with the current in vivo standard for ocular toxicity testing, the lens laser-scanner provides a direct assessment of the effects of potentially toxic exposures on the lens. Owing to the common embryological tissue origin of the lens and cornea, as well as functional similarities such as transparency and refraction, the primary culture of the lens represents a suitable model for ocular irritancy. Preliminary validation studies of the lens laser-scanner have shown comparable results with respect to the Draize test and have even been shown to be more sensitive without inflicting any discomfort onto a live animal18. These results are additionally collected objectively, without the interpretation of an observer.
The lens laser-scanner assay measurements are directly relevant to the natural function of the lens in vivo. Moreover, unlike assays that culture the cornea or cell lines, the bovine lens maintains its refractive function using the long-term cell culture method developed, and the optical quality assay can be performed while maintaining the lens in its environment. The result is that, unlike other assays that produce a single endpoint as a result of the test itself damaging the lens, the optical quality assay can be performed repeatedly while successfully culturing the lens for up to 1000 h24.
As the lens is largely devoid of organelles, with the exception of the anterior epithelium and superficial cortical fiber cells, these cells perform organelle functions for the entire lens37. It is straightforward then to understand the connection between cellular changes and the induction of lens cataracts, as observed in vitro and in vivo15,19. Lens metabolic activity essentially represents the activity of the anterior epithelial monolayer. While assays similar to resazurin are available, for example, tetrazolium salts including MTT, XTT, MTS, and WST, resazurin provides a non-toxic and sensitive assay highly compatible with primary lens culture. Unlike MTT, which requires the solubilization of precipitated crystals, the resazurin protocol does not involve solutions that are likely to induce lysis. Additionally, cell culture studies have implied that the resazurin endpoint is more sensitive than tetrazolium salt assays38.
This method is designed to model the response of the lens as an ocular tissue and optical device to various chemical and environmental exposures. The two representative compounds chosen for this investigation are benzalkonium chloride, a preservative in ophthalmic solutions, and lanosterol, a sterol previously studied as part of an effort to find pharmaceutical interventions for cataracts. The results demonstrate lens stress in response to the preservative and no significant response to lanosterol. This method could be used further to study the toxicity of potential pharmaceutical treatments for cataract.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
Thanks to the Natural Sciences and Engineering Research Council (NSERC) and the Canadian Optometric Education Trust Fund (COETF) for the funds for this project.
Materials
| Name | Company | Catalog Number | Comments |
| (2-Hydroxypropyl)-β-cyclodextrin | Sigma-Aldrich | H107 | Powder |
| 1 L bottle-top filtration system | VWR | 97066-204 | Full Assembly, bottle-top, 0.2 μm |
| 100 mm Petri dish | VWR | 89022-320 | Slippable, media saver style, sterile |
| 12 well-plate | Corning | 353043 | Sterile, clear-bottom |
| 35 mm petri dish | VWR | 25373-041 | Falcon disposable petri dishes, sterile, Corning |
| 96 well-plate | VWR | 29442-072 | Sterile, clear-bottom |
| Alamar blue (resazurin) | Fischer Scientific | DAL1100 | Molecular Probes cell viability reagent |
| Benzalkonium chloride solution | Sigma-Aldrich | 63249 | 50% in H20 |
| Biosafety cabinet | |||
| Cytation 5 plate reader | BioTek | CYT5MPV | Cell imaging multi-mode reader |
| Fetal bovine serum | ThermoFischer Scientific | 12484028 | Qualified, heat inactivated, Canada |
| HEPES | Sigma-Aldrich | H3375 | For cell culture, powder |
| Incubator | |||
| Lanosterol | Sigma-Aldrich | L5768 | ≥93%, powder |
| L-glutamine | Sigma-Aldrich | For cell culture, powder | |
| Medium (M-199) | Sigma-Aldrich | M3769 | Modified, with Earle′s salts, without L-glutamine, sodium bicarbonate, and phenol red, powder, suitable for cell culture |
| Pasteur pipettes | 5 3/4'', with and without cotton | ||
| Penicillin-Streptomycin | ThermoFischer Scientific | 15140122 | Liquid (10,000 U/mL) |
| Phospate buffer saline (PBS) | liquid, sterile, suitable for cell culture | ||
| Pipette tips (100 µL, 1,000 µL, 5,000 µL) | VWR | Sterile | |
| ScanTox (lens laser-scanner) | Specially developed in-house | N/A | Scans lens with a laser to determine lens optical quality |
| ScanTox culture chamber | Specially developed in-house | N/A | Holds bovine lens in place during testing and culturing |
| Sodium bicarbonate | Sigma-Aldrich | S5761 | For cell culture, powder |
| Sodium hydroxide | Sigma-Aldrich | S2770 | 1.0 N, BioReagent, suitable for cell culture |
References
- Khairallah, M., et al. Number of people blind or visually impaired by cataract worldwide and in world regions, 1990 to 2010. Investigative Ophthalmology & Visual Science. 56 (11), 6762-6769 (2015).
- Priority eye diseases. World Health Organization Available from: https://www.who.int/blindness/causes/priority/en/index1.html (2014)
- Wilhelmus, K. R. The Draize eye test. Survey of Ophthalmology. 45 (6), 493-515 (2001).
- Jester, J. V. Extent of corneal injury as a biomarker for hazard assessment and the development of alternative models to the Draize rabbit eye test. Cutaneous and Ocular Toxicology. 25 (1), 41-54 (2006).
- Vinardell, M. P., Mitjans, M. Alternative methods for eye and skin irritation tests: an overview. Journal of Pharmaceutical Sciences. 97 (1), 46-59 (2008).
- Bonneau, N., Baudouin, C., Reaux-Le Goazigo, A., Brignole-Baudouin, F. An overview of current alternative models in the context of ocular surface toxicity. Journal of Applied Toxicology. , (2021).
- Bree, M., Borchman, D. The optical properties of rat, porcine and human lenses in organ culture treated with dexamethasone. Experimental Eye Research. 170, 67-75 (2018).
- Leist, C. H., Meyer, H. P., Fiechter, A. Potential and problems of animal cells in suspension culture. Journal of Biotechnology. 15 (1-2), 1-46 (1990).
- Bassnett, S., Shi, Y., Vrensen, G. F. Biological glass: structural determinants of eye lens transparency. Philosophical Transactions of the Royal Society of London. Series B, Biological sciences. 366 (1568), 1250-1264 (2011).
- Alghamdi, A. H. S., Mohamed, H., Sledge, S. M., Borchman, D. Absorbance and light scattering of lenses organ cultured with glucose. Current Eye Research. 43 (10), 1233-1238 (2018).
- Tang, D., et al. Light scattering of human lens vesicles in vitro. Experimental Eye Research. 76 (5), 605-612 (2003).
- O'Brien, J., Wilson, I., Orton, T., Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry. 267 (17), 5421-5426 (2000).
- Bantseev, V., Sivak, J. G. Confocal laser scanning microscopy imaging of dynamic TMRE movement in the mitochondria of epithelial and superficial cortical fiber cells of bovine lenses. Molecular Vision. 11, 518-523 (2005).
- Remington, L. A., McGill, E. C. . Clinical Anatomy of the Visual system. , (1998).
- Michael, R. Development and repair of cataract induced by ultraviolet radiation. Ophthalmic Research. 32, 1-44 (2000).
- Hamid, R., Rotshteyn, Y., Rabadi, L., Parikh, R., Bullock, P. Comparison of alamar blue and MTT assays for high through-put screening. Toxicology In Vitro. 18 (5), 703-710 (2004).
- Bantseev, V., et al. Mechanisms of ocular toxicity using the in vitro bovine lens and sodium dodecyl sulfate as a chemical model. Toxicological Sciences. 73 (1), 98-107 (2003).
- Sivak, J. G., Herbert, K. L., Segal, L. Ocular lens organ culture as a measure of ocular irritancy: The effect of surfactants. Toxicology Methods. 4 (1), 56-65 (1994).
- Youn, H. Y., Moran, K. L., Oriowo, O. M., Bols, N. C., Sivak, J. G. Surfactant and UV-B-induced damage of the cultured bovine lens. Toxicology In Vitro. 18 (6), 841-852 (2004).
- Sivak, J. G., Stuart, D. D., Herbert, K. L., Van Oostrom, J. A., Segal, L. Optical properties of the cultured bovine ocular lens as an in vitro alternative to the Draize eye toxicity test: Preliminary validation for alcohols. Toxicology Methods. 2 (4), 280-294 (1992).
- Wong, W., Sivak, J. G., Moran, K. L. Optical response of the cultured bovine lens; testing opaque or partially transparent semi-solid/solid common consumer hygiene products. Toxicology In Vitro. 17 (5-6), 785-790 (2003).
- Sivak, J. G., Stuart, D. D., Weerheim, J. A. Optical performance of the bovine lens before and after cold cataract. Applied Optics. 31 (19), 3616-3620 (1992).
- Stuart, D. D., Sivak, J. G., Cullen, A. P., Weerheim, J. A., Monteith, C. A. UV-B radiation and the optical properties of cultured bovine lenses. Current Eye Research. 10 (2), 177-184 (1991).
- Dovrat, A., Sivak, J. G. Long-term lens organ culture system with a method for monitoring lens optical quality. Photochemistry and Photobiology. 81 (3), 502-505 (2005).
- Zhao, L., et al. Lanosterol reverses protein aggregation in cataracts. Nature. 523 (7562), 607-611 (2015).
- Daszynski, D. M., et al. Failure of oxysterols such as lanosterol to restore lens clarity from cataracts. Scientific Reports. 9 (1), 8459 (2019).
- Baudouin, C., Denoyer, A., Desbenoit, N., Hamm, G., Grise, A. In vitro and in vivo experimental studies on trabecular meshwork degeneration induced by benzalkonium chloride (an American Ophthalmological Society thesis). Transactions of the American Ophthalmological Society. 110, 40-63 (2012).
- Schartau, J. M., Kroger, R. H., Sjogreen, B. Short-term culturing of teleost crystalline lenses combined with high-resolution optical measurements. Cytotechnology. 62 (2), 167-174 (2010).
- Truscott, R. J. Age-related nuclear cataract-oxidation is the key. Experimental Eye Research. 80 (5), 709-725 (2005).
- Deeley, J. M., et al. Human lens lipids differ markedly from those of commonly used experimental animals. Biochimica et Biophysica Acta. 1781 (6-7), 288-298 (2008).
- Bantseev, V., et al. Effect of hyperbaric oxygen on guinea pig lens optical quality and on the refractive state of the eye. Experimental Eye Research. 78 (5), 925-931 (2004).
- Choh, V., Sivak, J. G. Lenticular accommodation in relation to ametropia: the chick model. Journal of Vision. 5 (3), 165-176 (2005).
- Oriowo, O. M., et al. Evaluation of a porcine lens and fluorescence assay approach for in vitro ocular toxicological investigations. Alternatives to Laboratory Animals: ATLA. 30 (5), 505-513 (2002).
- van Doorn, K. L., Sivak, J. G., Vijayan, M. M. Optical quality changes of the ocular lens during induced parr-to-smolt metamorphosis in Rainbow Trout (Oncorhynchus mykiss). Ocular lens optical quality during induced salmonid metamorphosis. Journal of Comparative Physiology. A, Neuroethology, Sensory, Neural, and Behavioral Physiology. 191 (7), 649-657 (2005).
- Herbert, K. L., Sivak, J. G., Bell, R. C. Effect of diabetes and fructose/non-fructose diet on the optical quality (cataracts) of the rat lens. Current Eye Research. 19 (4), 305-312 (1999).
- Wormstone, I. M., Collison, D. J., Hansom, S. P., Duncan, G. A focus on the human lens in vitro. Environmental Toxicology and Pharmacology. 21 (2), 215-221 (2006).
- Oyster, C. W. The human eye: structure and function. Sinauer Associates. , (1999).
- Xu, M., McCanna, D. J., Sivak, J. G. Use of the viability reagent PrestoBlue in comparison with alamarBlue and MTT to assess the viability of human corneal epithelial cells. Journal of Pharmacological and Toxicological Methods. 71, 1-7 (2015).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved