Method Article
Iatrogenic Injury Recapitulated: Electroexcision Technique for Urethral Stricture Modeling in Rats
In This Article
Summary
In this study, we present a novel, efficient, and stable rat model of urethral stricture created through electroexcision of the rat urethra, which effectively simulates iatrogenic injury observed in clinical settings.
Abstract
Urethral stricture (US) is a common clinical condition in urology, characterized by high prevalence and morbidity across all ages. Current treatments for US, such as urethral dilatation and internal urethrotomy, fail to fully resolve the condition and are associated with high rates of recurrence and complications.
Additionally, the pathogenesis of US is not well understood. To explore the pathogenesis of US and develop new therapeutic strategies, it is crucial to establish a standardized rat model that accurately reflects the clinical manifestations. This study outlines a straightforward and repeatable method for inducing US in rats using a high-frequency electric knife. The method involves making a longitudinal incision with the electric knife set to a unipolar mixed cutting mode at 4 W, which inflicts significant urethral damage. Histopathological analysis shows thickening of the urothelium, inflammatory infiltration, and disorganized collagen fibers. This model effectively replicates iatrogenic injury through electroexcision in the rat urethra. In summary, this study successfully establishes a new, efficient, and stable rat model of US that closely mimics the clinical scenario, providing a valuable tool for further research into the mechanisms and novel treatments for US.
Introduction
Urethral stricture (US) is among the oldest urologic conditions and continues to be widely prevalent. Recent data suggest that there are between 229 and 627 cases of US per 100,000 males1. Those suffering from US experience a range of symptoms including lower urinary tract symptoms2, pain3, and sexual dysfunction4. Several medical treatments are available, such as urethrotomy, urethroplasty, and dilation5. However, these treatments are often complicated by issues such as bleeding, infection, and incontinence, contributing to the disease burden and exhibiting varying rates of recurrence6,7. Consequently, identifying the most effective therapeutic approaches remains a critical challenge engaging researchers and clinicians.
US is generally characterized as a narrowing of the anterior urethra caused by fibrosis and cicatrization of the urethral mucosa and surrounding spongiosum tissue8. Despite its prevalence, the causes and mechanisms underlying US are poorly understood, and there is a lack of suitable animal models for in-depth study. Iatrogenic injury, primarily from transurethral surgery, is currently the leading cause of US, accounting for 41% of cases9. Therefore, an ideal animal model for US research should accurately replicate common clinical injuries, exhibit close genomic and proteomic similarities to humans, and demonstrate both efficiency and stability. Such a model would greatly facilitate deeper investigations into the pathogenesis of US and the development of more effective treatments.
To investigate the pathogenic process and mechanism of common clinical types, various animal models have been developed using large animals such as rabbits10,11, dogs12, and pigs13,14 employing techniques like electrocoagulation, electroresection15, and bleomycin injections16. However, these models often face challenges due to sample size limitations and genetic differences from humans. Additionally, the cost-effectiveness of using large animals must be considered; despite the high costs associated with daily care, large animals also carry significant risks of infection, necessitating extensive postoperative care and considerable expenses. It is well documented that rodents share physiological and pathological characteristics with humans in many organ systems. A recent study has shown homology between the urinary tract cells of rodent animals and humans17. Furthermore, the costs of purchasing, housing, and postoperative care of rats are significantly lower than those of large animals18. Consequently, a rat model of the US is deemed suitable; however, the development of such models in rats has been inadequately described.
Prior studies have used surgical tools such as blades or needles to induce US in rat models19. This approach has been associated with risks such as damaging periurethral blood vessels, leading to significant bleeding. The subjective nature of these surgical procedures can also result in variability in the extent of mechanical injury, lacking quantitative criteria for modeling, which may affect the assessment of urethral repair outcomes in subsequent therapeutic studies.
Given these considerations, there is a clear need to develop an additional US rat model. To establish an efficient, cost-effective, and stable US model in rats, we employed a high-frequency electric knife machine in our research. This model will facilitate further investigations into the mechanisms of US and the evaluation of new therapeutic approaches before proceeding to clinical trials.
Protocol
In this investigation, twenty 6-month-old male Sprague-Dawley rats, each weighing 400-500 g, were employed. All animal procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee at the Fifth Affiliated Hospital of Sun Yat-Sen University (Approval number: 00349). The animals were housed in a facility with controlled temperature and lighting conditions. A fundamental characteristic of urethral stricture is the development of scarring within the urethra. Based on the established timeline for scar formation, which typically occurs within 4 weeks of injury, we designated the presence of discernible scar tissue in the urethra at the 4-week postoperative mark as the experimental endpoint.
1. Preparation of surgical instruments
- Prepare the following surgical materials: Teflon coated catheter (0.6 x 1 mm), absorbable sutures (6-0), suture scissors, tissue forceps, needle-holding pliers, smooth forceps, high-frequency electrosurgical unit (Figure 1), including the panel of the high-frequency electric knife, surgical electrode high-frequency electrosurgical knife, and the anal conductive rod (see Table of Materials).
- Clean the surgical area with alcohol wipes containing 75% ethanol.
- Preparation for using a high-frequency electric knife in a unipolar electric cutting mode.
- Connect the high-frequency electric knife to the grounded 220 V main power supply using a power cord.
- Insert the sterilized hand-controlled knife handle and conductive rod into their respective sockets (Figure 1C).
NOTE: Ensure that all power cables are securely connected to maintain the safety of the surgery.
2. Preparation of the animal
- Draw up the sodium pentobarbital (60 mg/kg) into a syringe.
- Secure the rat by grasping its tail with the dominant hand and holding the skin of its ears and neck with the non-dominant hand to ensure it is firmly fixed.
- Expose the rat's lower abdomen and disinfect the area using cotton balls soaked in 75% ethanol.
- Insert the syringe needle approximately 1-2 mm beside the abdominal midline in the lower abdomen, directing it into the abdominal cavity at a 45° angle.
NOTE: You will feel a loss of resistance when the needle penetrates the peritoneum. - Gently pull back the syringe plunger to ensure there is no blood or fluid in the syringe.
- Slowly inject the sodium pentobarbital. Withdraw the needle, completing the injection, and disinfect the site again with cotton balls soaked in 75% ethanol.
- Monitor the rat's breathing rhythm and pinch its hind paw to check for reflexes.
NOTE: Confirm that the rat's breathing is steady and that it shows no reflexes before proceeding with surgery. - Squeeze out the erythromycin ointment and dip a cotton swab in the ointment. Apply the ointment with the cotton swab on the corneas of both eyes to prevent corneal drying during the procedure.
- Use an electric shaver to shave the lower abdomen and perineum after anesthesia is confirmed.
- Wipe off loose hair from the shaved area with a tissue dampened with saline.
- Disinfect the area by wiping it twice with a gauze sponge soaked in iodophor to minimize skin irritation.
- Place the deeply anesthetized rat on a heating pad set to 37°C in a supine position. Ensure sterility during the surgical procedures by wearing medical gloves.
3. Urethral catheterization and injury procedure
- Insert the conductive rod into the anus of the rat to establish an effective closed-loop circuit for the high-frequency electric knife machine (Figure 2A).
- Catheterize the urethra of the rat to aid in positioning and provide support during the surgery's post-processing steps.
- Lubricate the urinary catheter with paraffin oil.
- Expose the penis by squeezing the skin around it to protrude it from the abdomen.
- Disinfect the penis of the rat with a gauze sponge soaked in iodophor.
- Gently open the urethral orifice with smooth forceps and insert the lubricated catheter into the urethra.
- Carefully insert the Teflon-coated catheter in the urethra with the rhythm of urethral contraction; avoid any forceful insertion (Figure 2B).
- If resistance is encountered, gently pull the penis toward the ventral side to adjust the bending angle of the penis and continue advancing the catheter into the bladder until the urine flows out (Figure 2C).
- Confirm successful catheterization by ensuring the penis forms an approximate 45° angle with the abdomen (Figure 2D).
- Turn on the high-frequency electric knife machine, click the buttons of the high-frequency knife panel to select the unipolar mixed cutting mode, and set the power to 4 W for the following steps of electroexcising the penis layer by layer.
- Align the incision with the catheter. Press the yellow button on the electrosurgical knife handle and make an incision along the catheter marking line from the penile skin to the outer layer of the urethra using the electric knife until the urethra is exposed (Figure 3A,B).
NOTE: Ensure you press the yellow button for electroexcision, not the blue button for electrocoagulation. - Continue to make a longitudinal incision of 0.5 cm in the urethra with the electric knife, ensuring a transverse diameter of 0.1 cm, until the catheter is visible (Figure 3C).
- Suture the wound and the superficial skin layer by layer using absorbable sutures (6-0) with either continuous or interrupted stitches (Figure 3D,E).
- Remove the urethra catheter and reposition the penis (Figure 3F).
- Turn off the high-frequency electric knife and remove the conductive rod from the rat's anus.
- Clean the surgical materials with alcohol (75% ethanol) wipes.
4. Postoperative care
- Place the rat on a warmed pad (37 °C) and monitor its recovery from anesthesia closely. Pay careful attention to the rat's physical condition, including breathing rhythm, body temperature, and level of consciousness.
NOTE: Do not return the rat into its cage until full recovery is observed. - House the rats in a clean cage and fill the water bottle with morphine-spiked water (0.4 mg/kg) for daily pain management after recovery.
- Administer non-steroidal anti-inflammatory drugs (such as Carprofen, 0.5 mg/kg, subcutaneous injection) for the rats' full recovery from postoperative pain.
5. Histological evaluation
- Four weeks after the surgery, induce the micturition reflex by lifting the rats' tails. Place the rat in a euthanasia box, open the CO2 transmission pipe valve, and humanely euthanize the rats with an overdose of CO2.
- Turn off the valve after confirming that the rat is motionless and not breathing and observing that the pupil is dilated. Continue to observe the rat's physical condition for 2 min to confirm that euthanasia is successful.
- Remove the rat from the euthanasia box.
- Clean the surgical area and surgical materials with wipes soaked in 75% ethanol.
- Dissect the abdomen to observe the bladder-filling status and harvest the entire urethra.
- After harvesting the urethra, put the rat in a sealing bag and store it in a 4 °C refrigerator for processing by laboratory technicians.
- Morphologically evaluate the US for gross pathology, focusing on the color and smoothness of the tissue.
- Stain the urethra tissue with hematoxylin and eosin (H&E) to visualize the wound structure and urothelial cell layer.
- Dip the slides in xylene for deparaffinization (2 x 5 min).
- Rehydrate the slides in ethanol in order (100% 1 x 3 min, 95% 1 x 3 min, 85% 1 x 3 min, 75% 1 x 3 min).
- Wash the slides in deionized water (2 min).
- Stain the slides with hematoxylin (10 min).
- Wash the slides in deionized water (5 min).
- Differentiate the samples with differentiation solution (40 s).
- Wash the slides in deionized water (2 x 3 min).
- Stain the slides with eosin (2 min).
- Rehydrate the slides in ethanol in an order (100% 1 x 3 s, 95% 1 x 3 s, 85% 1 x 3 s, 75% 1 x 3 s)
- Dip the slides in 100% ethanol (1 min) and transparentize the slides with xylene (2 x 1 min).
- Add neutral balsam and seal with the coverslip.
- Apply Masson's trichrome stain to the urethra tissue to highlight the collagen fiber structure.
- Dip the slides in xylene for deparaffinization (2 x 5 min).
- Rehydrate the slides in ethanol in order (100% 1 x 3 min, 95% 1 x 3 min, 85% 1 x 3 min, 75% 1 x 3 min).
- Wash the slides in deionized water (2 min).
- Stain the slides in Weigert's Iron Hematoxylin solution (5 min).
- Wash the slides in deionized water (5 min).
- Stain the slides in Biebrich Scarlet-Acid Fucshin (5 min).
- Wash the slides in deionized water (2 x 3 min).
- Stain the slides with Phosphomolybdic Acid solution (5 min).
- Place slides in Aniline Blue solution (5 min). Discard solution.
- Rinse the slides, dehydrate through alcohol, and clear in xylene.
- Add neutral balsam and seal with the coverslip.
- Perform immunofluorescence staining using a TGF-β antibody to assess the urothelial cell layer.
- Add the appropriate antigen retrieval buffer to the pressure cooker. Place the pressure cooker on the hotplate and turn it on full power.
- Once boiling, transfer the slides from the tap water to the pressure cooker. Run cold water over the cooker and heat the slides for 10 min.
- Turn off the pressure cooker and remove the slides. Cool the slides to room temperature.
- Wash the slides with PBS solution.
- Mark the margins of samples with liquid blocker pen.
- Dip the slides in PBS plus 0.3% Triton X-100 for 30 min.
- Wash the slides in PBS with gentle agitation (2 x 5 min).
- Block in 10% goat serum in PBS at room temperature (60 min).
- Aspirate blocking buffer and incubate with primary antibody at 4 °C for overnight.
- Wash the slides with 1x PBS (3 x 5 min).
- Incubate with fluorochrome-labeled secondary antibody diluted in antibody dilution buffer.
- Wash the slides with 1x PBS (3 x 3 min).
- Mount and seal the slides.
- Digitally scan the stained slides using a 40x objective in a slide scanner.
- Analyze the images and measure the thickness of urothelium using the referenced software (Table of Materials).
- Open the software.
- Click Local Computer to select the stained slide images.
- Click Measure Distance to measure the thickness of the urothelium.
- Record the data on the thickness of urothelium.
Results
The protocol outlined in this study successfully established stable urethral stricture in rats and demonstrated high reproducibility. The average duration of the operations was 20 min, and no technical issues arose during the procedures. Urethral specimens were successfully harvested 4 weeks after the procedure.
In the experimental group, the rats' bladders showed signs of overdistension, in contrast to the control group where the bladders were empty (Figure 4). These observations indicate a significant impairment in the voiding function of rats following the experimental modeling.
Four weeks post-surgery, the wound sites had developed into waxy white scar tissue protruding from the urethra's surface (Figure 5B), while the urethral sections in the control group appeared smooth and pale red control (Figure 5A).
Histological examination with hematoxylin and eosin (H&E) staining of the urethral section revealed significant urothelium thickening and lymphocyte aggregation. Additionally, Masson staining identified a pronounced reddish area of hyperplastic and metaplastic urethral epithelial tissue, accompanied by disordered collagen fibers (see Supplemental Figure S1 for detailed images of Masson staining), indicating abnormalities in the structure of smooth muscle and collagen fibers (Figure 6).
Immunofluorescence analysis of cryosections showed increased TGF-β in the urethral tissue post-surgery (Figure 7), suggesting that the formation of urethral structure is associated with elevated expression of TGF-β.

Figure 1: Key instruments used to establish the US model. (A) Teflon-coated catheter (0.6 x 1 mm); (B) absorbable sutures (6-0), tissue forceps, needle-holding pliers, and smooth forceps (from left to right); (C) high-frequency electrosurgical pen and anal conductive rod; (D) the panel of the high-frequency electric knife. Please click here to view a larger version of this figure.
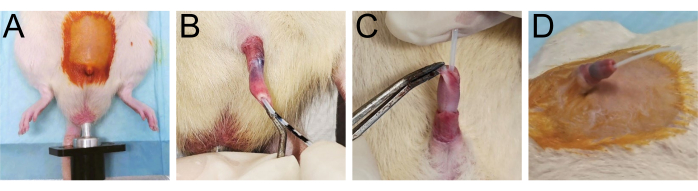
Figure 2: Preparations for the surgery. (A) Shave the lower half abdomen of the rat and connect the anal conductive rod. (B-D) Illustrate the placement of the urethral catheter within the urethra. Please click here to view a larger version of this figure.

Figure 3: The electroresection process. (A) Shows the creation of a longitudinal incision on the penile skin measuring 0.8 cm; (B) exposure of the urethra; (C) details the electrosection through all layers of the urethral wall until the catheter within the urethra is visible. (D,E) Depicts the removal of the urethral catheter and the repositioning of the penis. Please click here to view a larger version of this figure.
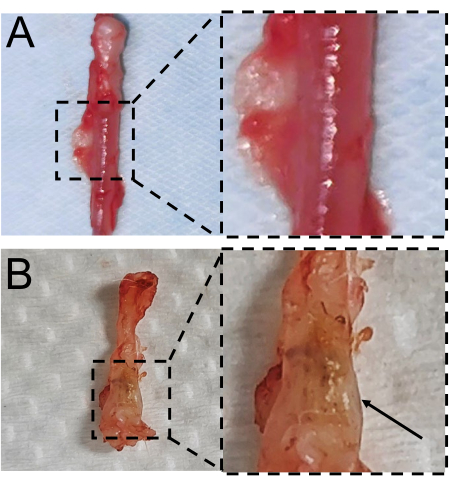
Figure 4: The filling status of the bladder in rats under anatomical conditions. (A) Features an empty bladder from rats in the control group; (B) shows an overfilled bladder from rats in the experimental group. The bladders are within the red dashed outline. Please click here to view a larger version of this figure.

Figure 5: Macroscopic findings of the resected urethral samples. (A) The normal urethral tissue. (B) The urethral scar tissue. Please click here to view a larger version of this figure.

Figure 6: Results of histology of the urethral samples. (A) Urethral tissue from the control group stained with H&E (original magnification, 100x). (B) Masson's trichrome staining of a urethral mass at 100x original magnification. (C) Enlargement and the measurement data of normal urothelium's thickness, which is 85.9 µm. Scale bar = 20 µm. (D) Shows H&E staining of urethral tissue from the experimental group at 100x original magnification. (E) Depicts Masson's trichrome staining of urethral tissue from the experimental group, also at 100x original magnification. Scale bars = 100 µm. (F) Enlargement of the urethra scar and the measurement data of thickened urothelium-231.2 µm. Scale bar = 20 µm. Please click here to view a larger version of this figure.

Figure 7: Immunohistochemistry findings on excised urethral samples. TGF-β antibody labeling highlights urothelial cells; a green fluorescence is emitted upon binding of the antibody to the cells. (A-C) Show lower signal intensity in urothelial cells of the control group compared to (D-F) higher signal intensity in urothelial cells of the experimental group. Please click here to view a larger version of this figure.
Supplemental Figure S1: The whole image of Masson staining of urethra samples. (A) Masson staining of the control group; (B) Masson staining of the experimental group. Please click here to download this File.
Discussion
US pose a significant healthcare burden with a substantial economic impact, adversely affecting both psychological and physical well-being20. There is still a need for a treatment that not only completely cures US but also effectively prevents its recurrence.
In this study, we utilized a rat model to develop a straightforward and reproducible method for mimicking urethral injury in patients, which was followed by transurethral surgery-induced urethral injury. Rats are favored as animal models because they are cost-effective, easy to handle, offer repeatability, and provide reliable data. The rat model used in this study has several advantages over other models: it is commonly used in various omics analyses and is easy to manage. Additionally, the similarity in gene expression of urinary tract epithelial cells between rodents and humans makes the rat US model particularly suitable for deeper investigations21.
As can be seen in Figure 7, TGF-β was expressed in the whole circle of the urethra both in control and experimental groups, and the expression of TGF-β in the experimental group was markedly higher than the control group. Prior work has documented that the resident urothelium cells virtually undergo a phenotypic switch into myofibroblasts after the injury, the process of which is mainly driven by TGF-β, which exerts significant effects on scar formation by regulating ECM (extracellular matrix) production, immune modulation, cell proliferation, migration and differentiation22,23. Our finding suggests that the overexpression of TGF-β may promote urethral scar formation, and the secretion of TGF-β is not confined to the resident cells in the wound surface but requires the participation of the whole urethra.
The small diameter and curvature of the male rat urethra present challenges in detecting changes in urodynamics after modeling. There is a scarcity of research on methods for assessing urinary function in male rats. In this study, we used indirect methods to observe changes in urinary function by assessing bladder filling status 4 weeks post-surgery. Upon stimulating the micturition reflex, the observation of the dissected rat abdomen showed that the experimental group exhibited persistent bladder fullness, indicative of significant impairment in voiding function post-modeling. These findings are consistent with the clinical symptoms of difficult urination experienced by patients with urethral stenosis24. However, developing methods to conduct physiological studies on urinary parameters in male rats, such as voiding frequency, average voided volume, and voiding efficiency, remains a challenge.
The choice of modes and power settings of high-frequency electric knives critically influences the extent of injury in surgical procedures. This study introduces a surgical technique that ensures an appropriate level of injury, and an adequate degree of the high-frequency electric knife which is 4 W for developing a US model in rats. The most common method in rat US models involves making incisions with a needle or scalpel25. However, using a scalpel tends to increase the likelihood of continuous bleeding due to the smooth surface and small diameter of the rat's urethra, which may inadvertently damage surrounding blood vessels.
The high-frequency knife offers a significant advantage by providing simultaneous hemostasis through the application of a stable and adjustable electric current to tissues, enhancing the stability and convenience of the procedure. Additionally, the controllability of the power setting allows for a quantifiable assessment of the damage inflicted.
An ideal experimental protocol would consistently produce urethral wounds of uniform size and depth. In this study, the unipolar mixed cutting mode was selected to induce urethra damage. Unlike the electrocoagulation mode, which primarily causes the volatilization of liquid components, drying, coagulation of tissues, and sealing of blood vessels without tissue rupture-leading to rapid tissue scorching and adhesion-the cutting mode enables precise, layer-by-layer incisions into the penile tissue. While electrocoagulation has been commonly used in rabbit US models and requires additional steps such as separating the penis from the radium and making incisions with other surgical instruments26, which increases animal trauma and complication risks, the unipolar mixed cutting mode allows for direct incisions into the foreskin to expose the urethra. This method enhances operational efficiency and minimizes trauma to the experimental animals.
The power setting in the previous study was not specified in detail, as it typically depends on the weight of the experimental animals and the contact area between the electronic knife and tissue. In this study, the experimental subjects were 6-month-old SD rats weighing between 400 g and 500 g. For these conditions, the optimal power setting was determined to be between 4-7 W, which allowed for precise control over the size and depth of the incision. We recommend that operators start with a lower power setting when first using the high-frequency knife to develop the US rat model, adjusting as needed to find the most suitable value for different conditions.
Furthermore, one critical step is the catheterization of the rats' urethras prior to surgery. Successful catheterization is confirmed when the catheter is correctly placed in the bladder. Although the catheter serves as a temporary scaffold, it must maintain the urethra's position and assist in identifying the surgical site. The male rat's urethra has a relatively small diameter, and there is limited space between the foreskin and penile tissue, making it easy to mistakenly insert the catheter into the wrong opening, potentially complicating subsequent procedures. The correct placement of the catheter is verified when urine flows out, indicating it is securely within the bladder. Additionally, the catheterized penis should be positioned at a 45° angle to enhance stability within the urethra and facilitate the surgical procedure.
In conclusion, this study demonstrates a novel, highly effective, and cost-efficient method for creating a US model in rats. This method is characterized by its simplicity, high success rate, and stability, offering a valuable tool for investigating a wide range of innovative treatments for urethral stricture.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This study was supported by grants from the Natural Science Foundation of Guangdong Province (No.2019A1515012116 and No.2022A1515012559).
Materials
| Name | Company | Catalog Number | Comments |
| absorbable sutures (6-0) | KERONG COMPANY | KR2230814 | |
| Animal operating pad | Provided by Guangdong Provincial Key Laboratory of Biomedical Imaging | NA | |
| CaseViewer 2.4 | 3DHISTECH Ltd. | ||
| Carprofen | Sigma-Aldrich | MFCD00079028 | |
| CoraLite488-conjugated Goat Anti-Rabbit IgG(H+L) | Proteintech | SA00013-2 | |
| H&E Stain Kit | Abcam | ab150669 | |
| high-frequency electrosurgical unit | Beijing Taktvoll Technology Company | ES-100v | |
| Masson staining kit | Merck | HT15 | |
| needle-holding pliers | RWD Life Science | S15001-11 | |
| Paraffin oil | NA | NA | |
| smooth forceps | RWD Life Science | F13019-12 | |
| Sodium pentobarbital | Guangdong Provincial Key Laboratory of Biomedical Imaging | NA | |
| Sprague–Dawley rat | Guangdong Medical Laboratory Animal Center | GDMLAC-035 | |
| suture scissors | RWD Life Science | S15001-11 | |
| Teflon coated catheter (0.6 mm x 1 mm) | DGZF new materials company | NA | |
| TGF Beta 1 Polyclonal antibody | Proteintech | 21898-1-AP | |
| Tissue scissors | RWD Life Science | S13029-14 |
References
- Rourke, K. F., et al. Canadian Urological Association guideline on male urethral stricture. Cuaj-Can Urol Assoc. 14 (10), 305-316 (2020).
- Cotter, K. J., et al. Prevalence of post-micturition incontinence before and after anterior urethroplasty. J Urol. 200 (4), 843-847 (2018).
- Bertrand, L. A., et al. Lower urinary tract pain and anterior urethral stricture disease: Prevalence and effects of urethral reconstruction. J Urol. 193 (1), 184-189 (2015).
- Mondal, S., Bandyopadhyay, A., Mandal, M. M., Pal, D. K. Erectile dysfunction in anterior urethral strictures after urethroplasty with reference to vascular parameters. Med J Armed Forces India. 72 (4), 344-349 (2016).
- Campos-Juanatey, F., et al. European association of urology guidelines on urethral stricture disease (part 2): Diagnosis, perioperative management, and follow-up in males. Eur Urol. 80 (2), 201-212 (2021).
- Hoy, N. Y., Chapman, D. W., Dean, N., Rourke, K. F. Incidence and predictors of complications due to urethral stricture in patients awaiting urethroplasty. J Urol. 199 (3), 754-759 (2018).
- Anger, J. T., Buckley, J. C., Santucci, R. A., Elliott, S. P., Saigal, C. S. Trends in stricture management among male medicare beneficiaries: Underuse of urethroplasty. Urology. 77 (2), 481-485 (2011).
- Milenkovic, U., Albersen, M., Castiglione, F. The mechanisms and potential of stem cell therapy for penile fibrosis. Nat Rev Urol. 16 (2), 79-97 (2018).
- . EAU guidelines on urethral-strictures Available from: https://uroweb.org/guidelines/urethral-strictures (2023)
- Faydacı, G., et al. Comparison of two experimental models for urethral stricture in the anterior urethra of the male rabbit. Urology. 80 (1), 225.e7-225.e11 (2012).
- Yang, M., et al. Urine-microenvironment-initiated composite hydrogel patch reconfiguration propels scarless memory repair and reinvigoration of the urethra. Adv Mater. 34 (14), 210952 (2022).
- Orabi, H., Aboushwareb, T., Zhang, Y., Yoo, J. J., Atala, A. Cell-seeded tubularized scaffolds for reconstruction of long urethral defects: A preclinical study. Eur Urol. 63 (3), 531-538 (2013).
- Sievert, K. -. D., et al. Introducing a large animal model to create urethral stricture similar to human stricture disease: A comparative experimental microscopic study. J Urol. 187 (3), 1101-1109 (2012).
- Sievert, K. -. D., et al. Urethroplasty performed with an autologous urothelium-vegetated collagen fleece to treat urethral stricture in the minipig model. World J Urol. 38 (9), 2123-2131 (2019).
- Hu, W. -. F., Li, C. -. L., Zhang, H. -. P., Li, T. -. T., Zeng, X. -. Y. An experimental model of urethral stricture in rabbits using holmium laser under urethroscopic direct visualization. Urol Int. 93 (1), 108-112 (2014).
- Hua, X., et al. An experimental model of anterior urethral stricture in rabbits with local injections of bleomycin. Urology. 116, 230.e9-230.e15 (2018).
- Strittmatter, F., et al. Expression of fatty acid amide hydrolase (faah) in human, mouse, and rat urinary bladder and effects of faah inhibition on bladder function in awake rats. Eur Urol. 61 (1), 98-106 (2012).
- Abdullahi, A., Amini-Nik, S., Jeschke, M. G. Animal models in burn research. Cell Mol Life Sci. 71 (17), 3241-3255 (2014).
- Castiglione, F., et al. Adipose-derived stem cells counteract urethral stricture formation in rats. Eur Urol. 70 (6), 1032-1041 (2016).
- Cheng, X., et al. The changing trend in clinical characteristics and outcomes of male patients with urethral stricture over the past 10 years in China. Front Public Health. 9 (794451), 1-8 (2021).
- Yu, Z., et al. Single-cell transcriptomic map of the human and mouse bladders. J Am Soc Nephrol. 30 (11), 2159-2176 (2019).
- Lichtman, M. K., Otero-Vinas, M., Falanga, V. Transforming growth factor beta (tgf-β) isoforms in wound healing and fibrosis. Wound Repair Regen. 24 (2), 215-222 (2016).
- Tatler, A. L., Jenkins, G. Tgf-β activation and lung fibrosis. Proc Am Thorac Soc. 9 (3), 130-136 (2012).
- Palminteri, E., et al. Contemporary urethral stricture characteristics in the developed world. Urology. 81 (1), 191-197 (2013).
- Hedlund, P., Streng, T., Lee, T., Andersson, K. -. E. Effects of tolterodine on afferent neurotransmission in normal and resiniferatoxin treated conscious rats. J Urol. 178 (1), 326-331 (2007).
- Yao, H. -. J., et al. Three new experimental models of anterior urethral stricture in rabbits. Transl Androl Urol. 11 (6), 761-772 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved