Method Article
Porcine Normothermic Isolated Liver Perfusion
In This Article
Summary
The porcine model of liver normothermic machine perfusion (NMP), described here, can be successfully used to study NMP as a preservation strategy, a tool for viability assessment, and a platform for organ repair. It holds a high translational value, however it is technically challenging and labor-intensive.
Abstract
Porcine models of liver ex situ normothermic machine perfusion (NMP) are increasingly being used in transplant research. Contrary to rodents, porcine livers are anatomically and physiologically close to humans, with similar organ size and bile composition. NMP preserves the liver graft at near-to-physiological conditions by recirculating a warm, oxygenated, and nutrient-enriched red blood cell-based perfusate through the liver vasculature. NMP can be used to study ischemia-reperfusion injury, preserve a liver ex situ before transplantation, assess the liver's function prior to implantation, and provide a platform for organ repair and regeneration. Alternatively, NMP with a whole blood-based perfusate can be used to mimic transplantation. Nevertheless, this model is labor-intensive, technically challenging, and carries a high financial cost.
In this porcine NMP model, we use warm ischemic damaged livers (corresponding to donation after circulatory death). First, general anesthesia with mechanical ventilation is initiated, followed by the induction of warm ischemia by clamping the thoracic aorta for 60 min. Cannulas inserted in the abdominal aorta and portal vein allow flush-out of the liver with cold preservation solution. The flushed-out blood is washed with a cell saver to obtain concentrated red blood cells. Following hepatectomy, cannulas are inserted in the portal vein, hepatic artery, and infra-hepatic vena cava and connected to a closed perfusion circuit primed with a plasma expander and red blood cells. A hollow fiber oxygenator is included in the circuit and coupled to a heat exchanger to maintain a pO2 of 70-100 mmHg at 38 °C. NMP is achieved by a continuous flow directly through the artery and via a venous reservoir through the portal vein. Flows, pressures, and blood gas values are continuously monitored. To evaluate the liver injury, perfusate and tissue are sampled at predefined time points; bile is collected via a cannula in the common bile duct.
Introduction
Liver transplantation is the sole definitive treatment for end-stage liver failure; however, its success is limited by a persistent imbalance between patients on the waitlist and the availability of potential donor organs1. To increase the donor pool, donor criteria have been gradually extended in the last decade, including older donor age, liver steatosis, and donation after circulatory death (DCD)2,3. During a DCD procedure, the liver invariably suffers a period of warm ischemia between the withdrawal of life-sustaining therapy, declaration of death, and in situ cooling and preservation, aggravating ischemia-reperfusion injury (IRI)4. As a result, DCD livers are associated with an increased incidence of early allograft dysfunction and biliary complications5,6.
For these high-risk donor livers, conventional preservation with static cold storage does not offer sufficient protection against IRI. Hereto, alternative preservation strategies such as normothermic machine perfusion (NMP) have gained considerable traction. During normothermic machine perfusion, the liver is connected ex situ to an isolated circuit and perfused with an oxygenated and nutrient-enriched perfusate at body temperature. Clinical trials suggest that NMP reduces hepatocellular injury, as reflected by reduced peak transaminase release and early allograft dysfunction7. However, little is known about liver cell biology during NMP8.
Animal models have been pivotal in the evolution of liver transplantation. In contrast to rodent models, the pig is considered to be of higher translational value as the porcine liver is anatomically and physiologically close to humans, with similar organ size and bile composition. Nevertheless, porcine liver transplant models are labor-intensive, difficult to standardize, and carry a significantly higher financial cost.
Porcine liver NMP can be used to serve different purposes. It can be applied to mimic transplantation ex situ when using a whole blood-based perfusate, to preserve a donor liver in a protective environment with a leukocyte-depleted red blood cell-based perfusate, to assess potential biomarkers predicting liver function ex situ prior to transplantation, or as a platform to investigate regenerative therapy9,10,11.
The adoption of porcine liver NMP models is challenging, while surgical and perfusion-related technical aspects are scarcely described. In our research laboratory, we adopted the NMP setup originally described by Butler et al.12 to develop and validate a 24 h porcine ex situ isolated liver perfusion model that could be used to both preserve a liver graft for transplantation and to mimic a transplant. Here, we describe a step-by-step protocol; a methodological framework and potential pitfalls are published elsewhere9.
Protocol
All experiments were conducted after KU Leuven animal care committee approval and in line with European guidelines.
1. Animal information
NOTE: Male TOPIGS TN70 pigs, aged 3 months, with a body weight of approximately 30 kg and liver weight of 600-700 g are used for this study protocol.
- Keep the animals under a 12 h day/night rhythm in single pens with free access to food and tap water and visual, olfactory, and auditory contact between them.
- Ensure that the animals arrive at least 2 days prior to the surgery to get accustomed to their surroundings. Fast the pigs for 12 h prior to surgery with free access to water.
NOTE: Anesthesia is maintained with isoflurane and analgesia with fentanyl. Throughout anesthesia, the electrocardiogram, pulse oximetry, capnography, and blood pressure are continuously monitored. The experiments are terminal; pigs are euthanized by exsanguination while procuring the liver under continued general anesthesia and analgesia.
2. Preparation of the perfusion setup
- Installation of the disposable perfusion kit
- Fixate the reservoir at a fixed height approximately 15 cm higher than the liver receptacle.
- Connect the pump head to the designated slot on the centrifugal pump. Connect the oxygen tube to the oxygenator. Connect the in- and outflow tubes of the heater/cooler to the designated slots on the oxygenator.
- Install one pinch valve on the outflow tube under the reservoir. Install the second pinch valve on the inflow tube to the reservoir after the Y-connection, where the other tube will feed the hepatic artery.
NOTE: The first pinch valve will control the inflow into the portal vein. Closing the second pinch valve will increase the flow, and therefore also the pressure in the hepatic artery. - Install the flow sensor distal from the first pinch valve on the reservoir outflow tube. Install the second flow sensor on the inflow tube to the pump head.
NOTE: The first flow sensor will measure the flow into the portal vein. The second flow sensor will measure the outflow from the vena cava. - Cut the outflow tube from the oxygenator and insert the arterial filter in the correct direction.
NOTE: Cut the tube to no longer than 2 cm after the oxygenator; if it is left too long, it will kink when the tubing becomes softer when it is perfused with a 37 °C perfusate. If it still kinks, support the arterial filter by tying it to the support of the reservoir.
- Installation of the leakage recirculation tube
- The perfusion kit contains two tubes with one 3/16 end and one 1/16 end. Connect the two tubes to each other by inserting the 1/16 end into the 3/16 end. Connect the 3/16 end to the reservoir.
- Install the tube in the roller pump, considering the rotation direction. Set up the correct tubing diameter and set the speed to 15-18 rotations per minute (rpm). Place the 1/16 end into the liver receptacle and fixate if necessary.
- Continuous in-line blood gas analysis calibration
- Switch on the gas analyzer and select Calibrate.
- Check the correct serial number on the sensor package and press OK.
- Take the arterial sensor holder out of the cassette and connect the sensor to it with a blue cap on top and a white filter on the bottom.
- Unscrew and remove the white cap under the filter; do not unscrew the filter itself. Loosen the blue venting cap on top, do not remove it. Firmly insert the sensor and sensor holder into the calibration cassette.
- Start calibration. When calibration is finished, remove the sensor and sensor holder from the calibration cassette. Remove the white filter on the bottom and tighten the blue venting cap on top.
- Insert the sensor in the sampling line of the perfusion kit. Do not start gas analysis until the perfusion circuit is primed.
- Priming of the circuit
- Insert an infusion line into a 500 mL bag of plasma expander and attach it to the reservoir. Place a tube clamp on the reservoir outflow line and fill the reservoir with 300 mL of plasma expander.
- Remove the tube clamp and let the plasma expander fill the circuit
- De-air the pump head and oxygenator. The circuit is now primed. Switch on the heater/cooler and set it to 38 °C
- Preparation of the infusion lines
- Draw 5 mL (25.000 U) of a 5 U/mL heparin solution into a 50 mL syringe. Draw an additional 25 mL of 0.9% NaCl solution to obtain a total volume of 30 mL of heparin solution (infusion rate: 1 mL/h).
- Dissolve 5 g of sodium taurocholate in 50 mL of 0.9% NaCl and draw it into 450 mL of 0.9% NaCl to reach a 1% concentration. A total volume of 168 mL is needed (infusion rate: 7 mL/h).
- Draw 2 mL (200 U) of a 100 U/mL insulin solution into a 50 mL syringe. Draw an additional 28 mL of 0.9% NaCl solution to obtain a total volume of 30 mL of insulin solution (infusion rate: 1 mL/h).
- Draw 10 mL of glycine buffer (diluent) and add it to the 0.5 mg vial of epoprostenol. Using the microbial filter provided in the epoprostenol kit, draw 5 mL out of the vial containing the epoprostenol reconstituted with glycine buffer in a 50 mL syringe. Draw an additional 25 mL of 0.9% NaCl solution to obtain a total volume of 30 mL of epoprostenol solution (infusion rate: 1 mL/h).
3. Induction of anesthesia
- Sedation
- Prepare a syringe with 2 mg/kg xylazine and 8 mg/kg Zoletil (4 mg/kg tiletamine and 4 mg/kg zolazepam), a syringe with 10 mL of 0.9% NaCl, a three-way valve, an extension line, and an intramuscular 21 G needle.
- Place the needle into the gluteal muscle, inject the xylazine and tiletamine mixture, and flush the extension line with the 0.9% NaCl. After 15 min, the pig is sedated.
- Weigh the pig and transport it to the operating room.
- Anesthesia
- Turn on the ventilator
- Place the pig in a supine position on the operating table and fixate the extremities.
- Pre-oxygenate with a ventilation mask with 1.5 L of O2, 1.5 L of air, and 1% isoflurane.
- Attach a saturation probe to the tail or ear. Connect the three electrocardiogram leads for continuous monitoring.
- Insert a 22 G catheter in an ear vein, connect to a three-way valve, and start a drip of intravenous (IV) fluids (Plasmalyte) at a rate of 400 mL/h.
- Place a 60 mL syringe with 50 µg/mL fentanyl into an automatic syringe driver. Give a bolus of 1 mL and start a continuous infusion at a rate of 0.16 mL/kg/h.
- Remove the ventilation mask and insert the laryngoscope, lifting the epiglottis. Insert an endotracheal tube into the trachea and inflate the balloon to prevent air leakage. Fixate the tube with tape to the pig's snout.
NOTE: Close off the isoflurane when removing the ventilation mask. - Connect the endotracheal tube to the ventilator.
NOTE: Ventilator settings: 0.4 L tidal volume; 0.5 kPa mean airway pressure; 2.5 kPa airway pressure; 0.5 kPa positive end expiratory pressure; 15/min frequency; 4.7-5.3 kPa end-tidal CO2. - Connect the capnograph to the endotracheal tube.
4. Surgery
- Deep venous catheter and arterial line
- Disinfect the surgical field with betadine and place drapes on both sides of the midline.
- Make a 7 cm long incision from the left side of the top of the sternum laterally to the sternocleidomastoid muscle and place an orthostatic retractor.
- Dissect the subcutaneous tissue off the muscle in the lateral direction and identify the external jugular vein. Dissect free the vein, ligating side branches if present.
- Place two 2/0 ligatures around the external jugular vein and tie off the cranial ligature.
- Cut open the vein caudal from the tied ligature and insert a 12 French venous catheter.
NOTE: Make sure the venous catheter is flushed with heparinized saline prior to insertion. - Fixate the catheter with the second ligature. If the venous catheter in the ear vein is fragile or too small, switch the IV fluid and fentanyl lines to the deep venous catheter. Otherwise, do not use it until blood collection to the cell saver.
- Dissect free the medial edge of the sternocleidomastoid muscle and replace the orthostatic retractor opening the plane between the sternocleidomastoid muscle on the lateral side and the trachea on the medial side.
- Remove the thymus to expose the carotid artery. Repeat step 4.1.4 for the artery.
- Insert the arterial line in the carotid artery in a caudal direction and fixate. Connect the arterial line to the line from the pressure monitor.
- Dissection of the aorta and vena cava
- Perform a midline laparotomy from the xiphoid to the pubic bone.
NOTE: In male pigs, caudal from the penis, incise 1 cm lateral from the midline to avoid damaging the subcutaneous urethra. - Transect the umbilical ligament. Place an abdominal retractor. Pull the intestines left laterally and cranially to visualize the aorta and vena cava.
NOTE: Porcine intestine is non-rotated; therefore, no colon mobilization is necessary to gain access to the retroperitoneum. - Dissect free approximately 3 cm of the aorta just cranial of the iliac bifurcation and place two ligatures around the aorta.
NOTE: There is a large lymphatic vessel near the aorta; be careful not to damage it as this will blur the surgical field and complicate dissection. - Dissect free the vena cava at the same level as the aorta and place two ligatures.
- Perform a midline laparotomy from the xiphoid to the pubic bone.
- Gastroduodenal ligament dissection
- Start the dissection at the lateral side of the gastroduodenal ligament, dissect free the common bile duct, and encircle with a vessel loop.
- Retract the bile duct to the medial side, exposing the portal vein. Remove a large lymph node on the lateral side of the portal vein
- Free up the portal vein toward the pancreas; there is often a branch from the stomach and pancreas that need to be ligated and transected. Encircle the portal vein with a vessel loop on the side of the liver and a ligature on the side of the pancreas. Retract the portal vein laterally, making sure not to close it off.
- Identify the common hepatic artery and encircle it with a vessel loop.
- Thoracic aorta dissection
- Pull the liver caudal and open the central tendinous part of the diaphragm ventrally from the suprahepatic vena cava. Retract the esophagus to the right side, exposing the thoracic aorta.
- Dissect free from the surrounding tissue and be careful not to damage the azygos vein.
NOTE: It is not necessary to encircle the thoracic aorta; dissection should be extended sufficiently to place a vascular clamp.
- Preparation of the cell saver
- Hang the reservoir in the black ring, remove the cap from the single leg of the adapter, and attach the tube to the 3/8 inch outlet port on the bottom of the blood collection reservoir.
- Uncap the suction/anticoagulant tube and connect it to one of the 1/4 inch blood inlet ports on the top edge of the blood collection reservoir.
- Insert the bowl of the wash kit by turning; make sure a click is heard.
- Place the tube in the roller pump and the tube divider.
- Hang a citrate phosphate dextrose adenine (CPDA)-1 blood collection bag on the pole, and make sure the connection is tight.
NOTE: CPDA-1 blood bag composition (63 mL): 2.99 g/L anhydrous citric acid; 26.3 g/L dyhidrous sodium citrate; 2.22 g/L monohysrate sodium phosphate; 31.9 g/L dextrose monohydrate; 0.275 g/L adenine. - Hang the waste bag on the side of the machine (make sure it is closed properly) and connect it to the wash kit (yellow cap).
- Connect both Y-shaped tubes (white caps) to a bag of 3 L of 0.9% NaCl.
- Attach the tube with the blue cap to the adapter tube on the bottom of the blood collection reservoir. Switch on the autotransfusion system.
- Cannulation of the aorta and vena cava
- Administer 500 IU/kg heparin and allow it to circulate for 2 min. Tie the caudal ligature around the aorta and insert a 20 French cannula in the aorta and fixate. The same procedure is repeated for the vena cava.
- To mimic a DCD procedure, warm ischemia is induced by clamping the thoracic aorta for a period of time, in this case 60 min.
NOTE: During warm ischemia, the caval and jugular drainage are opened, and blood collection to the cell saver is commenced. This results in exsanguination of the pig. - Flushing the liver
- At the end of warm ischemia, initiate a cold flush with 2 L of ice-cold (4-6 °C) preservation solution through the aortic cannula, and cool the abdomen with a topical application of slush ice.
NOTE: During the cold flush, all remaining blood is flushed out and collected to the cell saver. - When the first 2 L is flushed, remove the cannula from the aorta, tie the ligature on the portal vein, and cannulate the portal vein. Then, fixate it with the vessel loop.
- Flush the liver via the portal vein with another 2 L of ice cold (4-6°C) preservation solution.
- At the end of warm ischemia, initiate a cold flush with 2 L of ice-cold (4-6 °C) preservation solution through the aortic cannula, and cool the abdomen with a topical application of slush ice.
- Hepatectomy
- Remove the ice from the abdomen. Transect the bile duct close to the pancreas.
- Remove the portal cannula and divide the portal vein. Retract the intestines to the left to expose the infrahepatic vena cava.
- Dissect the vena cava free from the retroperitoneum and divide just cranial from the renal veins. Dissect the common hepatic artery up to the celiac artery and origin from the aorta.
NOTE: Cutting the right diaphragmatic crus can improve exposure. - Divide the gastroduodenal artery and cut out the celiac artery with a patch of the aorta. Transect the lesser omentum close to the stomach cranially up to the esophagus
- Mobilize the left liver by cutting the left triangular ligament. Cut the diaphragm on the left side of the vena cava.
- Mobilize the right liver by cutting down the right diaphragm from ventral to dorsal, ending just caudally from the infrahepatic vena cava transection.
- Cut the suprahepatic vena cava and cut any remaining attachments. The liver is now free; remove it and place it in a bowl with ice water.
- Back-table procedure
- Weigh the liver. Cannulate the portal vein with a 25 French cannula and secure with ligatures. Cannulate the hepatic artery with a 14 French reinforced cannula and fixate with ligatures.
- Cannulate the infrahepatic vena cava and position the tip of the cannula at the level where the hepatic veins drain in the vena cava. Fixate with ligatures.
- Put a purse string around the edge of the diaphragm to prevent bleeding from any diaphragmatic veins and tie off the suprahepatic vena cava.
- De-air the portal cannula and perform a back-table flush of the portal vein with 250 mL of cold plasma expander. Check for any leaks.
NOTE: Check for adequate outflow through the caval cannula. - After the portal flush, place a tube clamp on the portal cannula, ensuring no air enters the cannula and portal vein.
- De-air the arterial cannula and flush 250 mL of cold plasma expander through the hepatic artery. Check for any leaks and clip any side branches. Place a tube clamp on the arterial and caval cannula.
5. Normothermic machine perfusion
- Perfusate
- During the back-table preparation, add the cell saver-produced, washed red blood cells to the circuit to obtain the desired hematocrit of 30%. Start the pump to mix the red blood cells with the plasma expander. Start the continuous gas analyzer.
NOTE: The formula for red blood cell volume to obtain desired hematocrit: (liver weight + priming volume) x desired hematocrit/hematocrit after washing. The continuous gas analyzer also provides feedback on the perfusion temperature, which normally matches the setting of the heater at 38 °C. - Add 10 mL of 10% calcium gluconate, 2 mL of heparin (10.000 IU), and 750 mg of cefuroxime in 10 mL of 0.9% NaCl to the perfusate. Set the manual gas blender to 0.5 L/min FiO2 of 21%.
- During the back-table preparation, add the cell saver-produced, washed red blood cells to the circuit to obtain the desired hematocrit of 30%. Start the pump to mix the red blood cells with the plasma expander. Start the continuous gas analyzer.
- Initiation of perfusion
- Switch on the pressure sensors, flow sensors, and roller pump for leakage recirculation.
- Place the liver in the receptacle. Place the tube clamps on the portal and arterial inflow tube and caval outflow tube of the circuit, and cut out the Y-connector.
- Connect the cannulas to their respective in- and outflow tubes with a T-connection piece in between. Prevent air from entering the circuit.
- Install three-way taps on the T-connection pieces and connect pressure lines to them. Zero pressure the lines and start continuous pressure monitoring.
- Set the pinch valves, almost closing them completely, to prevent supraphysiological flows and endothelial stress.
- Start perfusion by removing the tube clamps from the portal inflow. Immediately after the portal inflow has started, remove the clamps from the caval outflow and start the pump. The pump speed is pressure controlled, so aim for a pressure in the caval outflow between -5 mmHg and -2 mmHg. Aim for a portal flow of 0.75 mL/min/g liver.
- When portal perfusion is stable and caval pressures are adequate, remove the clamps from the arterial tube. Aim for pressures around 55-60 mmHg and flows around 0.25 mL/min/g liver.
- Maintaining stable perfusion hemodynamics
- Cover the liver with a glass dome or plastic wrap to prevent heat loss from the surface.
- If the portal flow is too high, close the pinch valve on the portal inflow tube.
- If caval pressure becomes too negative, the risk of creating a vacuum inside the vena cava increases. Excessive negative pressures can be countered by slowing down the pump speed. Alternatively, increasing inflow through the portal vein reduces the negative outflow pressure by providing more volume to the vena cava.
- If arterial pressure is too low, it can be increased by increasing the pump speed or by closing the pinch valve toward the portal reservoir to push more flow through the arterial inflow tube.
- Sampling
- Obtain the perfusate samples from the caval outflow three-way valve or a designated sampling line between the oxygenator and the portal reservoir.
- Obtain needle biopsies throughout perfusion. Needle holes must be sutured as there is no coagulation due to the heparinization of the circuit.
- Collect bile by fixating an 8 French cannula in the bile duct. Make sure to ligate the cystic duct.
Results
The perfusion protocol presented uses the self-regulation of the liver's blood flow to achieve stable hemodynamic conditions for up to 24 h and simulate the physiological distribution of blood flow in the portal vein and hepatic artery. Figure 1 represents a schematic overview of the perfusion circuit. Figure 2A shows a consistent distribution of blood flow, with the portal vein and hepatic artery contributing approximately 75% and 25% of total hepatic flow (measured at the inferior vena cava level), respectively. Figure 2B shows stable intrahepatic vascular resistance. The portal vein has lower resistance compared to the hepatic artery, similar to normal physiological conditions.
Stable and consistent perfusion with adequate oxygenation ensures the preservation of liver function for 24 h. Figure 3A shows that the release of aspartate transaminase (a marker of liver injury) in the perfusate reaches a steady level within 6-12 h of perfusion. Additionally, the perfusate pH is self-regulated by the liver and maintained within a normal range without the need for bicarbonate supplementation (Figure 3B). The perfusate lactate is also cleared to normal levels within the first hour of perfusion (Figure 3C), and bile secretion is maintained for 24 h (Figure 3D).
The most common cause of unstable and unsuccessful liver perfusion in this protocol is improper placement of the inferior vena cava cannula, which can lead to impaired outflow9. This can cause the portal vein resistance to double (Figure 4A), resulting in a decrease in portal vein blood flow and a compensatory increase in hepatic artery flow, disrupting the normal 75%:25% distribution of blood flow between the two hepatic inflows (Figure 4B). Additionally, outflow obstruction is associated with intrahepatic hemorrhage and hepatocellular necrosis, as evidenced by a steady increase in the concentration of aspartate transaminase in the perfusate (Figure 4C).
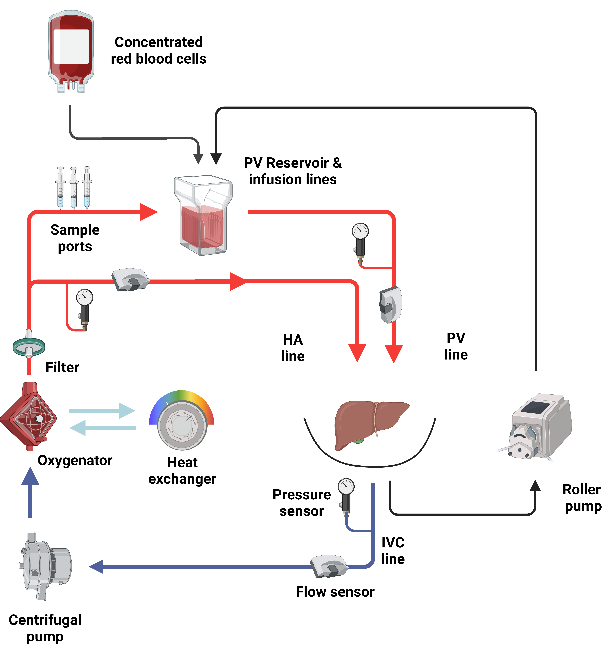
Figure 1: Schematic overview of the perfusion circuit. The isolated liver perfusion is performed in a closed circuit driven by a centrifugal pump. After the oxygenator, the circuit splits into a portal side, where the portal inflow is provided by gravity through a reservoir, and an arterial side, where the arterial inflow is directly provided by the pump. Please click here to view a larger version of this figure.
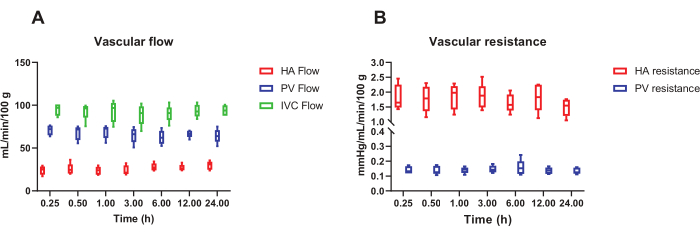
Figure 2: Hemodynamic of 24 hour porcine liver NMP. (A) Stable total blood flow through the liver during 24 h NMP, with a distribution of 75% through the portal vein (PV) and 25% through the hepatic artery (HA) that approximates the normal physiological distribution. (B) Equally stable intrahepatic resistance throughout perfusion. n = 5 animals. Box plots represent median and interquartile range, whiskers represent the total range. Abbreviation: IVC = inferior vena cava. Please click here to view a larger version of this figure.

Figure 3: Overview of liver function during 24 hour porcine liver NMP. (A) The perfusate concentration of aspartate transaminase (AST) reaches a steady state between 6-12 h of NMP. (B) The perfusate pH quickly reaches and is maintained within normal ranges throughout 24 h of perfusion. (C) The perfusate lactate concentration is cleared to normal levels within the first hour of NMP. Panel D depicts the volume of bile secreted hourly at fixed time points during perfusion. n = 5 animals. Box plots represent median and interquartile range, whiskers represent the total range. Please click here to view a larger version of this figure.
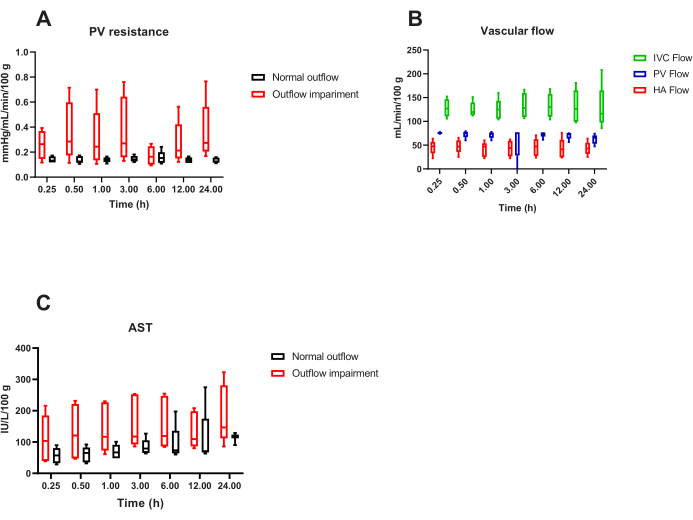
Figure 4: Representative overview of suboptimal 24 hour porcine liver NMP. (A) Unstable and doubled portal vein (PV) resistance in the case of incorrect cannulation of the inferior vena cava and outflow obstruction compared to normal outflow. This condition disrupts the near-to-physiology (B) distribution of portal vein and hepatic artery blood flow and is usually associated with (C) intrahepatic hemorrhage and a higher release of aspartate transaminase in the perfusate when compared to perfusion with normal outflow. n = 5 animals per group. Box plots represent median and interquartile range, whiskers represent the total range. Please click here to view a larger version of this figure.
Discussion
Here, we have detailed our experience with porcine liver NMP. The advantages of this technique include high translational value and versatility. Porcine liver NMP can be applied either to investigate and increase one's understanding of this enhanced preservation technique, or alternatively, to mimic transplantation. This setup allows manual control over every aspect of the perfusion, enabling adjusting both portal and arterial pressure and flow in various ways.
To simulate clinical practice as close as possible, hepatectomy in the pig is performed similarly as it would be done in human donors, which implies that some back-table preparation is required. As it is a closed circuit, attention should be given to meticulous hemostasis during the back-table flush of the liver. Although the recirculation tube allows for some leakage, excessive hilar bleeding might result in increased hemolysis. Another key step in the preparation is the position of the outflow cannula in the vena cava, as suboptimal placement might lead to collapse of the vena cava, thereby creating a vacuum resulting in outflow obstruction and congestion of the liver. Therefore, a multiperforated tip is used to prevent obstruction, and a servo regulator is used to control the pressure in the inferior vena cava. Additionally, upon reperfusion, the caval clamp is opened a few seconds after initiating portal inflow, and the pump speed should be increased slowly until the desired outflow pressures are reached. We also recommend securing the outflow cannula to the liver receptacle to ensure that the outflow cannula remains in the correct position, even when manipulating the liver when taking biopsies.
During NMP, adequate oxygenation is paramount. In this circuit, we included a plasma-tight hollow fiber membrane oxygenator certified for extended use, which was key to allowing a 24 h perfusion with stable oxygenation without significant leakage or erythrocyte aggregation.
The manual operation of this NMP setup might lead to some degree of operator-dependent outcomes. Nevertheless, the results of these NMP experiments are in line with those published by Butler et al.12. Surgical and technical complexity may restrain wider implementation; however, the technical failure rate is low in our experience. Less than 10% of experiments cannot be completed, usually due to the instability of the pig during anesthesia, of which we notice seasonal changes. Large animal experiments remain more costly than rodent models, and a disadvantage of this setup is that the circuit itself is disposable, and therefore, a repeating cost. However, one perfusion without downstream analyses costs around €500, which is still considerably less than a transplant model.
Increasing knowledge of NMP preservation and reports of successful perfusion of up to several days have brought the field of organ transplantation to a crossroads with regenerative medicine13,14,15. Therefore, future applications of these large animal perfusion models will likely include the investigation of active therapeutic interventions in livers not otherwise considered transplantable, in order to increase the potential donor pool and organ utilization rates.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank all research students from the faculty of medicine of KU Leuven involved in these experiments.
Materials
| Name | Company | Catalog Number | Comments |
| Alaris GH Plus syringe pump | BD Care Fusion | 80023 UN 01-G | |
| Anesthesia device | Dräger | Titus | |
| Arterial catheter Cavafix Certo | Braun, Melsungen, Germany | BRAU4152557 | |
| Blood gas analyzer | Radiometer | ABL815 | |
| Calcium gluconate 10% | Braun, Melsungen, Germany | 570/13596667/1214 | |
| Capnograph | Dräger | Scio | |
| Cell saver | Medtronic | AutoLog | |
| Centrifugal pump Biomedicus | Medtronic | 85315 REV 3.0 | |
| Centrifuge Rotina 420R Hettich | VWR | 521-1156 | |
| Custom made perfusion circuit | Medtronic | M323901C | |
| Disposable set cell saver | Medtronic | ATLS24 | |
| DLP Single stage venous cannula, straight 20F | Medtronic | 66120 | |
| Epoprostenol | GlaxoSmithKline Belgium, Wavre, Belgium | Flolan | |
| Fentanyl-Janssen 0.05 mg/mL | Janssen | HK-08700 | |
| Flow sensor BioPro TT | Em-Tec | 12271 | |
| Formaldehyde 4% | VWR | VWRK4078.9005 | |
| Freezer -80 °C | New Brunswick Scientific | U570-86 | |
| Fridge | Liebherr | CUP 3513 | |
| Geloplasma | Fresenius-Kabi, Bad Homburg, Germany | freeflex | |
| Heater cooler | Stöckert-Shiley, Sorin group | 16-02-1950 | |
| Heparin 5000 IE/mL | Leo Pharma, Ballerup, Denmark | HeparinLeo | |
| Hepatic artery canula | Medtronic | BIO-MEDICUS 12F | |
| IGL-1 organ preservation solution | Institut Georges Lopez | IGL-1/1000/D | |
| In-line blood gas analyzer | TERUMO | Calibrator 3MCDI 540/CDI 500 | |
| Insulin 200 IU Actrapid | Novo Nordisk, Dagsvaerd, Denmark | MEDI-00018 | |
| Isoflurane 1000 mg/g Inhalation vapour | Chanelle Pharma | Iso-Vet | |
| IV catheter BD Insyte-W 20 G | BD | 381334 | |
| Liquid nitrogen tank | KGW Isotherm | S22 | |
| Mersilene 250CM M3 USP2/0 non needled ligapak | JNJ medical | F4503 | |
| Mersilene 250CM M3.5 USP0 non needled ligapak | JNJ medical | F4504 | |
| Mersilene 5X70CM M3.5 USP0 non needled | JNJ medical | EH6935H | |
| Mersilene 6X45CM M3 USP2/0 non needled | JNJ medical | EH6734H | |
| Micro pipettes 1000 µL | Socorex | 82,51,000 | |
| Monitoring | Siemens | SC 8000 | |
| Plasmalyte Viaflo | Baxter | Plasmalyte Viaflo | |
| Portal vein canula | CALMED LABS | 18F RV-40018 | |
| Pressure sensor | Stöckert-Shiley, Sorin group | 22-06-2000 | |
| Pressure servo regulator | Medtronic | BM 9505-2 | |
| Prolene 4-0 | JNJ medical | EH7151H | |
| Roller pump | Cobe Century USA | 468048-000 REV C | |
| Sodium bicarbonate 8.4% | Braun, Melsungen, Germany | 362 2339 | |
| Sodium taurocholate | Sigma Aldrich, Burlington, USA | 86339 | |
| Surgical scalpel nr 24 | Swann Morton | 0211 | |
| Venous catheter, 3-lumen; 12FR | ARROW | AK-12123-F | |
| Vicryl Vio 250CM M2 USP3/0 non needled gigapak | JNJ medical | V1205G | |
| Xylazine 2% | VMD Livestock pharma | XYL-M 2% | |
| Zinacef Cefuroxime 750 mg | GlaxoSmithKline Belgium, Wavre, Belgium | NDC 0173-0353-32 | |
| Zoletil 100 | Virbac | Zoletil 100 |
References
- Dunson, J. R., Bakhtiyar, S. S., Joshi, M., Goss, J. A., Rana, A. Intent-to-treat survival in liver transplantation has not improved in 3 decades due to donor shortage relative to waitlist growth. Clinical Transplantation. 35 (10), e14433 (2021).
- Monbaliu, D., Pirenne, J., Talbot, D. Liver transplantation using donation after cardiac death donors. Journal of Hepatology. 56 (2), 474-485 (2012).
- Croome, K. P., Taner, C. B. The changing landscapes in DCD liver transplantation. Current Transplantation Reports. 7 (3), 194-204 (2020).
- Coffey, J. C., et al. The influence of functional warm ischemia time on DCD liver transplant recipients' outcomes. Clinical Transplantation. 31 (10), (2017).
- Meurisse, N., et al. Outcomes of liver transplantations using donations after circulatory death: A single-center experience. Transplantation Proceedings. 44 (9), 2868-2873 (2012).
- Ruck, J. M., et al. Temporal trends in utilization and outcomes of DCD livers in the United States. Transplantation. 106 (3), 543-551 (2022).
- Nasralla, D., et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 557 (7703), 50-56 (2018).
- Blondeel, J., Monbaliu, D., Gilbo, N. Dynamic liver preservation: Are we still missing pieces of the puzzle. Artificial Organs. 47 (2), 248-259 (2022).
- Gilbo, N., et al. Porcine liver normothermic machine perfusion: Methodological framework and potential pitfalls. Transplantation Direct. 8 (1), e1276 (2021).
- Maione, F., et al. Porcine isolated liver perfusion for the study of ischemia reperfusion injury: A systematic review. Transplantation. 102 (7), 1039-1049 (2018).
- Gilbo, N., et al. Coagulation factors accumulate during normothermic liver machine perfusion regardless of donor type and severity of ischemic injury. Transplantation. 106 (3), 510-518 (2021).
- Butler, A. J., et al. Successful extracorporeal porcine liver perfusion for 72 hr. Transplantation. 73 (8), 1212-1218 (2002).
- Eshmuminov, D., et al. An integrated perfusion machine preserves injured human livers for 1 week. Nature Biotechnology. 38 (2), 189-198 (2020).
- Xu, J., Buchwald, J. E., Martins, P. N. Review of current machine perfusion therapeutics for organ preservation. Transplantation. 104 (9), 1792-1803 (2020).
- Martins, P. N., Turco, S. D., Gilbo, N. Organ therapeutics during ex-situ dynamic preservation. a look into the future. European Journal of Transplantation. 1, 63-78 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved