Method Article
Intracavernous Pressure Recording in a Cavernous Nerve Injury Rat Model
* These authors contributed equally
In This Article
Summary
This protocol describes developing a stable bilateral cavernous nerve injury rat model of radical prostatectomy associated with erectile dysfunction and intracavernous pressure measurement.
Abstract
The bilateral cavernous nerve (CN) injury rat model has been extensively used to simulate clinical cavernous nerve injury associated with erectile dysfunction (ED) for evaluating the effect of clinical therapeutic methods. However, the methods of CN injury model construction are flawed and varied in the ED research field. It is CN crush injury that is the most commonly used method in recent years. This study aims to provide a detailed description of the procedure of bilateral CN injury rat model construction and measurement of intracavernous pressure (ICP) recording, providing a reliable and reproducible CN injury rat model. This work successfully developed the CN injury method of hemostat crush injury using a syringe needle as hard support and a hemostat with a rubber sleeve. Also, this method concludes that a voltage of 1.0 V, frequency of 20 Hz, and pulse-width of 5 ms are the optimized stimulation parameters for ICP recording in a bilateral CN injury rat model.
Introduction
ED is one of the common diseases in adult men. It is estimated that the number of ED patients in the world will reach 322 million by 20251. One multicenter extensive sample survey in China shows that the proportion of ED caused by pelvic surgery or trauma is about 8%2. Despite the continuous improvement of surgical techniques and surgical instruments, the incidence of ED is still high. It has been considered that the development and progression of ED after nerve-sparing radical prostatectomy (RP) contributes to cavernous nerve injury resulting in atrophy of corpus cavernosum smooth muscle, apoptosis of endothelial cells, and pathological remodeling3,4.
For studying the mechanism of hemodynamics and histopathology changes of CN injury associated with ED, several different types of CN injury animal models have been developed and assessed, including rodent, dog, cat, and monkey5,6,7. Relying on the advantages in expenditure and reproducibility, the bilateral CN injury rat model has become the most common model for assessing ED after radical pelvic surgery8. However, various forms of nerve injury have been reported in numerous literature whose principal differences are nerve injury approaches (crush, freezing, transection, and excision)9,10,11. Furthermore, the diversity of nerve injury approaches might lead to inconsistency in intracavernous pressure (ICP) recording parameters in the rat model, which determines the accuracy and evaluation of ICP8. Nevertheless, there is not a standardized method for inducing nerve injury and recording ICP of the model yet.
Therefore, this study aims to build a more reliable and reproducible bilateral CN injury rat model. This method provides a detailed description of the procedure of model construction and ICP measurement, which might be beneficial to study the mechanisms of ED and develop effective treatments in the future.
Protocol
Fifteen adult male Sprague-Dawley rats (3-month-old) weighing between 300-350 g were used in this study. All animal procedures were performed following the NIH Guidelines for the Care and Use of Laboratory Animals and with the approval of The fifth affiliated hospital of Sun Yat-Sen University Institutional Animal Care and Use Committee. Animals were housed in a comfortable facility with temperature and light controlled.
1. Preparation for surgical procedure materials
- Prepare the following instruments: scalpel, tissue scissors, thread scissors, bending forceps, tissue forceps, microsurgery forceps, Hartman mosquito hemostatic forceps, sterile surgical sheets, a microneedle holder, rat abdominal retractors, and biological signal acquisition and processing system (see Table of Materials).
- Sterilize all surgical instruments before operation. Use alcohol (70% ethanol) wipes to clean the surgical area.
NOTE: The surgical instruments should be sterilized by alcohol immersion overnight.
- Sterilize all surgical instruments before operation. Use alcohol (70% ethanol) wipes to clean the surgical area.
- Prepare the pressure recording system
- Connect a 10 mL syringe containing heparin saline and a hypodermic 25 G needle to a 3-way stopcock with a tube (20 cm length). Flush the sterilized tube with sterile heparin saline (200 U/mL).
NOTE: Filling the tube with heparin saline avoids introducing air bubbles into the system.
- Connect a 10 mL syringe containing heparin saline and a hypodermic 25 G needle to a 3-way stopcock with a tube (20 cm length). Flush the sterilized tube with sterile heparin saline (200 U/mL).
- Lift the 25 G needle 20 cm (just the tube length) above the animal operating pad. Then examine the measurement accuracy of the pressure recording system by flushing or tapping.
2. Preparation of the animal
- Anesthetize rats by sodium pentobarbital (60 mg/kg) intraperitoneal injection (see Table of Materials).
NOTE: To confirm sufficient depth of anesthesia, an evaluation of spontaneous breathing rhythm and the reflexes of a rat via pinching hind paw was performed. - Apply ointment on bilateral eyes to avoid corneal dryness.
- After confirming a proper anesthetization, shave the lower half of the abdomen, neck, and perineum using an electric shaver. Place the rat in the supine position on a heating pad (37 °C). Wear medical gloves to maintain sterile conditions during surgical procedures.
3. CN isolation and injury procedure
- Use a scalpel to make a 4 cm incision through the skin at the lower, midline abdominal. To fully expose the bladder and the prostate, use tissue scissors and tissue forceps to make a proper length incision through the subcutaneous fascia, the muscular tissue, and the peritoneum.
- Use a rat abdominal retractor to enlarge the operative field map. Use absorbent cotton swabs to separate the prostate from the adjacent tissues, such as ligaments.
NOTE: Major pelvic ganglion (MPG) and CN could be found at one of two the dorsolateral areas of the prostate. - Use angled micro scissors to incise the fascia overlying CN 1-6 mm distal to MPG. Then slide a 9-0 suture under the CN with the use of microsurgery forceps.
- Place a syringe needle (25 G) underneath the CN, 5 mm distal to MPG. Then put the hemostat in the light of the "hemostat tip-syringe needle-nerve-hemostat tip" sandwich structure (Figure 1 and Figure 2).
NOTE: The syringe needle needs to be ground flat. - Apply the hemostat with full tip closure at 5 mm distal from the ganglion for 1 min, then withdraw the hemostat and the syringe needle (Figure 2).
- Uplift the nerve slightly via a 9-0 suture, and place the hooks of the bipolar electrode (see Table of Materials) around the CN 2-4 mm distal to MPG (Figure 3).
NOTE: Two pairs of MPG and CN wereoperated in the same way.
4. Catheterization of the corpus cavernosum and stimulation of the CN for ICP measurement
- Flush the tube with sterile heparin saline (200 U/mL) before introducing it into the corpus cavernosum.
- Hold the 25 G needle and keep the insert direction parallel with the course of the corpus cavernosum (Figure 3).
NOTE: The tunica albuginea should be stretched to facilitate the insertion. - Push the 25 G needle 6 mm into the corpus cavernosum (Figure 3). Flush the tube and press the corpus cavernosum lightly to evaluate the sensitivity of the transducer (Figure 4). To prevent accidental falling off, fix the pipe on the worktable with adhesive tape.
- Use the following parameters for CN stimulation: voltage at 1.0 V, frequency at 20 Hz, pulse width at 5 ms. Apply 1 min of stimulation with 5 min of rest between the following stimulation.
NOTE: Turn the 3-way stopcock to the pressure transducer channel when starting the measurement.
5. Postoperative Care
- Place the rats on a warmed pad (37 °C) and monitor them carefully for anesthesia recovery.
- For postoperative pain control, provide non-steroidal anti-inflammatory drugs (such as Carprofen, 0.5 mg/kg, subcutaneous injection) (see Table of Materials) when the rats fully recover.
- Move rats to the aseptic cage and monitor them 2 days to evaluate the incisional wound's nourishment state, mental state, and infection.
Results
The surgery procedure produced a typical ICP response curve using this protocol with the recommended stimulation settings. The ICP response curve rises instantly when stimulating the nerve and drops when the stimulation is withdrawn (Figure 5). It is essential to examine the intracavernous pressure line before measuring the ICP, which affects the evaluation of increased ICP values (Figure 4).
As illustrated in Figure 6, there is no significant difference between the peak ICP and plateau of ICP when voltage is above 1.0 V on normal rats (without cavernous nerve injury). However, the peak ICP and plateau of ICP increase with increasing stimulation voltage above 1.0 V after cavernous nerve injury (Figure 7). The ICP measurement was assessed at pre-operation, 0, 7, and 28 days following CN crush. There was a significant difference of ICP between 0 days and 7 or 28 days of post-operation, but no statistical difference between 7 days and 28 days (Figure 8). It indicates that the CN injury rat model following the current method is reliable.
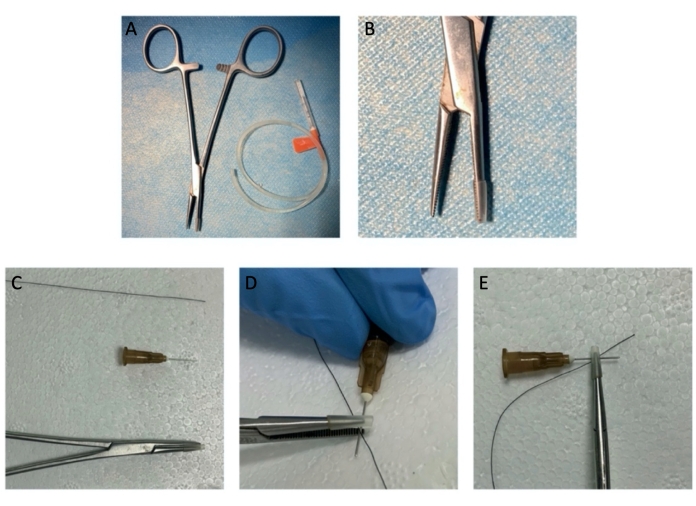
Figure 1: The instruments of hemostat crush injury. (A, B) The hemostat with a rubber sleeve. (C-E) The simulative structure of "hemostat tip-syringe needle-nerve-hemostat tip" is shown. Please click here to view a larger version of this figure.
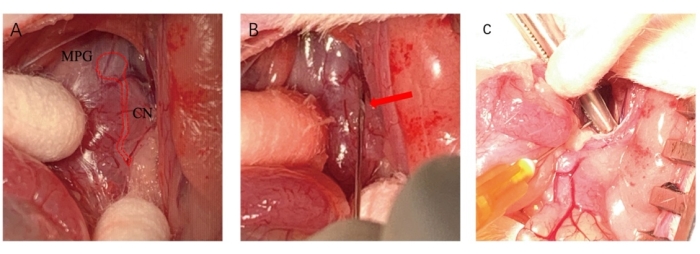
Figure 2: The procedure of cavernous nerves injury. (A) The anatomical structure of the MPG and CN (marked by a red line). (B) Placing a syringe needle underneath the CN with a certain angle (red arrow). (C) A hemostat was applied to CN to perform injury. Please click here to view a larger version of this figure.
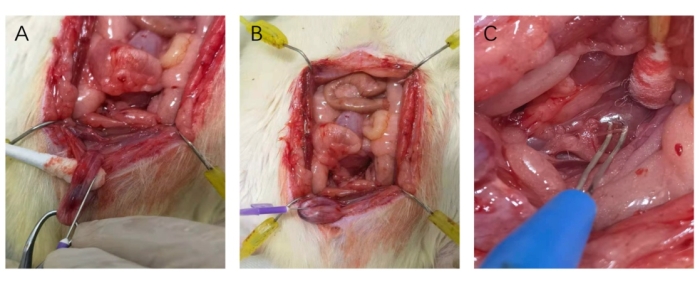
Figure 3: Catheterization of the corpus cavernosum and Hooking of the nerve. (A) 25 G needle was parallel with the course of the corpus cavernosum when catheterizing. (B) Pushing the 25 G needle into the corpus cavernosum. (C) Placing the nerve on the hooks of the bipolar electrode. Please click here to view a larger version of this figure.
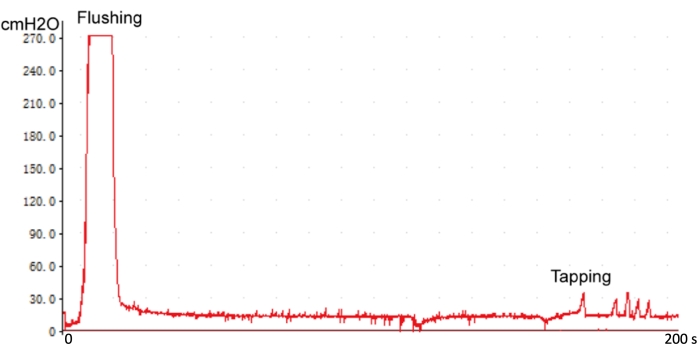
Figure 4: Examing the intracavernous pressure line. The sensitive response curve suggests that the 23 G needle is in the correct position of intracavernous. Please click here to view a larger version of this figure.
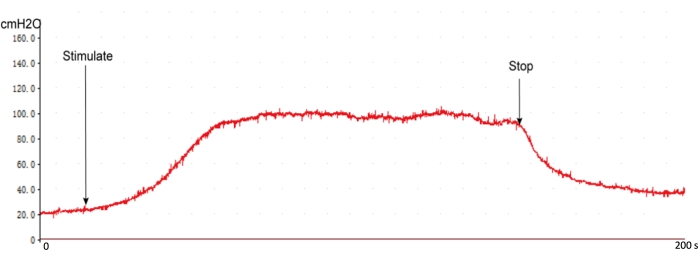
Figure 5: The typical ICP response curve of normal rats. When starting stimulating CN, the ICP quickly rises and enters a plateau. The ICP decreased to baseline without stimulation. Please click here to view a larger version of this figure.

Figure 6: The effect of voltage gradient stimulation on ICP without cavernous nerve injury. With increasing stimulation voltage above 1.0 V, the peak ICP and plateau of ICP don't increase. Please click here to view a larger version of this figure.
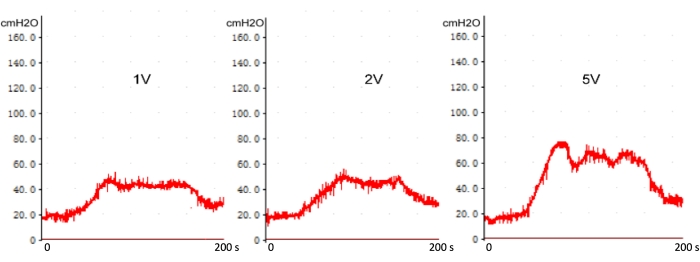
Figure 7: The voltage gradient stimulation on ICP with real-time cavernous nerve injury. With increasing stimulation voltage above 1 V, the peak ICP, and plateau of ICP increase. Please click here to view a larger version of this figure.

Figure 8: The measurement of ICP at different post-operation times. ICP decreases maintains a lower ICP level up to 28 days. Please click here to view a larger version of this figure.
Discussion
ED is a severe complication of pelvic surgery or trauma. Although undergoing a nerve-sparing operation, the incidence rate of ED is approximately 14-90% in radical prostatectomy (RP)12. Due to the problematic regeneration of injury CN, the clinical curative effect is less than satisfactory. Thus, a stable CN injury animal model for exploring treatments of ED is essential. Quinlan et al. first reported the CN injury rat model for the study of RP-associated ED13. Several studies developed CN injury rat models based on the Quinlan model, including transection, excision, crush, and freezing of the CN8,14,15,16,17. Each type of injury could be performed unilaterally or bilaterally for a particular experiment design.
Despite the least severe degree of injury, crush type can reserve the perilemma epineurium of the CN. Bilateral CN crush injury is the best analogy to nerve-sparing RP18,19.Nevertheless, there exist some problems with the methods of CN crush injury reported in the current study. Lack of a sufficient degree of injury and multiple injuries limit the application of the model. A single-point injury model with an adequate degree has an unparalleled advantage in basic research. Therefore, we had developed a more stable bilateral CN injury rat model of RP-associated ED.
CN is liable to neurotmesis because of its' slender size. This study first proposed an operating skill to ensure adequate injury degree and avoid nerve transection, using a syringe needle as rigid support and a hemostat with a rubber sleeve. Nevertheless, different compression forces and times would determine the degree of injury that influences the success rate of model construction. The current study found that applying a hemostat with full tip closure at 5 mm distal from the ganglion for 1 min might be the most appropriate operating mode.
For evaluating the stability and reliability of the model, erectile function recovery was assessed at 0, 7, and 28 days following CN crush. It was found that there was a significant difference of ICP between 0 days and 7 or 28 days; however, there was no significant difference between the ICP values of the 7 days and 28 days. It indicates that erectile function degenerates gradually and appears to maintain a lower ICP level up to 28 days. This suggests that the Bilateral CN crush injury rat model is suitable for a one-month experiment design.
The CN stimulation voltage in studies doesn't have a general agreement, which varies from 1.0 to 12 V. Firstly, the effect of voltage gradient stimulation on the ICP was explored in normal rats. With increasing stimulation voltage above 1.0 V, the peak ICP and plateau of ICP don't rise. Our result is in accordance with Hox, M. et al.'s work20. This phenomenon suggests that the current conducted via the nerve is above the threshold and sufficient to trigger the reflex resulting in a complete physiological response. After being injured, CN was instantly stimulated by gradient voltage, and ICP was recorded. Compared with 1.0 V, the peak ICP and plateau of ICP increase with increasing stimulation voltage above 1 V. Using a higher stimulation voltage might lead to a "false positive" ICP response curve. In general, using a voltage of 1.0 V, frequency of 20 Hz, and pulse-width of 5 ms as stimulation parameters for ICP recording in a bilateral CN injury rat model is recommended.
As with other animal models, the bilateral CN injury rat model via the current method also has some limitations compared with clinical patients. Rat model with the better regenerative ability of the peripheral nervous system might influence the evaluation of nerve regeneration and recovery. In contrast, it provides an acceptable research method in the current study. Therefore, it is necessary to establish a more stable bilateral CN injury rat model of ED, contributing to achievements transformation in clinical treatment.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant NO. 82071636).
Materials
| Name | Company | Catalog Number | Comments |
| 25 G needle | BD Bioscience | 367391 | |
| Abdominal retractor | RWD Life Science | R22009-01 | |
| Animal operating pad | Provided by Guangdong Provincial Key Laboratory of Biomedical Imaging | NA | |
| Bending forceps | RWD Life Science | F12011-10 | |
| Biological signal acquisition and processing system | Techman Software | BL-420S | |
| Bipolar electrode | Techman Software | AC0047 | |
| Carprofen | Sigma-Aldrich | MFCD00079028 | |
| HARTMAN mosquito hemostatic forceps | RWD Life Science | F22002-10 | |
| Heparin | Shanghai Aladdin Biochemical Technology | 2608411 | |
| Micro needle holder | RWD Life Science | F31047-12 | |
| Microsurgery forceps | RWD Life Science | F11001-11 | |
| Scalpel | RWD Life Science | S32003-12 | |
| Sodium pentobarbital | Guangdong Provincial Key Laboratory of Biomedical Imaging | NA | |
| Sprague–Dawley rat | Guangdong Medical Laboratory Animal Center | GDMLAC-035 | |
| Thread scissors | RWD Life Science | S15001-11 | |
| Tissue forceps | RWD Life Science | F13019-12 | |
| Tissue scissors | RWD Life Science | S13029-14 |
References
- Ayta, I. A., McKinlay, J. B., Krane, R. J. The likely worldwide increase in erectile dysfunction between 1995 and 2025 and some possible policy consequences. BJU International. 84 (1), 50-56 (1999).
- Li, D., et al. Multicenter pathophysiologic investigation of erectile dysfunction in clinic outpatients in China. Urology. 79 (3), 601-606 (2012).
- Montorsi, F., et al. Recovery of spontaneous erectile function after nerve-sparing radical retropubic prostatectomy with and without early intracavernous injections of alprostadil: results of a prospective, randomized trial. The Journal of Urology. 158 (4), 1408-1410 (1997).
- Mulhall, J. P., Graydon, R. J. The hemodynamics of erectile dysfunction following nerve-sparing radical retropubic prostatectomy. International Journal of Impotence Research. 8 (2), 91-94 (1996).
- Lue, T. F., Takamura, T., Schmidt, R. A., Palubinskas, A. J., Tanagho, E. A. Hemodynamics of erection in the monkey. Journal of Urology. 130 (6), 1237-1241 (1983).
- Lue, T. F., Takamura, T., Umraiya, M., Schmidt, R. A., Tanagho, E. A. Hemodynamics of canine corpora cavernosa during erection. Urology. 24 (4), 347-352 (1984).
- Semans, J. H., Langworthy, O. R. Observations on the neurophysiology of sexual function in the male cat. The Journal of Urology. 40 (6), 836-846 (1938).
- Canguven, O., Burnett, A. Cavernous nerve injury using rodent animal models. TheJournal of Sexual Medicine. 5 (8), 1776-1785 (2008).
- Sezen, S. F., Hoke, A., Burnett, A. L., Snyder, S. H. Immunophilin ligand FK506 is neuroprotective for penile innervation. Nature Medicine. 7 (10), 1073-1074 (2001).
- Leungwattanakij, S., et al. Cavernous neurotomy causes hypoxia and fibrosis in rat corpus cavernosum. Journal of Andrology. 24 (2), 239-245 (2003).
- Burnett, A. L., Becker, R. E. Immunophilin ligands promote penile neurogenesis and erection recovery after cavernous nerve injury. Journal of Urology. 171 (1), 495-500 (2004).
- Mulhall, J. P. Defining and reporting erectile function outcomes after radical prostatectomy: challenges and misconceptions. Journal of Urology. 181 (2), 462-471 (2009).
- Quinlan, D. M., Nelson, R. J., Partin, A. W., Mostwin, J. L., Walsh, P. C. The rat as a model for the study of penile erection. Journal of Urology. 141 (3), 656-661 (1989).
- Burnett, A. L., Lowenstein, C. J., Bredt, D. S., Chang, T. S., Snyder, S. H. Nitric oxide: a physiologic mediator of penile erection. Science. 257 (5068), 401-403 (1992).
- Carrier, S., et al. Regeneration of nitric oxide synthase-containing nerves after cavernous nerve neurotomy in the rat. Journal of Urology. 153 (5), 1722-1727 (1995).
- El-Sakka, A. I., et al. Effect of cavernous nerve freezing on protein and gene expression of nitric oxide synthase in the rat penis and pelvic ganglia. Journal of Urology. 160 (6), 2245-2252 (1998).
- Mullerad, M., Donohue, J. F., Li, P. S., Scardino, P. T., Mulhall, J. P. Functional sequelae of cavernous nerve injury in the rat: is there model dependency. TheJournal of Sexual Medicine. 3 (1), 77-83 (2006).
- Hayashi, N., et al. The effect of FK1706 on erectile function following bilateral cavernous nerve crush injury in a rat model. Journal of Urology. 176 (2), 824-829 (2006).
- Hsieh, P. S., et al. The effect of vascular endothelial growth factor and brain-derived neurotrophic factor on cavernosal nerve regeneration in a nerve-crush rat model. BJU International. 92 (4), 470-475 (2003).
- Hox, M., Mann-Gow, T., Lund, L., Zvara, P. Cavernous Nerve Stimulation and Recording of Intracavernous Pressure in a Rat. Journal of Visualized Experiments. (134), e56807 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved