Method Article
Enforced Activation of Enhancer RNAs In Situ through the dCas9 Synergistic Activation Mediator System
In This Article
Summary
Enhancer RNAs (eRNAs) are non-coding RNAs produced from active enhancers. An optimal approach to study eRNA functions is to manipulate their levels in the native chromatin regions. Here we introduce a robust system for eRNA studies by using CRISPR-dCas9-fused transcriptional activators to induce the expression of eRNAs of interest.
Abstract
Enhancers are pivotal genomic elements scattered through the mammalian genome and dictate tissue-specific gene expression programs. Increasing evidence has shown that enhancers not only provide DNA binding motifs for transcription factors (TFs) but also generate non-coding RNAs that are referred to as eRNAs. Studies have demonstrated that eRNA transcripts can play significant roles in gene regulation in both physiology and disease. Commonly used methods to investigate the function of eRNAs are constrained to “loss-of-function” approaches by knockdown of eRNAs, or by chemical inhibition of the enhancer transcription. There has not been a robust method to conduct “gain-of-function” studies of eRNAs to mimic specific disease conditions such as human cancer, where eRNAs are often overexpressed. Here, we introduce a method for precisely and robustly activating eRNAs for functional interrogation of their roles by applying the dCas9 mediated Synergistic Activation Mediators (SAM) system. We present the entire workflow of eRNA activation, from the selection of eRNAs, the design of gRNAs to the validation of eRNA activation by RT-qPCR. This method represents a unique approach to study the roles of a particular eRNA in gene regulation and disease development. In addition, this system can be employed for unbiased CRISPR screening to identify phenotype-driving eRNA targets in the context of a specific disease.
Introduction
The human genome contains a constellation of regulatory elements1,2,3. Among these, enhancers emerge to be one of the most critical categories4,5,6. Enhancers play essential roles in regulating development, and are responsible for generating spatial-temporal gene expression programs to determine cell identity5,6,7. Conventionally, enhancers are only considered to be DNA elements that provide binding motifs for transcription factors (TFs), which then control target gene expression6,8. However, a series of studies found that many active enhancers also transcribe non-coding enhancer RNAs (i.e., eRNAs)4,9,10.
The level of eRNA transcription was found to correlate with the activity of an enhancer4,10. Active enhancers produce more eRNA transcripts and show higher levels of epigenome markers associated with active transcription, such as H3K27ac and H3K4me19,11,12. Some studies have demonstrated that eRNA transcripts can play important roles in transcriptional activation of target genes10,12. A large number of eRNAs were identified to be deregulated in human cancers13,14,15,16, many of which exhibited high cancer type specificity and clinical relevance. These findings bring opportunities that the elucidation of eRNAs that can drive/promote tumorigenesis may offer novel targets for therapeutic intervention13,15.
Current methods to study eRNA functions are almost exclusively based on knockdown strategies that used small interference RNAs (siRNA), short hairpin RNAs (shRNAs), or antisense oligonucleotides (ASOs, of which locked nucleic acids (LNAs) are the commonly used type in research)10,12,17. However, human diseases such as cancer predominantly show overexpression of eRNAs as compared to their adjacent normal tissue15, demanding tools to “overexpress” eRNAs to mimic their disease-relevant expression patterns for functional studies. To achieve this, a plasmid-based ectopic overexpression system is not optimal because the exact transcription start and termination sites of eRNAs remain largely unclear. In addition, a plasmid expression system may alter the location of eRNAs, causing potential artifacts of their functions18. Here we provide a detailed protocol to facilitate the functional characterization of eRNAs by enforcing their “overexpression” in the native genomic locus of their production (i.e., in situ), which is based on the CRISPR/dCas9-Synergistic Activation Mediators System (SAM).
The SAM system was initially developed for activating coding genes and long intergenic non-coding RNAs (lincRNAs) associated with BRAF inhibitor resistance in melanoma cells19. Unlike other CRISPR activation (CRISPRa) technologies, the SAM system consists of a combination of transcription activators to confer robust transcriptional activation of target regions. These activators include: an enzymatically dead Cas9 (dCas9) fused with VP64 (i.e., dCas9-VP64); a guide RNA containing two MS2 RNA aptamers, and an MS2-p65-HSF1 fusion activator protein. The presence of the MS2 aptamers in the gRNA can recruit the MS2-p65-HSF1 fusion protein to the vicinity of dCas9/gRNA binding sites. Among these, VP64 is an engineered tetramer of the herpes simplex VP16 transcriptional activator domain, which has been shown to strongly activate gene transcription by recruiting general transcription factors20,21,22. The MS2-p65-HSF1 fusion protein consists of three parts. The first part, the MS2-N55K, is a mutant form of MS2 binding protein that has a stronger affinity23; the other two parts of this fusion protein are the transactivation domain of p65 and heat shock factor 1 (HSF1), both of which are transcription factors that possess strong transactivation domains and can induce robust transcription programs24,25. Therefore, the SAM system essentially created a highly potent activator complex to activate transcription of designated coding genes and lincRNAs19.
Protocol
The entire workflow of this protocol is shown in Figure 1.
1. Enhancer RNA (eRNA) selection
- Identify a putative enhancer region of interests by using binding peaks of chromatin immunoprecipitation sequencing (ChIP-Seq) data, i.e., of histone modifications (e.g., H3K4me1 and H3K27ac), or of transcription coactivators (e.g., p300).
- Identify the eRNA of interest by intersecting the ChIP-Seq peak with RNA-seq signals (e.g., from total RNA-seq or from nascent RNA-seq such as Global Run-On Sequencing (GRO-Seq).
NOTE: The region chosen for designing gRNAs should be usually limited to the “enhancer core” region, where TFs or coactivators (e.g., p300) showed clear ChIP-seq peaks (Figure 2A). The dCas9 and its fusion coactivators will be recruited by a gRNA to this region to mimic the native binding of coactivators (Figure 2A). If specific datasets such as Cap Analysis of Gene Expression (CAGE)4 or GRO-cap26 are available, they can be used to precisely determine the “enhancer core”, the region between two transcription start sites of the eRNAs transcribed to opposite directions4,10,26.
2. gRNA design
- Use common CRISPR gRNA design tools such as CRISPOR27 to select gRNAs with low potential off targeting (http://crispor.tefor.net/).
NOTE: Other tools like Benchling28, or CHOPCHOP29 can also be used as additional options for gRNA design. - Paste the enhancer core DNA sequence into the Step 1 column in the CRISPOR website, then click the dropdown button to choose the corresponding genome (e.g., human) in the Step 2 column. Click the dropdown button to set the Protospacer Adjacent Motif (PAM) sequence as “NGG” in the Step 3 column and then click “Submit” button to generate guide sequences with a length of 20 bp.
- Choose guides with highest specificity scores in CRISPOR tool, i.e., low off-target potential, then add “CACCG” to the 5’ end, and “C” to the 3’ end, respectively.
NOTE: Select the guides with highest specificity scores (>85 in CRISPOR is preferred). - Order oligonucleotides for each sense and antisense sequence from commercial sources.
NOTE: Additional instructions on CRISPR/Cas9 gRNA design can be found in other studies30,31. The overhangs in step 2.2 will make the gRNA compatible with the SAM gRNA backbone (Addgene #61427), which uses the BsmBI restriction enzyme.
3. Clone gRNAs into a lentiviral construct
- To anneal oligos mix 1 µL of each paired oligo at 100 µM, 1 µL of 10x T4 ligation buffer, 0.5 µL of T4 DNA Ligase (400,000 units/mL) and 6.5 µL of H2O to reach a total volume of 10 µL. Incubate at 37 °C for 30 min, then 95 °C for 5 min, and ramp down to 25 °C at 5 °C/min. Dilute to 100 µL using H2O.
- Digest the gRNA backbone by mixing 2 µL of 10x restriction enzyme (RE) Buffer, 300 ng of lenti_gRNA(MS2)_zeo backbone plasmid (Addgene #61427) in 1 µL, 1 µL of BsmBI enzyme, and 16 µL of H2O to reach a total volume of 20 µL. Incubate at 55 °C for 15 min.
- Mix ligation components with 20 µL digestion product by adding 2.5 µL of 10x T4 ligation buffer, 1 µL of diluted annealing product and 1.5 µL of T4 DNA ligase (400,000 units/mL) into a 25 µL system. Incubate at room temperatures for 30 min.
- Transform 2 µL of the ligation mix from step 3.3 into Stbl3 competent E.coli cells. Plate them on an ampicillin LB-agar plate and incubate overnight at 37 °C.
- Pick and inoculate a single bacteria colony and extract plasmid. Send it for Sanger sequencing to confirm that the gRNA sequence is correctly inserted.
NOTE: Sequences for the primers and gRNAs are available in Supplementary Table 1. Stbl3 chemically competent E.coli cells are recommended to be used here because they have a higher plasmid DNA yield and higher plasmid stability when generating instability-prone lentivirus plasmids32.
4. gRNA efficiency test
NOTE: Although it may not be necessary for every gRNA, it is recommended that researchers examine the quality of gRNA by performing Surveyor assay (i.e., mismatch cleavage assay) to detect indels or mutations that can only be efficiently generated by good quality gRNAs33,34. Other methods such as Tracking of Indels by Decomposition (TIDE) can also be used to determine gRNA efficiency30,35. Surveyor nuclease is a member of a family of mismatch-specific endonucleases that can cut double-strand DNA with mismatches (Figure 3A). The quality of gRNAs can be revealed by the efficacy of producing smaller DNA species. Practically, surveyor cutting efficacy can also be affected by the transfection efficiency of gRNAs and Cas9.
- Transfect gRNA together with the pSpCas9(BB)-2A-Puro (Addgene #62988) plasmid that expresses the Cas9 protein into 293T cells using a lipid-based transfection reagent. Use 1.2 µg/mL for each plasmid per well in a 6 well plate. Continue to culture the cells for 3 days after transfection. Harvest and extract genomic DNA according to the manufacturer’s protocol36.
- Use polymerase chain reaction (PCR) to amplify the targeted enhancer region from genomic DNA. Use PCR conditions shown in Supplementary Table 2. Use primers in Supplementary Table 1 for an example enhancer, NET1e. Denature the PCR products by incubating at 95 °C for 10 min, and re-hybridize them by ramping down from 95 °C to 25 °C at the speed of -0.3 °C/s to allow the single-strand DNA (ssDNA) with and without gRNA-induced indels or mutations to anneal to each other.
- Digest the hybridized DNA by the Surveyor nuclease (Figure 3A) following the manufacturer’s protocol37. Mix 400 ng of hybridized DNA from step 4.2, 1 µL of Surveyor nuclease, 1 µL of Surveyor enhancer and 5 µL of 0.15 M MgCl2 in a 50 µL system. Incubate at 42 °C for 60 min. Analyze the DNA on an agarose gel. Use negative control, such as cells with Cas9 only but without targeting gRNAs, in the assay and gel electrophoresis (Figure 3B).
5. Lentivirus generation
- Add psPAX2, pMD2.G, and a target plasmid (e.g., lenti_dCas9-VP64_Blast, Addgene #61425; or lenti_MS2-p65-HSF1_Hygro, Addgene #61426) at the ratio of 3 µg : 1 µg : 4 µg in a 1.5 mL polypropylene tube. Mix them with 500 µL of Opti-MEM and incubate for 5 min at room temperature.
- In a separate tube, put 10 µL of the lipid-based transfection reagent in 500 µL of Opti-MEM, and incubate for 5 min at room temperature.
- Combine the products from steps 5.1 and 5.2 and incubate for 20 min at room temperature.
- Plate 293T cells one day before the transfection and let them reach a confluence of ~30% in a 10 cm dish at the time of transfection. Add 4 mL of regular medium (DMEM with 10% FBS), then add the complex from the step 5.3 dropwise to cells. Add DMEM with 10% FBS to make the final volume up to 6 mL. Incubate overnight in a cell incubator at 37 °C with 5% CO2.
- The next day, change the medium to 10 mL of new DMEM with 10% FBS. Harvest the medium 24 h after the medium change. Use a syringe filter (e.g., 0.45 µm) to filter the virus-containing medium and then proceed to step 6 or store the virus in -80 °C.
NOTE: Lentivirus operations require a BioSafety Level II cabinet. Caution needs to be taken to safely handle the virus-associated experiments; and if any container has direct contact with the viral medium, it needs to be bleached for more than 20 min before disposal as biohazard.
6. Cell culture
- Maintain cells in a CO2 cell culture incubator at 37 °C with 5% CO2.
- Culture MCF7 and 293T cells in Dulbecco’s Modified Eagle Medium (DMEM) medium with 10% FBS.
- Grow cells in 10 cm dishes and split at a 1:3 to 1:5 ratio when confluent.
7. Cell infection and selection
- Seed the target cells (e.g., MCF7) directly in viral medium mixture containing 0.5 mL of lenti_dCas9-VP64_Blast (Addgene #61425) and 0.5 mL lenti_MS2-p65-HSF1_Hygro (Addgene #61426). Add 8 µg/mL Hexadimethrine bromide to increase the efficiency of infection. Use a well of uninfected cells as a negative control for examining the efficacy of antibiotic selection.
NOTE: The amount of viral medium is recommended as follows: 6 mL viral medium for 10 cm dish, 1 mL for a well of a 6 well plate, and 0.5 mL for a well of a 12 well plate. - At 24 h post-infection, add to the cells fresh medium containing Blasticidin (5 µg/mL for MCF7 cells) and Hygromycin (200 µg/mL for MCF7 cells).
- Keep cells in antibiotic selection medium until the negative control cells die out.
NOTE: Time may vary for different cell lines to become stable. For MCF7, it usually takes 5-7 days for non-infected cells to completely die out. A killing curve using a range of antibiotic concentrations should be tested for a new cell line prior to the experiments. It is acceptable to use a cell mixture for the next steps, but an alternative is to pick single-cell colonies that are homogeneous in terms of expression of the two effector proteins. The stable line obtained is referred to as the SAM-effector parental line (e.g., MCF7 SAM-effector line) in this paper. It is recommended that a shared SAM-effector parental cell line be used for infection by different targeting or non-targeting gRNAs, especially if their effects will be compared. - Use western blotting to determine whether the cells stably express the two effector proteins (an example is shown in Figure 4A). Use reverse transcription of total RNAs followed by quantitative PCR (RT-qPCR) as an alternative method to examine the expression levels of the two mRNAs (dCas9-VP64 and MS2-p65-HSF1, Figure 4B).
NOTE: Primers for examining the mRNA levels of the two dCas9 effectors are available in Supplementary Table 1. - Generate lentivirus of gRNAs constructed in step 3.5 and infect the stable SAM cell line dually expressing dCas9-VP64 and MS2-p65-HSF1 with individual gRNA lentivirus. Add Zeocin (100 µg/mL for MCF7 cells) to the medium 24 h post gRNA viral transduction. Generate a negative control that expresses non-targeting gRNA (NT-gRNA) in the parental SAM cell line.
NOTE: Ensure that the selection drug is specific for the construct of interest.
8. RNA extraction and quantitative RT-PCR to examine eRNA levels
- Extract total RNAs from SAM cell lines expressing either NT-gRNA or targeting gRNAs using an RNA extraction kit38, or other phenol chloroform based method. Use cells of ~80% confluency in one well of a six-well plate for RNA extraction.
NOTE: No significant difference was observed in our practice for eRNA detection when RNAs are extracted either by commercial binding columns or by phenol chloroform reagents. - Make complementary DNA (cDNA) from the purified RNA by reverse transcription reaction with random hexamer, following the manufacturer's protocol39. Use conditions in Supplementary Table 2.
NOTE: Because the majority of eRNAs are non-polyadenylated1,2,4,9,10, random hexamer is routinely used for cDNA generation. - Design primers for RT-qPCR measuring target eRNAs using a reputable primer designing tool (e.g., Primer 3). Test the amplification linearity of the primer pairs by examining if the primers will linearly amplify serial diluted cDNAs and show expected qPCR cycle differences. Use conditions in Supplementary Table 2.
NOTE: The primers for RT-qPCR should target the highly transcribed region in the nascent RNA-seq, and the linearity test of primers should include a broad range of cDNA dilutions to ensure that all possibly encounterable eRNA levels are tested. An example of linearity test is shown in Figure 5A. - Perform RT-qPCR and analyze the eRNA expression levels in control cells (SAM cell line with NT-gRNA) and in SAM cell line with eRNA-targeting gRNAs (e.g., NET1e gRNA#1) (e.g., Figure 6A). Primers for NET1e RNA are shown in Supplementary Table 1. Use conditions in Supplementary Table 2.
9. dCas9 ChIP and qPCR
NOTE: This step is an optional experiment to validate the binding of dCas9/SAM-gRNA complex to the target enhancer by the specific gRNAs. While it is encouraged that users perform this step, it is not necessary to test every single gRNA. Refer to an example shown in Figure 5B. Refer to primers listed in Supplementary Table 1.
- Cross-link cells
- Remove the medium from cells and add 1% formaldehyde dissolved in phosphate buffered saline (PBS). Leave for 10 min.
- Add 2.5 M glycine at 1:20 volume to quench the cross-linking and wash cells twice with ice-cold PBS. Add 700 µL of ice-cold PBS and scrape the cells to a 1.5 mL tube.
- Centrifuge at 2,000 x g for 5 mins at 4 °C. Proceed to step 9.2, or snap freeze and store at -80 °C.
- Sonicate
- Make fresh LB1, LB2 and LB3 buffers. LB1: 50 mM Hepes-KOH (pH 7.5), 140 mM NaCl, 1 mM EDTA, 10% glycerol, 0.5% NP-40, 0.25% Triton-X 100 and 1x Protease inhibitor. LB2: 10 mM Tris-HCl (pH 8.0), 200 mM NaCl, 1 mM EDTA, 0.5 mM EGTA and 1x Protease inhibitor. LB3: 10 mM Tris-HCl (pH 8.0), 100 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 0.1% Na-Deoxycholate, 0.5% N-lauroylsarcosine and 1x Protease inhibitor. Supplement 1x Protease inhibitor freshly to the buffer before the experiment.
- Add 1 mL of buffer LB1 to cell pellets, pipette well, rotate at 4 °C for 10 min, and spin at 2,000 x g for 5 mins at 4 °C. Pour off the supernatant, add 1 mL of LB2 buffer, rotate at 4 °C for 10 min, and spin at 2,000 x g for 5 min at 4 °C. Pour off the supernatant and remove the excess of the LB2 buffer. Add 300 µL of buffer LB3.
- Sonicate and fragment the chromatin DNA to an average size of ~200-400 bp using a proper sonicator system. After the sonication, add 30 µL of 10% Triton-X 100 and mix well. Test the proper sonication time if a new cell line is used.
- Centrifuge at 14,000 x g to remove the pellet. Transfer the supernatant to the new tube and add 630 µL of LB3 and 70 µL of 10% Triton X-100 to a total volume of 1 mL. Add 2 µg of the Cas9 antibody to the supernatant and rotate at 4 °C overnight.
- Immuno-complex capture and reverse cross-linking
- Prepare RIPA buffer (50 mM HEPES pH 7.6, 1 mM EDTA, 0.7% Na-deoxycholate, 1% NP-40, 0.5 M LiCl) and elution buffer (1% SDS and 0.1 M NaHCO3).
- Wash Protein G dynabeads with 1% BSA in PBS 3 times and LB3 buffer once.
- Add 30 µL of Protein G dynabeads to the sample and incubate at 4 °C for 4 h.
- Wash the beads-immuno-complex in 500 µL of RIPA buffer 6x. Do not let beads dry out.
- Wash the beads-immuno-complex once with TE buffer; remove the buffer.
- Add 200 µL of elution buffer to the beads-immuno-complex and vortex; then put it in a temperature-adjustable heated shaker set to 65 °C with 600 rpm shaking for 6-16 h to reverse crosslink.
- DNA extraction
- Remove tubes from the shaker, briefly spin down the beads and put them on a magnetic stand. Transfer 200 µL of the supernatant to a fresh tube and add 200 µL of TE buffer.
- Add 1 µL of RNase A (1 mg/mL) to the tube and incubate at 37 °C for 1 h.
- After 1 h of incubation, add 2 µL of Proteinase K (20 mg/mL) to the sample and incubate it at 65 °C for 2 h.
- Centrifuge briefly and add 400 µL of phenol-chloroform-isoamyl alcohol mixture. Mix well, then centrifuge for 5 min at 10,000 x g.
- Move 400 µL of the upper layer to a 1.7 mL tube containing 16 µL of 5 M NaCl and 1 µL glycogen (20 µg/µL) and mix well.
- Add 800 µL of 100% ethanol and leave overnight at -20 °C.
- Next morning, centrifuge the tubes at 4 °C at 14,000 x g for 15 min.
- Remove the supernatant and wash the pellet with 1 mL of 70% ethanol. Spin down at 14,000 x g for 10 min.
- Remove ethanol, air dry pellet, and resuspend in 50 µL of TE (10 mM Tris-HCl, pH 8.0, 1 mM EDTA).
- Perform ChIP-qPCR to examine the recruitment of the SAM complex to the targeting site by a pair of enhancer core-targeting primer. Use a primer pair targeting an irrelevant region as a negative control.
10. Cell growth assay and other functional tests of eRNA over-activation
- Trypsinize SAM cell lines expressing the non-targeting gRNA or eRNA-targeting gRNAs and plate ~3,000 cells per well in a 96 well plate.
- Measure cell growth by using a live-cell imager or other methods (e.g., cell counting, or water-soluble tetrazolium salt-1 assay).
- Use the half-maximal inhibitory concentration (IC50) to test the cellular responses to specific cancer drugs in cells with or without NET1e overexpression by SAM15.
NOTE: Results of cell growth assays and drug sensitivity tests of breast cancer cells are presented after overexpression of an eRNA transcribed adjacent to NET1 gene, which was referred to as NET1e15. Other assays can be conducted at the cellular or organismal levels based on the need of each specific project.
Results
Figure 1 illustrates the overall workflow of this protocol. Our focus was on a representative eRNA, NET1e15, which is overexpressed in breast cancer, for which SAM system was used to activate and study it’s biological role in regulating gene expression, cell proliferation and cancer drug response. For this NET1 enhancer, several p300 ChIP-Seq peaks, flanked by transcribed eRNA transcripts (Figure 2A,B) were marked. Based on strong GRO-Seq signals, one of the p300 ChIP-Seq peaks was selected as the enhancer core (where bi-direction eRNA transcripts originate, Figure 2B). NET1 enhancer region was also marked by dense ChIP-Seq peaks of H3K4me1 and H3K27ac. Multiple gRNAs were designed within this enhancer core p300 peak, with two of the gRNAs denoted by arrows in the inlet (Figure 2B). A chromatin loop between NET1 enhancer core and NET1 promoter can be detected in the data of Chromatin Interaction Analysis by Paired-End Tag Sequencing (ChIA-PET, black arch, Figure 2B), suggesting the direct regulatory relationship between the NET1e and the NET1 gene.
The quality of designed gRNA can be tested by two different methods i.e., Surveyor assay (Figure 3) or ChIP-qPCR (Figure 5). In Figure 3A, a schematic is used to illustrate the procedure of surveyor assay used to examine the quality of gRNAs. In surveyor assay, a PCR product spanning the gRNA recognition site was amplified from 60 ng genomic DNA, which was subjected to denaturing and rehybridization, and subsequently surveyor cleavage. In the control cells transfected with Cas9 plasmid only (pSpCas9(BB)-2A-Puro, Addgene #62988), only one band of 1,205 bp (indicated by the blue asterisk, Lane 1 in Figure 3B) was detected. By contrast, the assay using genomic DNAs from cells transfected with NET1e gRNA#1 together with Cas9 generated two cleavage products of 777 bp and 428 bp (denoted by orange asterisks), respectively (lane 2, Figure 3B). Similarly, shown in lane 3, the co-transfection of NET1e gRNA#2 with Cas9 caused Surveyor digestion of the 1,205 bp PCR product into two digested products of 905 bp and 300 bp (marked by red asterisks), respectively (Figure 3B). The sizes of these cleavage products matched the expected sizes of digestion due to the in-dels or mutations generated by the gRNAs, indicating that gRNAs and Cas9 can cut our targeted enhancer DNA in cells.
MCF7 parental SAM cell line was successfully generated, which expresses the two effector proteins as shown both by western blotting (Figure 4A) and RT-qPCR (Figure 4B). After infecting this parental line with NT-gRNAs or specific gRNAs targeting NET1 enhancer, ChIP-qPCR was conducted to test if the gRNA can recruit dCas9-VP64 (one of the effectors) to our target site. Prior to ChIP-qPCR, the amplification linearity of ChIP-qPCR primers needs to be tested by a PCR-cycle versus diluted-cDNA/ChIP-DNA standard curve, as shown in Figure 5A. For each pre-diluted standard shown in the x axis of Figure 5A, four folds of serial dilution were conducted to generate them from sonicated genomic DNAs. A standard curve was plotted between delta cycle threshold (∆CT) values (y axis) against the relative quantity (log2) of each diluted standard to the initial amount of genomic DNA (Figure 5A). This specific primer set conferred an R square of 0.994, and we generally recommend primer sets with an R square higher than 0.95. We then conducted ChIP by using a ChIP-quality antibody targeting Cas9 and found that the dCas9-VP64 protein was recruited by our NET1e gRNA to the target NET1 enhancer region, but not to another irrelevant genomic region (Figure 5B).
RT-qPCR was used to examine the expression levels of the targeted NET1e RNA after SAM activation by two different gRNAs in MCF7 SAM cells. Figure 6A shows that in cells with engineered SAM system targeting NET1 enhancer, >30-fold of NET1e upregulation was successfully achieved with two different gRNAs. Because NET1e was overexpressed in breast tumors than adjacent normal tissue based on our analysis of the Cancer Genome Atlas (TCGA) datasets15, and because its overexpression correlated with poor survival and altered response to a set of cancer drugs15, we tested if the enforced overexpression of NET1e by SAM can directly alter cell proliferation or cellular response to cancer drugs. We conducted cell growth assay and measured cell confluence by a live cell imager. The confluences were measured every 6 h and normalized to that at 0 h, which showed that SAM-enforced overexpression of NET1e accelerated cell growth (Figure 6B). We selected the BEZ235 and the Obatoclax to examine cell response to cancer drugs because NET1e expression is positively correlated with IC50 of these two drugs based on the analysis from Cancer Therapeutics Response Portal (CTRP) and Genomics of Drug Sensitivity in Cancer (GDSC). The result suggested that NET1e overexpression directly conferred resistance to specific cancer drugs (Figure 6C).

Figure 1: A work-flow chart demonstrating procedures used to generate the SAM system for enforced activation of eRNAs of interest. Please click here to view a larger version of this figure.

Figure 2: Epigenetic features of NET1 enhancer and its neighborhood in MCF7 cells. (A) A diagram showing the structure of the “Enhancer core” to denote the DNA region bound by transcription factors and cofactors, which is used for gRNA design to anchor SAM system in step 2 of the protocol. (B) A snapshot of genome browser tracks of ChIP-Seq, GRO-Seq, and ChIA-PET of indicated factors at NET1 and NET1e loci in MCF7 cells. GRO-Seq is stranded (Red: Watson strand, Orange: Crick strand). The zoom-in inlet to the right demonstrates the specific “enhancer core” used for designing two gRNAs (red lines and pointed by arrows). ChIA-PET track indicates a potential regulatory relationship between NET1e and NET1 gene, with the black and grey arch denoting chromatin loops. Figure 2B is modified from Zhang, Z. et al.15. The MCF7 ChIA-PET dataset is from ENCODE data portal1. Please click here to view a larger version of this figure.

Figure 3: Surveyor nuclease digestion assay to test gRNA quality. (A) The workflow of the Surveyor assay. The red dot on the DNA indicates in-dels or mutations generated by CRISPR/Cas9 to the target region. The forward primer was designed to be ~300-400 bp from the cutting site and the reverse primer ~800-900 bp from the cutting site, allowing easy discerning of their cutting in gel electrophoresis. (B) An agarose gel picture of Surveyor digestion assay. Blue asterisk indicates uncleaved DNA from PCR, while orange and red asterisks indicated cleaved products by Surveyor. Please click here to view a larger version of this figure.

Figure 4: Examination of dCas9-VP64 and MS2-p65-HSF1 expression in stable MCF7 SAM cell line. (A) Western blots with a Cas9 antibody in parental MCF7 cells (MCF7 WT), and in the MCF7 cell line expressing dCas9-VP64 and MS2-p65-HSF1 proteins (MCF7 SAM). GAPDH was used as a loading control. (B) RT-qPCR results show the mRNA levels of dCas9-VP64 and MS2-p65-HSF1 in MCF7 WT and MCF7 SAM, respectively. The values were normalized to GAPDH. Error bars represent mean ± SD; N=3. P value: Student’s t-test. Please click here to view a larger version of this figure.

Figure 5: ChIP-qPCR to validate dCas9-VP64 recruitment to NET1 enhancer. (A) Amplification linearity test of the primer set used for the NET1 enhancer ChIP-qPCR. (B) ChIP-qPCR based on Cas9 antibody using primers specific for the NET1 enhancer region, and an irrelevant genomic region as a negative control (hg19, chr14:35,025,431-35,025,595). Error bars represent mean ± SD; N=3. P value: Student’s t-test. Please click here to view a larger version of this figure.
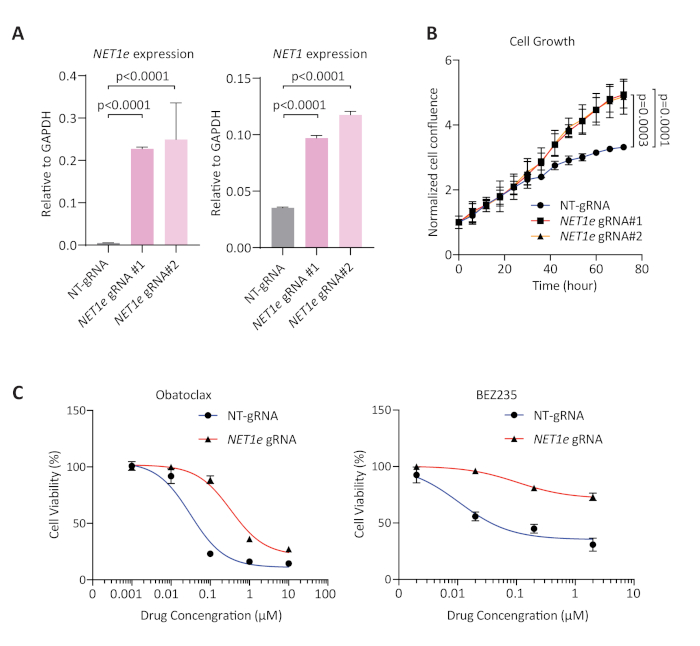
Figure 6: NET1e expression level, cell growth assay, and responses to anti-cancer drugs in MCF7 NET1e-SAM cells. (A) RT-qPCR result showing the expression levels of NET1e, or its neighboring gene, NET1, in the SAM cell lines expressing non-targeting gRNA (NT-gRNA), NET1e gRNA#1 or NET1e gRNA#2. The values are normalized to GAPDH. Error bars represent mean ± SD; N=3. P value: Student’s t-test. (B) Normalized cell confluence of MCF7 SAM cells expressing gRNAs as indicated. (C) The IC50 of two drugs (Obatoclax and BEZ235) in SAM cell lines expressing NET1e gRNA#1 or NT-gRNA. The relative cell amount was measured with live cell imager. Error bars represent mean ± SD. Student’s t-test was used to calculate the p values. This figure is modified from Zhang et al15. Please click here to view a larger version of this figure.
Supplementary Table 1: List of primer sequences for gRNAs, Surveyor assay, RT-qPCR, and ChIP qPCR. Please click here to download this file.
Supplementary Table 2: PCR and RT-qPCR experimental setup and reaction conditions. Please click here to download this file.
Discussion
Based on our data, we conclude that the SAM system is suitable for studying the role of eRNAs in regulating cellular phenotypes, e.g., cell growth or drug resistance. However, careful gRNA designing is required for robust eRNA activation, due to the following reasons. First of all, the transcription start site (TSS) of eRNA in each specific cell lines/types remains less clearly annotated. Due to this, epigenomic information (e.g., ChIP-Seq of H3K27ac, of transcription factors, or of p300), transcriptional activity depicted by GRO-Seq (or additional CAGE4 or GRO-cap26 datasets if available) need to be utilized to deduce the transcription starting sites so that gRNAs can be designed in a way to avoid the potential impediment of normal enhancer transcription by the dCas9/SAM complex. Second, gRNAs provided by current design tools may show differential eRNA activation potency in SAM systems, the basis of which remains mechanistically unclear. It can be due to different binding dynamics of distinct gRNA sequences30, or due to the relative location of the gRNA/dCas9 binding site versus the other critical regulatory DNA elements near the enhancer core region. To ensure robust eRNA activation and to reduce off-targeting, we recommend readers to design multiple gRNAs (n>3) and test their actual activation efficacy by RT-qPCR. If a consistent result is generated by multiple gRNAs, it is considered conclusive. Furthermore, the process of generating stable lines also varies based on the choice of cell lines, which may require different selection time, length, and antibiotic concentrations. Finally, we found it important to ensure the quality of primer sets for examining eRNAs in RT-qPCR, as their abundance is sometimes one or two magnitudes lower than common mRNAs. The primers’ performance in qPCR and their linear range of amplification should be carefully examined. It is also important and necessary to remove the trace of genomic DNAs in RNA samples when the levels of eRNAs are examined, because these DNAs may confound RT-qPCR results.
We largely followed the original method developed by the group of Feng Zhang et al.19. The modifications here is to target the dCas9-SAM complex to enhancers and to activate eRNAs instead of lincRNAs or mRNAs.
Here we introduce a highly effective method to activate the transcription of targeted enhancers at its native chromosomal sites (i.e., in situ). This method complements conventional approaches to study eRNAs in gene regulation that often emphasize on the knockdown of eRNAs by shRNAs, siRNAs, or ASOs10,17. Indeed, it has been largely infeasible to apply strategies to activate the transcription of eRNAs for functional interrogation until the advent of CRISPR mediated epigenetic tools. This is because ectopic expression of an eRNA driven by a heterologous promoter may not be optimal in two aspects: 1) the full-length transcript of eRNAs from their transcriptional start to end sites (particularly the ends) are difficult to be determined or are possible of mixed species in a cell; 2) the ectopic expression may alter the location of eRNAs, or may not contain proper structural features of the RNAs due to the lack of genomic/epigenomic contexts. CRISPR-dCas9 based epigenetic activation of eRNAs overcomes the above problems and provides an ideal method to “over-express” eRNAs in situ. Importantly, the enhanced eRNA expression recapitulates the disease-relevant enhancer over-activation commonly observed in human cancers13,14,15,16, permitting subsequent studies of the pathological consequence.
Here we used NT-gRNA as a control for eRNA-targeting gRNAs to deduce the effects of the eRNA over-activation. However, there are other controls that can be employed. For example, cells with eRNA-targeting gRNAs with or without one of the two effector proteins may also be considered to deduce the genes affected by the effector proteins. Additional genome-wide tools such as ChIP-seq may be utilized to examine the global binding patterns of dCas9 effectors together with a targeting gRNA to further test the specificity of the SAM activator. Regardless, we consider that a robust conclusion should always be based on at least two separate gRNAs generating consistent results. Currently, little knowledge is available in terms of how the enforced binding of dCas9 effectors may impede the normal binding of transcription factors or cofactors on enhancers, which should be taken into consideration when users are designing and/or interpreting their experiments. This should be an important research question for future work to better take advantage of this robust tool. While our mainly focus was on the oncogenic functions of eRNAs in this paper, the SAM system can be applied to examine eRNA functions in other biological or pathological settings40,41,42. One limit of this system is that it is not rapidly inducible, nor is it reversible, which may elicit indirect effects on cell growth that is not directly caused by eRNA activation. Therefore, one of the future directions to improve this protocol is to make it inducible and reversible. This will permit our manipulation of enhancers and eRNAs with higher temporal or spatial precision.
Disclosures
The content is solely the responsibility of the authors and does not necessarily represent the official views of the Cancer Prevention and Research Institute of Texas.
Acknowledgements
This work is supported by grants to W.L (Cancer Prevention and Research Institute of Texas, CPRIT RR160083 and RP180734; NCI K22CA204468; NIGMS R21GM132778; The University of Texas UT Stars Award; and the Welch foundation AU-2000-20190330) and a post-doctoral fellowship to J.L (UTHealth Innovation for Cancer Prevention Research Training Program Post-doctoral Fellowship, CPRIT RP160015). We acknowledge the original publicataion15 where some of our current figures were adopted from (with modifications), which follows the Creative Commons license (https://creativecommons.org/licenses/by/4.0/).
Materials
| Name | Company | Catalog Number | Comments |
| Blasticidin | Goldbio | B-800-100 | |
| BsmBI restriction enzyme | New England BioLabs Inc. | R0580S | |
| Cas9 mAb | Active Motif | 61757 | Lot: 10216001 |
| Deoxynucleotide (dNTP) Solution Mix | New England BioLabs Inc. | N0447S | |
| Dulbecco’s Modified Eagle Medium | Corning | 10-013-CM | |
| Dynabeads Protein G | Thermo Fisher Scientific | 65002 | |
| EDTA | Thermo Fisher Scientific | BP118-500 | |
| EGTA | Sigma | E3889 | |
| Fetal Bovine Serum | GenDEPOT | F0900-050 | |
| Glycogen | Thermo Fisher Scientific | 10814010 | |
| Hepes-KOH | Thermo Fisher Scientific | BP310-100 | |
| Hexadimethrine Bromide | Sigma | H9268 | |
| Hygromycin B | Goldbio | H-270-25 | |
| IGEPAL CA630 | Sigma | D6750 | |
| IncuCyte live-cell imager | Essen BioScience | IncuCyte S3 Live-Cell Analysis System | |
| lenti_dCAS-VP64_Blast | Addgene | 61425 | |
| lenti_gRNA(MS2)_zeo backbone | Addgene | 61427 | |
| lenti_MS2-p65-HSF1_Hygro | Addgene | 61426 | |
| LiCL | Sigma | L9650 | |
| Lipofectamine 2000 | Thermo Fisher Scientific | 11668-500 | |
| NaCl | Sigma | S3014 | |
| Na-Deoxycholate | Sigma | D6750 | |
| NaHCO3 | Thermo Fisher Scientific | BP328-500 | |
| N-lauroylsarcosine | Sigma | 97-78-9 | |
| Opti-MEM Reduced Serum Medium | Thermo Fisher Scientific | 31985070 | |
| PES syringe filter | BASIX | 13-1001-07 | |
| Protease Inhibitor Cocktail Tablet | Roche Diagnostic | 11836145001 | |
| pSpCas9(BB)-2A-Puro | Addgene | 62988 | |
| Q5 High-Fidelity DNA Polymerase | New England BioLabs Inc. | M0491S | |
| Q5 Reaction Buffer | New England BioLabs Inc. | B9027S | |
| Quick-DNA Miniprep | ZYMO Research | D3025 | |
| Quick-RNA Miniprep | ZYMO Research | R1054 | |
| Restriction enzyme buffer | New England BioLabs Inc. | B7203S | |
| RT-qPCR Detection System | Thermo Fisher Scientific | Quant Studio3 | |
| SDS | Thermo Fisher Scientific | BP359-500 | |
| Sonicator | Qsonica | Q800R2 | |
| Sso Advanced Universal SYBR Green Supermix | Bio-Rad Laboratories | 1725274 | |
| Stbl3 competent cell | Thermo Fisher Scientific | C7373-03 | |
| Superscript IV reverse transcript | Thermo Fisher Scientific | 719000 | |
| Surveyor Mutation Detection Kits | Integrated DNA Technologies | 706020 | |
| T4 DNA Ligase | New England BioLabs Inc. | M0202S | |
| T4 DNA Ligase Reaction Buffer | New England BioLabs Inc. | B0202S | |
| TE buffer | Thermo Fisher Scientific | 46009CM | |
| Thermal cycler | Bio-Rad Laboratories | T100 | |
| Thermomixer | Sigma | 5384000020 | |
| Zeocin | Thermo Fisher Scientific | ant-zn-1p |
References
- ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 489 (7414), 57-74 (2012).
- Djebali, S., et al. Landscape of transcription in human cells. Nature. 489 (7414), 101-108 (2012).
- Kundaje, A., et al. Integrative analysis of 111 reference human epigenomes. Nature. 518 (7539), 317-330 (2015).
- Andersson, R., et al. An atlas of active enhancers across human cell types and tissues. Nature. 507 (7493), 455-461 (2014).
- Heinz, S., Romanoski, C. E., Benner, C., Glass, C. K. The selection and function of cell type-specific enhancers. Nature Reviews Molecular Cell Biology. 16 (3), 144-154 (2015).
- Ong, C. T., Corces, V. G. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nature Reviews Genetics. 12 (4), 283-293 (2011).
- Hnisz, D., et al. Super-enhancers in the control of cell identity and disease. Cell. 155 (4), 934-947 (2013).
- Grossman, S. R., et al. Systematic dissection of genomic features determining transcription factor binding and enhancer function. Proceedings of the National Academy of Sciences of the United States of America. 114 (7), 1291-1300 (2017).
- Kim, T. K., et al. Widespread transcription at neuronal activity-regulated enhancers. Nature. 465 (7295), 182-187 (2010).
- Li, W., Notani, D., Rosenfeld, M. G. Enhancers as non-coding RNA transcription units: recent insights and future perspectives. Nature Reviews Genetics. 17 (4), 207-223 (2016).
- Creyghton, M. P., et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proceedings of the National Academy of Sciences of the United States of America. 107 (50), 21931-21936 (2010).
- Li, W., et al. Functional roles of enhancer RNAs for oestrogen-dependent transcriptional activation. Nature. 498 (7455), 516-520 (2013).
- Lee, J. H., Xiong, F., Li, W. Enhancer RNAs in cancer: regulation, mechanisms and therapeutic potential. RNA Biology. , 1-10 (2020).
- Sur, I., Taipale, J. The role of enhancers in cancer. Nature Reviews Cancer. 16 (8), 483-493 (2016).
- Zhang, Z., et al. Transcriptional landscape and clinical utility of enhancer RNAs for eRNA-targeted therapy in cancer. Nature Communications. 10 (1), 4562 (2019).
- Chen, H., et al. A Pan-Cancer Analysis of Enhancer Expression in Nearly 9000 Patient Samples. Cell. 173 (2), 386-399 (2018).
- Kopp, F., Mendell, J. T. Functional Classification and Experimental Dissection of Long Noncoding RNAs. Cell. 172 (3), 393-407 (2018).
- Lubelsky, Y., Ulitsky, I. Sequences enriched in Alu repeats drive nuclear localization of long RNAs in human cells. Nature. 555 (7694), 107-111 (2018).
- Konermann, S., et al. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 517 (7536), 583-588 (2015).
- Beerli, R. R., Segal, D. J., Dreier, B., Barbas, C. R. Toward controlling gene expression at will: specific regulation of the erbB-2/HER-2 promoter by using polydactyl zinc finger proteins constructed from modular building blocks. Proceedings of The National Academy of Sciences of the United States of America. 95 (25), 14628-14633 (1995).
- Gilbert, L. A., et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell. 154 (2), 442-451 (2013).
- Hirai, H., Tani, T., Kikyo, N. Structure and functions of powerful transactivators: VP16, MyoD and FoxA. International Journal of Developmental Biology. 54 (11-12), 1589-1596 (2010).
- Lim, F., Spingola, M., Peabody, D. S. Altering the RNA binding specificity of a translational repressor. Journal of Biological Chemistry. 269 (12), 9006-9010 (1994).
- Schmitz, M. L., Baeuerle, P. A. The p65 subunit is responsible for the strong transcription activating potential of NF-kappa B. EMBO Journal. 10 (12), 3805-3817 (1991).
- Vihervaara, A., Sistonen, L. HSF1 at a glance. Journal of Cell Science. 127, 261-266 (2014).
- Core, L. J., et al. Analysis of nascent RNA identifies a unified architecture of initiation regions at mammalian promoters and enhancers. Nature Genetics. 46 (12), 1311-1320 (2014).
- Haeussler, M., et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biology. 17 (1), 148 (2016).
- . Benchling [Biology Software] Available from: https://benchling.com (2020)
- Labun, K., et al. CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Research. 47, 171-174 (2019).
- Hanna, R. E., Doench, J. G. Design and analysis of CRISPR-Cas experiments. Nature Biotechnology. , (2020).
- Nageshwaran, S., et al. CRISPR Guide RNA Cloning for Mammalian Systems. Journal of Visualized Experiments. (140), e57998 (2018).
- Al-Allaf, F. A., Tolmachov, O. E., Zambetti, L. P., Tchetchelnitski, V., Mehmet, H. Remarkable stability of an instability-prone lentiviral vector plasmid in Escherichia coli Stbl3. 3 Biotech. 3 (1), 61-70 (2013).
- Qiu, P., et al. Mutation detection using Surveyor nuclease. Biotechniques. 36 (4), 702-707 (2004).
- Ran, F. A., et al. Genome engineering using the CRISPR-Cas9 system. Nature Protocols. 8 (11), 2281-2308 (2013).
- Brinkman, E. K., Chen, T., Amendola, M., van Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. Nucleic Acids Research. 42 (22), 168 (2014).
- Quick-DNA Miniprep Kit. ZYMO Available from: https://files.zymoresearch.com/protocols/_d3024_d3025_quick-dna_miniprep_kit.pdf (2020)
- SURVEYOR Mutation Detection Kit. IDT Available from: https://sfvideo.blob.core.windows.net/sitefinity/docs/default-source/user-guide-manual/surveyor-kit-for-gel-electrophoresis-user-guide.pdf?sfvrsn=a9123407_6 (2020)
- Quick-RNA Miniprep Kit. ZYMO Available from: https://files.zymoresearch.com/protocols/_r1054_r1055_quick_rna_miniprep_kit.pdf (2020)
- SuperScript IV Reverse Transcriptase Product Manual. Thermo Fisher Scientific Available from: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/SSIV_Reverse_Transcriptase_UG.pdf (2020)
- Ounzain, S., et al. Functional importance of cardiac enhancer associated noncoding RNAs in heart development and disease. Journal of Molecular and Cellular Cardiology. 76, 55-70 (2014).
- McCleland, M. L., et al. CCAT1 is an enhancer-templated RNA that predicts BET sensitivity in colorectal cancer. Journal of Clinical Investigation. 126 (2), 639-652 (2016).
- Miao, Y., et al. Enhancer-associated long non-coding RNA LEENE regulates endothelial nitric oxide synthase and endothelial function. Nature Communications. 9 (1), 292 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved