Palladium-Catalyzed Cross Coupling
Overview
Source: Vy M. Dong and Faben Cruz, Department of Chemistry, University of California, Irvine, CA
This experiment will demonstrate the concept of a palladium-catalyzed cross coupling. The set-up of a typical Pd-catalyzed cross coupling reaction will be illustrated. Pd-catalyzed cross coupling reactions have had a profound effect on how synthetic chemists create molecules. These reactions have enabled chemists to construct bonds in new and more efficient ways. Such reactions have found widespread applications in the fine chemical and pharmaceutical industries. Pd-catalyzed cross coupling reactions add another tool to the chemist's toolbox for constructing carbon-carbon bonds, which are central to organic chemistry. The combination of the importance of making carbon-carbon bonds and the impact of Pd-catalyzed cross coupling have resulted in these reactions being the subject of the 2010 Nobel Prize in Chemistry. Ei-ichi Negishi, one of the recipients of the 2010 Nobel Prize in chemistry, explained in his Nobel lecture that one of his motivations for developing this chemistry was to develop "widely applicable straightforward Lego-like methods for hooking up two different organic groups".
Principles
Pd-catalyzed cross coupling reactions consist of an electrophile (typically an organohalide), a nucleophile (typically an organometallic compound or an alkene), and a palladium catalyst. Regardless of the electrophile or nucleophile used, all Pd-catalyzed cross couplings rely on the Pd-catalyst to activate and combine both partners. Generally speaking, a Pd(0) species reacts with the organohalide via an oxidative addition to form an organopalladium species (RPdX). This organopalladium species then reacts with the nucleophilic partner to generate a new organopalladium species and ultimately construct a new carbon-carbon bond. Depending of the nucleophilic partner, the Pd-catalyzed cross coupling is given a specific name (see Table 1 below).
| Nucleophile | Reaction name |
| Organoboron | Suzuki |
| Organostannane | Stille |
| Organozinc | Negishi |
| Organomagnesium (Grignard reagent) | Kumada |
| Organosilane | Hiyama |
| Olefin/alkene | Heck |
| Alkyne | Sonogashira |
Table 1: List of Pd-catalyzed cross coupling reaction names and their nucleophilic partners.
There are two general mechanisms associated with these Pd-catalyst cross couplings. One for the Heck reaction, and one for the other cross coupling reactions. Overall, the Heck reaction couples an alkene with an organohalide to generate a now more substituted olefin (Figure 1). The first step of a Heck reaction is the same as all other Pd-catalyzed cross couplings. To begin, oxidative addition occurs between the Pd(0)-catalyst and the organohalide to generate an organopalladium(II) species. Next, the olefin coordinates to this newly formed organopalladium(II) species. After olefin coordination, carbopalladation occurs to generate a new carbon-carbon and carbon-palladium bond. Next, beta-hydride elimination occurs to generate a Pd(II)-hydride species and the olefin product. Finally, reductive elimination of HX regenerates the Pd(0)-catalyst, which can continue coupling to another molecule of organohalide and olefin.
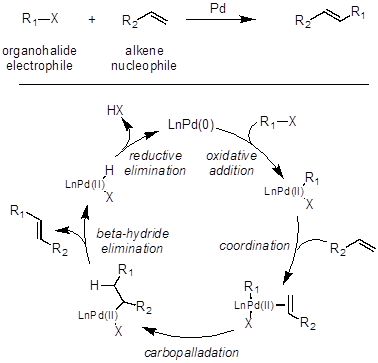
Figure 1: The Heck reaction couples an alkene with an organohalide to generate a now more substituted olefin.
For the remaining cross coupling reactions, the mechanism is as follows (Figure 2). Oxidative addition between the organohalide and Pd(0) catalyst results in the formation of an organopalladium(II) species. This organopalladium(II) species reacts with the nucleophilic organometallic compound in a step called transmetalation to generate an organopalladium(II) species with two carbon-palladium bonds. Finally, reductive elimination occurs to create a new carbon-carbon bond and regenerate the Pd(0) catalyst.

Figure 2: Mechanism for the remaining cross coupling reactions.
Procedure
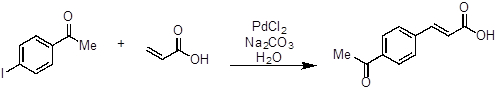
- Add 4-iodoacetophenone (246 mg, 1 equivalent, 1 mmol), acrylic acid (100 μL, 1.5 equivalents, 1.5 mmol), sodium carbonate (Na2CO3, 318 mg, 3 equivalents, 3 mmol), PdCl2 (2 mg, 0.01 equivalents, 0.01 mmol), and water (5 mL, 0.2 M) to a round bottom flask (~ 20 mL) equipped with a magnetic stir bar.
- Heat the reaction to approximately 100 °C and stir until complete consumption of 4-iodoacetophenone (approximately 1 h).
- The reaction can be monitored by TLC.
- Cool the reaction mixture to room temperature after completion.
- Acidify the reaction mixture with 1 M aqueous HCl to ~ pH of 1.
- The pH of the reaction mixture can be checked with litmus paper.
- A solid should precipitate.
- Collect the solid via filtration.
- Purify the crude material by recrystallization using a 1:1 mixture of methanol/water.
Results
The product should be a solid with the follow 1H NMR spectrum: 1H NMR (400 MHz, DMSO-d6): δ (ppm) 2.60 (s, 3H), 6.67 (d, J = 16.0 Hz, 1H), 7.65 (d, J = 16.0 Hz, 1H). 7.83 (d, J = 8.4 Hz, 2H). 7.97 (d, J = 8.4 Hz, 2H).
Application and Summary
These Pd-catalyzed cross coupling reactions have changed the way molecules are synthesized in academic and industrial settings. The impact of this technology can be seen in how chemists construct complex structures for pharmaceuticals, agriculture chemicals, and materials. Beyond Pd-catalyzed cross couplings, transition metal catalysis has changed (and is continuing to change) the way synthetic chemists prepare molecules that can have an impact on society through their potential therapeutic use.
Many molecules of interest for treating diseases have linkages connecting aromatic or heteroaromatic rings. Palladium cross coupling reactions, like the Suzuki reaction, have found widespread use in the pharmaceutical industry for making these types of structures. For example, Crizotinib (Xalkori), an anti-cancer drug for the treatment of non-small cell lung carcinoma, is synthesized on a several kilo-scale using a Suzuki coupling.

Figure 3: Crizotinib (Xalkori), an anti-cancer drug.
Palladium cross couplings have also been applied towards the synthesis of Taxol (an anticancer drug), Varenicline (an anti-smoking drug), and precursors for high performance electronic resins.

Figure 4: Taxol (an anticancer drug), Varenicline (an anti-smoking drug), and precursors for high performance electronic resins.
Skip to...
Videos from this collection:

Now Playing
Palladium-Catalyzed Cross Coupling
Organic Chemistry II
34.2K Views

Cleaning Glassware
Organic Chemistry II
123.3K Views

Nucleophilic Substitution
Organic Chemistry II
99.4K Views

Reducing Agents
Organic Chemistry II
43.0K Views

Grignard Reaction
Organic Chemistry II
148.9K Views

n-Butyllithium Titration
Organic Chemistry II
47.7K Views

Dean-Stark Trap
Organic Chemistry II
100.0K Views

Ozonolysis of Alkenes
Organic Chemistry II
66.9K Views

Organocatalysis
Organic Chemistry II
16.6K Views

Solid Phase Synthesis
Organic Chemistry II
40.9K Views

Hydrogenation
Organic Chemistry II
49.5K Views

Polymerization
Organic Chemistry II
93.7K Views

Melting Point
Organic Chemistry II
149.7K Views

Infrared Spectroscopy
Organic Chemistry II
214.3K Views

Polarimeter
Organic Chemistry II
99.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved