Method Article
Detergent-Free Decellularization of the Human Pancreas for Soluble Extracellular Matrix (ECM) Production
In This Article
Erratum Notice
Summary
The protocol described in this manuscript explains the steps for the fabrication of a soluble extracellular matrix (ECM) from the human pancreas. The solubilized ECM powder obtained through this protocol may be used for the recapitulation of pancreatic islets’ microenvironment in vitro and, potentially, in vivo settings.
Abstract
Islet transplantation (ITx) has the potential to become the standard of care in beta cell replacement medicine but its results remain inferior to those obtained with whole pancreas transplantation. The protocols currently used for human islet isolation are under scrutiny because they are based on the enzymatic digestion of the organ, whereby the pancreas is demolished, its connections to the body are lost and islets are irreversibly damaged. Islet damage is characterized by critical factors such as the destruction of the extracellular matrix (ECM), which represents the 3D framework of the islet niche and whose loss is incompatible with islet euphysiology. Researchers are proposing the use of ECM-based scaffolds derived from the mammalian pancreas to address this problem and ultimately improve islet viability, function, and lifespan. Currently available methods to obtain such scaffolds are harsh because they are largely detergent based. Thus, we propose a new, detergent-free method that creates less ECM damage and can preserve critical components of pancreatic ECM. The results show that the newly developed decellularization protocol allowed the achievement of complete DNA clearance while the ECM components were retained. The ECM obtained was tested for cytotoxicity and encapsulated with human pancreatic islets which showed a positive cellular behavior with insulin secretion when stimulated with glucose challenge. Collectively, we propose a new method for the decellularization of the human pancreas without the use of conventional ionic and non-ionic chemical detergents. This protocol and the ECM obtained with it could be of use for both in vitro and in vivo applications.
Introduction
The isolation of pancreatic islets is a meticulous process carried out through the enzymatic digestion of the connections between islets and their extracellular surrounding supportive structure. This destruction of the extracellular matrix (ECM) is one of the critical factors in characterizing islets' damage taking place during isolation processes1,2,3,4. The peri-islet ECM is an essential acellular component of the endocrine pancreas. It is composed of proteins and polysaccharides, which interact and cross-link to form a three-dimensional net that structurally and biochemically supports the physiological homeostasis, and helps in the recreation of this in vitro microenvironment2,5,6. The loss of fundamental signaling processes between islets and ECM is recognized as one of the contributing factors, which limits islets' survival in vitro and in vivo2,7,8,9,10.
Animal- and human-derived ECM have been widely used for the recapitulation of the pancreatic islets' microenvironment11,12,13,14,15,16,17,18,19. Since the ability of the ECM to enhance rat islet cells attachment, proliferation, and long-term culture maintenance was first reported in ref.20, many other studies have provided strong evidence that the restoration of native ECM interaction with human islets enhances islet function21,22. For instance, recent data has shown that islets encapsulated with ECM significantly improved glycemic control in diabetic mice, enhancing and facilitating the delivery of insulin in a novel cell-based insulin delivery platform23. Furthermore, studies have demonstrated that incorporation of critical components of pancreatic ECM can significantly improve the endocrine function of β-cells24,25,26,27.
ECM manufacturing protocols present in the literature are based on the application of chemical detergents, e.g., Triton X-100 or sodium dodecyl sulfate (SDS). Despite providing excellent DNA clearance, chemicals used in decellularization processes are cytotoxic, expensive, and residues on the decellularized tissue bring concerns in view of potential clinical application.
Based on these observations, the objectives of this study were three-fold: First, to develop a decellularization method for the human pancreas with minimal use of ionic or non-ionic chemical detergents; Second, to produce a soluble ECM scaffold for tissue culture; Third, to characterize the pancreatic ECM in order to assess its cytotoxicity and impact on islet cell function. The characterization is necessary for all the cell-culture-based applications, as it demonstrates that solubilized pancreatic ECM could be beneficial in recapitulating the pancreatic microenvironment for isolated islets. Described herein is an effective, detergent-free decellularization method for the human pancreas, characterization of the ECM, and the effect of ECM on viability and function of encapsulated isolated human pancreatic islets.
Protocol
This research study was approved by the human research committee of Wake Forest Baptist Medical Center. Human pancreases were ethically obtained from organ donors through Carolina Donor Services. Organ donors were screened for infectious diseases relevant to humans, according to UNOS regulations. Organs were received in sterile preservation solution where they were kept until use. Upon delivery to the lab, all organs were inspected, and samples of the native pancreas were collected for histological purposes. The organs were then frozen and stored in sterile conditions at -20 °C until further use.
1. Surgical preparation of the human pancreas
NOTE: Frozen pancreases were thawed overnight at 4 °C.
- Before initiating surgical dissection of the human pancreas, prepare 1 L of washing solution, consisting of 950 mL of deionized water (dH2O), 50 mL of iodine-based reagent and 1% Pen/Strep solution.
- Wear sterile gloves and a lab coat during the surgical dissection. Perform all surgeries under a cell-culture-grade hood to avoid possible contamination.
- Transfer the pancreas from the sterile transport bag into the sterile placenta basin. Ensure the pancreas is oriented with the head of the organ to the left and the tail to the right.
- Carefully dissect the duodenum from the head of the pancreas using sterile scissors and non-toothed forceps. Be careful not to make a hole in the duodenum, as this increases the chances of contamination.
NOTE: If a hole is made in the gastrointestinal tract, quickly clamp the hole, and proceed with the dissection. - Once the dissection of the head of the pancreas is completed, dispose of the duodenum into an appropriate biohazard bag and follow institutional guidelines for its disposal.
- Dissect and discard all extra-pancreatic tissue remaining around the head of the pancreas, including major blood vessels and the common bile duct, which are usually marked at the time of organ procurement with non-absorbable sutures. Dispose of the tissue following institutional guidelines.
- Dissect and discard all extra-pancreatic tissue and peripancreatic adipose tissue surrounding the tail of the pancreas, exposing the splenic blood vessels.
- Once the vascular pedicle of the spleen is exposed, remove the spleen, and dissect the splenic blood vessels in their entirety, including those attached to the posterior surface of the pancreas (the splenic artery and vein). Dispose of the spleen and remove tissue following institutional guidelines.
- Appropriately dissect and dispose any extra-pancreatic fat clearly visible, especially peripancreatic fat.
NOTE: At the level of the tail of the pancreas, the retroperitoneum may be visible. This should be lifted with non-toothed forceps, removed accordingly with surgical forceps, and disposed of properly. The pancreas should now be free of any extra-pancreatic tissue. - Dissect the pancreas to obtain 1 cm3 cubes of pancreatic tissue using surgical scissors, sterilized histological blades, or surgical scalpels. Pancreatic tissue cubes obtained after this dissection will undergo the decellularization process. The average number of pancreatic cubes that undergo decellularization depends on intrinsic characteristics of the organ. On an average, between 40–60 pancreatic cubes underwent decellularization in the presented study.
- Proceed to step 2.1
2. Decellularization of the pancreatic tissue
NOTE: This protocol was used with pancreas with an average weight of 100 g; therefore, it is not recommended to exceed this weight when using this decellularization technique.
- Place all the pancreatic tissue previously dissected in step 1.10 in a sterile plastic container (see Table of Materials) with the washing solution and incubate for 15 min at room temperature (RT).
- Remove the pancreatic tissue with the help of sterile forceps and place it in a new sterile container that contains sterile, endotoxin-free deionized water. Firmly close the cap of the container and place it on a refrigerated orbital shaker with a set temperature of 4 °C and a shaking speed between 180–200 rpm for 24 h.
- Remove the container with the pancreatic tissue and clean the outside appropriately before placing it into the hood for continuation of the process.
- Remove the pancreatic tissue using surgical forceps and place it into a new sterile container, along with 1 L of deionized water, 50 mg of DNase I, and 0.0025% magnesium chloride. Firmly close the cap of the container and place it in an orbital shaker, with a preset temperature of 37 °C and shaking speed of 100 rpm for 6 h.
- Prepare the EDTA-Trizma solution
- Gather a new sterile 1 L container and clean it appropriately before introducing it into the hood. To prepare 1 L of EDTA-Trizma solution, add the following: 985 mL of sterile deionized water, 7.5 mL of Tris-HCl Buffer, and 7.5 mL of EDTA solution. Store it at 4 °C until use.
- Remove the container enclosing the pancreatic tissue and clean the outside appropriately before introducing it into the hood for the continuation of the process.
- Remove the pancreatic tissue with the help of surgical forceps and place it into a new sterile container with 1 L of EDTA-Trizma-based solution. Firmly close the cap of the container and place it in an orbital shaker, with a preset temperature of 4 °C and a shaking speed between 180–200 rpm for 18 h.
- Remove the container containing the pancreatic tissue and clean the outside appropriately before introducing it into the hood for the process.
- Remove the pancreatic tissue with the help of sterile forceps and place it in a new sterile container with sterile endotoxin-free deionized water. Firmly close the cap of the container and place it on a refrigerated orbital shaker, with a set temperature of 4 °C and shaking speed between 180 and 200 rpm for 24 h.
NOTE: During this processing period, there will be a noticeable change in color, in which the tissue will appear brighter. - Collect the pancreatic tissue and store it in sterile 50 mL centrifugation tubes, reaching a maximum of 25 mL per tube for subsequent freezing and lyophilization. The number of centrifugation tubes varies depending on the size of the pancreas. Store samples at -80 °C.
3. Production of pancreatic ECM powder – lyophilization and cryo-milling
- After a minimum of 24–40 h of storage of the decellularized material at -80 °C, transfer the pancreatic ECM into a freeze dryer.
- Transfer the frozen material into a freeze dryer, preventing the material from thawing.
NOTE: The lyophilization step takes approximately 3–5 days depending on several factors, such as the amount of material in each tube undergoing the process and the surface exposed for lyophilization. It is recommended that the decellularized tissue does not exceed the level of 25 mL in each tube and that the tubes are frozen at a 45° angle, allowing more surface to be readily exposed once the lyophilization starts. Remove the lids from the tubes. - After lyophilization, transfer the materials into a cell-culture-grade hood.
- Cut each piece of the dried ECM into half of its size using sterile scissors. These dried ECM pieces will be subjected to an automated milling process to produce an ECM powder. Use a cryo-milled automatic processor to avoid a rise in temperature and subsequent denaturation of the biological properties of the ECM. Make sure to adjust the setting of the cryo-milling machine to obtain a fine powder after the milling process.
NOTE: In this study, pancreas from donors with a BMI < 30 were used. Pancreata from donors with a BMI > 30 showed a tendency to contain more intraparenchymal fat. The process of lyophilization is not considered optimal and may be inhibited by fat content. If after lyophilization the tissue is visibly oleaginous, it must be discarded as it will not be optimal for manufacturing the ECM powder.
4. Solubilization of the pancreatic ECM
NOTE: At this point, from an average size pancreas (100 g), the yield of the decellularized pancreatic powder is 1–2 g.
- Weigh 1 g of ECM powder with the aid of a sterile plastic spatula.
- Achieve solubilization following protocols previously described: Solubilize 1 g of ECM in 100 mL of 0.01 M HCl and 100 mg of pepsin for 48 h at RT with constant stirring28.
- After 48 h, use NaOH to neutralize the pH (pH = 7) of the acidic ECM while keeping the beaker containing the acidic ECM on ice to avoid gelation.
NOTE: Gelation kinetics varies according to the temperature, collagen content, pH, and neutralizing solution. In this research study, 0.01 M of NaOH was used. The volume of NaOH added for pH neutralization was approximately 100 mL. Consider measuring the pH multiple times while neutralizing the acidic ECM solution. - Transfer the neutral ECM solution into sterile 50 mL centrifuge tubes. Close the tubes and place them into a centrifuge. Centrifuge the tubes containing the solubilized ECM at maximum speed (12,000 x g) for 15 min at 4 °C. Following centrifugation, transfer the tubes into a cell culture hood.
NOTE: Following centrifugation of the sample, the insoluble component of the ECM will be deposited at the bottom of the tube, while the solubilized ECM-solution will be found at the top in the supernatant. - Using sterile techniques, collect the supernatant of the tube containing the solubilized ECM solution and transfer it into fresh 50 mL tubes, reaching a maximum of 25 mL per tube.
- Store the ECM-solution tubes at -80 °C.
5. Production of pancreatic ECM powder – lyophilization and storage
- After at least 24–40 h of storage of the soluble ECM-solution at -80 °C, transfer the tubes into a freeze dryer.
- Transfer the frozen material into a freeze dryer, ensuring that the material does not thaw.
NOTE: The lyophilization step takes approximately 3–5 days depending on several factors, such as the amount of material in each tube undergoing the process and the surface exposed for lyophilization. It is recommended that the solubilized ECM solution does not exceed the level of 25 mL in each tube and that the tubes are frozen at a 45° angle to allow more surface to be readily exposed once the lyophilization starts. Remove the lids from the falcon tube before lyophilization. - After lyophilization of the solubilized ECM-solution, transfer the material into a cell culture-grade hood. After complete lyophilization, a fine ECM powder will be visible.
NOTE: The obtained yield is 700 mg from 1 g of decellularized tissue. - Store the solubilized ECM powder or use for the cell culture purposes.
NOTE: It is advised to store the ECM at 4 °C for immediate use, at -20 °C for later use, and at -80 °C for long-term storage.
6. Characterization of the pancreatic ECM with histological staining
- Collect a piece of native pancreas soon after the delivery of the organ and before the decellularization. Fix this for 24 h in 10% formalin. Wash the samples in deionized water. Dehydrate it in graded alcohol before embedding it in paraffin and slice into 5 mm sections.
- At the end of the decellularization process, collect a sample of the decellularized material and fix it for 24 h in 10% formalin. Wash the sample in deionized water. Dehydrate it in graded alcohol before embedding it in paraffin and slice into 5 mm sections.
- To verify a successful decellularization, perform H&E and DAPI staining on both the native pancreas and the decellularized counterpart.
- For histological characterization, perform Masson’s Trichrome (MT) and Alcian Blue (AB) stainings, which will highlight the collagenous and GAGs structures, respectively.
7. Characterization of the soluble ECM powder
- Perform DNA content and total collagen analysis of the native pancreatic tissue and the soluble ECM powder as described previously29.
- Quantify sulfated glycosaminoglycans (sGAG) based on the protocol provided by a commercially available kit (see Table of Materials). Use a microplate spectrophotometer to measure sulfated sGAG content in the pancreatic ECM at a wavelength of 595 nm30.
NOTE: Report DNA content as ng/mg of dry tissue and the collagen and GAGs content as μg/mg of dry tissue.
8. Human Islets encapsulation with the ECM and culture
NOTE: Human pancreatic Islets were commercially obtained (see Table of Materials).
- Upon arrival, culture the human pancreatic islets in non-tissue culture treated plates for 24 h under standard condition with an approximate density of 2,000 IEQ in each 10 cm plate.
- Manipulate the human islets to test the effect of the ECM on the islets’ functionality and viability with the following experimental groups: Free Islets, Islets encapsulated in alginate, and Islets encapsulated in alginate-ECM.
NOTE: The above-mentioned groups of islets were cultured in vitro under standard condition for up to 8 days to assess the effect of the ECM on islets functionality and viability. - Weigh alginate on a scale and suspend it with HBSS into a centrifugation tube in order to obtain a ratio of 1.5% (w/v). Place the centrifuge tube on a rotator overnight at 4 ˚C.
NOTE: A molecular weight between 75–200 kDA and a G/M ratio of ≤ 1 were reported by the manufacturer for the alginate. - Weigh 0.1 mg of ECM and suspend it in 1 mL of the alginate previously prepared in order to have ECM concentration of 0.1 mg/mL.
- Encapsulate human pancreatic islets in alginate and alginate-ECM according to a previously described protocol31. The procedure is described in brief below.
- Gently collect and mix 3,000 IEQ in 1 mL of alginate and 3,000 IEQ in 1 mL of alginate-ECM at an ECM concentration of 0.1 mg/mL previously prepared in step 8.4.
- Pump the obtained suspensions through a microfluidic encapsulation device set to 0.2 mL/min and flow rate and air pressure of 2.0 Pa, respectively.
- Upon production, collect the microcapsules in 100 mM of CaCl2 bath with 10 mM of HEPES. Allow alginate to crosslink for 10 min.
- Prior to being cultured, wash the encapsulated islets with HBSS under standard condition, for 2–3 min at 37 ˚C, with 5% CO2.
- Add the culture media (see Table of Materials) and change two-third of the media every other day until the end of the experiment.
NOTE: The above steps must be performed both for the Islets in alginate and Islets in alginate-ECM.
- On day 8, post-encapsulation, perform glucose-stimulated insulin secretion (GSIS) and DNA analysis to assess the production of insulin and islets viability, respectively.
- Obtain brightfield images and Live/Dead staining of free and encapsulated islets to assess cellular morphology and viability.
NOTE: Qualitative assessment of the images was performed to assess the islets’ health. Parameters taken into consideration include shape, border, integrity, and diameter of the islets, as well as the presence of single cells in culture.
9. Glucose-stimulated insulin release (GSIS) and DNA measurement
NOTE: On day 8, post-encapsulation, human pancreatic islets were collected and incubated with Kreb’s buffer, containing low (2.8 mM) and high (16.8 mM) glucose concentrations, followed by KCl depolarization solution (25 mM). The glucose challenge was performed with modification of a protocol previously described32.
- Insert gel filtration resin in polypropylene columns.
- Insert treatment groups (free islets or encapsulated islets) in the middle portion of the gel filtration resin within the polypropylene columns.
- Pipette glucose and depolarizing solutions into the polypropylene columns described above and incubate for 1 h, in the following order: perform a pre-incubation period with low molarity glucose solution, then proceed with a series of incubations with low, high, low, and depolarizing glucose solution as mentioned in the NOTE at the beginning of section 9.
- Collect the medium from each incubation phase and store it at -80 °C for subsequent analysis.
- Following incubation and collection of the depolarizing solution, incubate human pancreatic islets with 1 mL of DNA extraction buffer and store it until further analysis.
- Measure the insulin content from the glucose and the depolarizing solution incubations with an Insulin ELISA assay, following the manufacturer’s protocol.
- Measure the DNA content from the extraction buffer collected in step 9.5 using a DNA assay kit following the manufacturer’s protocol.
- Normalize the insulin measurements according to the DNA content.
10. Statistical analysis
NOTE: Group comparisons refer to the same batch of human islets with n = 3 independent assessment conducted for each assay described in this manuscript. Values are expressed as Mean ± SD.
- Use a statistical software to perform the Mann-Whitney test for the assessment of decellularization and DNA remnants analyses, compare the native, the decellularized pancreas, and the solubilized ECM.
- Perform an unpaired t-test for the assessment of the glycosaminoglycans and collagen between the native, the decellularized pancreas, and the solubilized ECM.
- Perform a 2-way ANOVA with post-hoc Turkey’s multiple comparisons for the Glucose Stimulation Test in the assessment of the islets stimulation on day 8.
- Consider statistical significance at p < 0.05 with designation of *p < 0.05, **p<0.01, ***p < 0.001, and ****p < 0.0001.
Results
Native and acellular pancreatic samples were processed for histological staining with H&E, MT, and AB. The H&E staining showed complete absence of nuclear material and cells, confirming successful decellularization. MT and AB stainings showed the framework of the ECM, highlighting qualitatively collagenous and stromal components, respectively (Figure 1).
This method enabled the consistent generation of an ECM powder from the human pancreas. DNA quantification tests confirmed satisfactory cell clearance33. Native, acellular, and solubilized pancreatic ECM were biochemically characterized in order to assess basic biological composition. The pancreatic ECM was determined to be acellular and DNA-free (DNA < 50 ng.mg-1 of dry tissue), with consistent preservation of collagen and glycosaminoglycans as reported by others11,15. DNA analysis demonstrated clearance of deoxyribonucleic acid in both acellular and solubilized pancreatic ECM (from 4.56 μg/mg ± 3.42 μg/mg to 30.05 ng/mg ± 22.89 ng/mg for the acellular pancreas p = 0.0001; from 4.56 μg/mg ± 3.42 μg/mg to 22.81 ng/mg ± 11.31 ng/mg for the solubilized pancreatic ECM). (Figure 2).
Total collagen was quantified in the native pancreas, in the acellular and in the solubilized pancreatic ECM. Results showed a statistically significant increase in collagen in the acellular pancreas and in the soluble pancreatic ECM compared to the native tissue (from 7.35 μg/mg ± 1.68 μg/mg to 27.74 μg/mg ± 2.35 μg/mg for the acellular pancreas p < 0.0001; from 7.35 μg/mg ± 1.68 μg/mg to 26.08 μg/mg ± 3.63 μg/mg for the solubilized pancreatic ECM p < 0.001). This increase in collagen content is due to the isolation and purification of the ECM from the cellular components, which depict an overall enrichment in collagen and sGAG in the ECM compared to the native organ (Figure 2).
Quantification of glycosaminoglycans showed a statistically significant decrease of GAG content in the acellular tissue and solubilized pancreatic ECM comparable to our previous study11,15, with a decrease from 15.08 μg/mg ± 3.03 μg/mg to 4.87 μg/mg ± 1.20 μg/mg for the acellular pancreas p < 0.001 and from 15.08 μg/mg ± 3.03 μg/mg to 3.45 μg/mg ± 0.20 μg/mg for the solubilized pancreatic ECM (p < 0.01) (Figure 2).
Encapsulated islets cultured in non-tissue culture treated plates were viable 8 days post-encapsulation. Both live and dead staining showed that islets cultured in the three different conditions were viable; however, there was an increased presence of dead cells when un-encapsulated (Figure 3). Encapsulated islets maintained their spherical shape with a well-rounded border, a stable diameter, and almost no single cells in culture. At the time point analyzed, free islets showed a tendency to aggregate, to develop irregularities at the borders and shapes, and to exhibit a darker core, suggestive of a necrotic event.
Both free and encapsulated islets were found to be glucose responsive at the time point analyzed (Figure 3). Islets encapsulated in ECM-alginate showed a significant increase in insulin secretion following high glucose stimulation and KCl depolarization compared both to free and alginate-only encapsulated islets.

Figure 1: Representative H&E, Masson’s Trichrome and Alcian Blue histological images. Native human pancreas (A,C,E) and human pancreatic ECM (B,D,F) decellularized with the newly developed experimental protocol. H&E panel demonstrated a complete loss of nuclear structures compared to the native pancreatic tissue. Masson’s Trichrome staining highlight the framework of the extracellular matrix and Alcian Blue staining highlight the connective tissue framework of the extracellular matrix. Scale bar = 100 μm for panels A,C,D,E,F; Scale bar = 25 μm for panel B. Please click here to view a larger version of this figure.

Figure 2: Biochemical characterization of native pancreas, acellular, and solubilized pancreatic ECM. (A) Satisfactory removal of DNA in the acellular pancreas and in the solubilized pancreatic ECM was confirmed. (B,C) Glycosaminoglycans and collagen quantification performed in the native pancreas compared to the acellular and solubilized ECM. Statistical analysis was performed by the t-test of native versus decellularized pancreas and native vs solubilized ECM; **** = p < 0.0001; ***p < 0.001; **p < 0.01. Please click here to view a larger version of this figure.
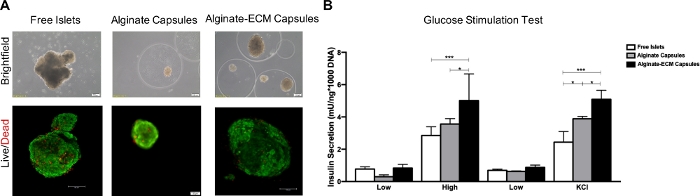
Figure 3: Qualitative assessment of viability of human islets and quantitative assessment of insulin secretion. (A) Brightfield and Live/dead images of human isolated islets cultured as free, in alginate capsules and in alginate-ECM capsules on day 6 post-encapsulation. (B) Glucose stimulation assessment of human isolated islets cultured on non-tissue culture treated plate in three different settings: free, in alginate capsules and in alginate-ECM capsules on day 8 post-encapsulation. Values of the insulin secretion were reported after DNA normalization. Statistical comparisons of the insulin secretion are made between the three culture conditions in high glucose and after KCl depolarizing solution, respectively; *p < 0.0 and ***p < 0.001. Please click here to view a larger version of this figure.
Discussion
The aim of this work was to develop a gentler, detergent-free decellularization protocol to produce pancreatic ECM. Attention was paid to the preservation of ECM components of the pancreatic parenchyma and the avoidance of a lengthy tissue exposure to conventional ionic or non-ionic chemical detergents during the decellularization process.
The most innovative aspect of the developed decellularization method is the avoidance of classic ionic and non-ionic chemical detergents. Our previous experience with the production of human pancreatic ECM11,12,13,22,34,35 set the baseline for the production of acellular pancreatic supportive media. Nevertheless, the infusion with hundreds of liters of chemical detergents through the pancreatic duct, the superior mesenteric artery, and the splenic artery11,13, requires specific surgical techniques and is not always feasible in large-scale production. Most importantly, decellularizing the tissue in an orbital shaker rather than infusing detergents through the vasculature represents a paradigm shift in methodology, contributing enormously to technical ease, consistency, and feasibility of decellularization, which enhance ECM production for translation. Moreover, organs procured for research purposes are often damaged due to inconsistencies in the procurement process, sometimes attributable to abnormal anatomy that frustrates cannulation. Therefore, our method would allow for all organs to be processed for decellularization. We infer that this method could be of interest for the decellularization and the production of ECM from various organs and potentially for both in vitro and in vivo applications.
Development of the present decellularization method was influenced by our previous experience using Triton X-100 as the main chemical detergent11,13,36. Being unable to quantify the remnant Triton X-100 on ECM post-decellularization and the lack of feasibility in scaling up the manufacturing process in a cGMP environment led our group to investigate the feasibility of obtaining decellularization by mechanical shaking rather than a perfusion of the whole pancreas. We hypothesized that utilization of a hypotonic solution would be efficient in precipitating osmotic damage in residing cells and exposing cellular nuclear material for a subsequent enzymatic treatment, a process aimed at the clearance of deoxyribonucleic acid residues. Following initial approaches to decellularize human pancreases by shaking diced tissue in chemical detergents for 48 h, we noticed that deionized water was effective in providing the cellular damage needed for the subsequent enzymatic step and cellular removals. We then explored the option of reducing the detergent-free phase at 24 h and confirmed that cellular mechanical damage due to the hypotonic solution, deionized water, was consistently able to provide a successful decellularization while avoiding the use of potential cytotoxic agents. Our data on the quantification of collagen and glycosaminoglycans showed consistent trends with our previous experiences and other research groups’ recent observations of the human pancreas11,15. Moreover, we found that a solubilization step did not reduce the quantity of critical components of the ECM, as their preservation is critical for ECM scaffolds to exert the function15,21,24,36. Pancreas decellularization methods currently discussed in literature37 are characterized by the use of ionic or non-ionic detergents and the consequent loss of the innate ECM composition, allowing us to infer that a more gentle method, possibly detergent-free, would better preserve the pancreatic ECM basic components.
The ECM is the 3D framework that provides structural and biochemical support to the cells in multicellular organisms. ECM proteins and structures play vital roles in the determination, differentiation, proliferation, survival, polarity, and migration of cells6. The rationale for incorporating the pancreatic ECM in both in vitro and in vivo applications is based on the evidence that: in mature islets, interactions with ECM or other matrix materials regulate cell survival, proliferation, and insulin secretion, and aid in the preservation and restoration of the spherical islet morphology3,21,22,38; partially digested islets that retain some of their native ECM connections display markedly reduced apoptosis rates and significantly higher GSIS functionality compared to highly purified and ECM-free islets38,39,40; and ECM enhances islet function by preventing leukocyte infiltration and up-regulating the expression of α3 integrin and focal adhesion kinase, a crucial characteristic for beta cell-ECM attachment and interaction41,42,43.
Glucose-stimulated insulin secretion following the encapsulation of human islets in alginate microcapsules shows how the reconstitution of a tridimensional setting provides a healthier environment for islets in comparison to an in vitro culture on non-tissue culture treated plates. Encapsulation with alginate provides maintenance of the initial structure of the islets, avoiding cellular clumping and dispersion of single cells, therefore constituting the physiological structure of human islets. It is well known that islet function gradually declines over time when cultured in vitro under normal conditions, and alginate, an inert biomaterial, does not provide sufficient biochemical signaling for the maintenance of optimal islet function. Our ECM biomaterial proved to have a positive in vitro impact on the viability and the functionality of the islets when encapsulated with alginate compared to alginate only. Further investigation is necessary to prove whether the addition of this biomaterial when solubilized could serve as a step toward the improvement of islet-based technologies for in vitro culture systems and for potential in vivo applications44,45.
Some critical steps can be highlighted in the presented decellularization protocol: (1) a non-optimal surgical dissection of the human pancreas can lead to an excessive presence of adipose tissue, which will drastically impair the decellularization process; (2) an effective lyophilization may not be achieved when excessive adipose tissue is present; (3) this will inevitably affect the cryo-milling step, which will be non-efficient.
Several limitations were found using the presented protocol: (1) human-to-human organ variability before and after decellularization was observed; (2) there were also some non-optimal outcomes of decellularization in organs derived from donors with history of alcohol abuse, patients with BMI > 30 and excessive peri- and intra-pancreatic adipose tissue accumulation. We concluded that human pancreata from donors with BMI < 30 were generally deemed suitable for decellularization.
The results of this study show that the human pancreas can be decellularized with an osmotic-based, detergent-free process, to obtain ECM scaffolds for application in beta cell replacement medicine, and islet manipulation in vitro. The manufacturing process presents a feasible and reproducible approach for translational purposes and the avoidance of both chemical detergents and the use of a perfusion-based system represent efficient and cost-effective solutions to produce an ECM-based biomaterial, which when solubilized could be used in research and potentially in clinical settings. There is potential in assessing the efficacy of this biomaterial through recapitulating an optimal niche for the pancreatic islets and for insulin-producing cells to improve long-term cell maintenance, viability, and cellular differentiation.
Disclosures
There are no competing financial interests to declare by any of the coauthors.
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 Research and Innovation Program under grant agreement No. 646272. Human Pancreatic Islets were obtained from Prodo Laboratories, Aliso Viejo, CA 92656.
Materials
| Name | Company | Catalog Number | Comments |
| Corning 1L Easy Grip Polystyrene Storage Bottles with 45mm Caps | ThermoFisher | 430518 | Container use for decellularization |
| Cryogenic Mill | SPEX Certiprep | 6870-230 | |
| Deoxyribonuclease I from bovine pancreas | Sigma Aldrich | DN25 | |
| Distilled Water | ThermoFisher | 15230147 | |
| Falcon 50mL Conical Centrifuge Tubes | Corning | 352070 | |
| Human Pancreas | na | na | |
| Insulin-Elisa | Mercodia | 10-1113-01 | |
| Magnesium Chloride, 1M, Sterile | Bio-World | 41320004-1 | |
| Pepsin from porcine gastric mucosa | Sigma Aldrich | P7012-5G | |
| Placenta Basin w/o Lid, Sterile | DeRoyal | 32-881 | |
| Polypre chromatography tubes | Bio-rad | 731-1500 | Polypropylene columns |
| Quant-iT PicoGreen dsDNA Assay Kit | Invitrogen | P7589 | DNA Kit |
| SamplePrep Large-Capacity Freezer/Mill Accessory | SPEX | 6801 | Large Grinding Vial Set |
| Sephadex G-10 beads | Cytiva | 17001001 | Gel Filtration Resin |
| Surgical kit | na | na | |
| UltraPur 0.5M EDTA, pH 8.0 | ThermoFisher | 15575020 | |
| UltraPur 1 M Tris-HCI Buffer, pH 7.5 | ThermoFisher | 15567027 | |
| UltraPure DNase/RNase-Free Distilled Water | ThermoFisher | 10977023 |
References
- Korpos, E., et al. The peri-islet basement membrane, a barrier to infiltrating leukocytes in type 1 diabetes in mouse and human. Diabetes. 62 (2), 531-542 (2013).
- Daoud, J., Petropavlovskaia, M., Rosenberg, L., Tabrizian, M. The effect of extracellular matrix components on the preservation of human islet function in vitro. Biomaterials. 31 (7), 1676-1682 (2010).
- Rosenberg, L., Wang, R., Paraskevas, S., Maysinger, D. Structural and functional changes resulting from islet isolation lead to islet cell death. Surgery. 126 (2), 393-398 (1999).
- Paraskevas, S., Maysinger, D., Wang, R., Duguid, T. P., Rosenberg, L. Cell loss in isolated human islets occurs by apoptosis. Pancreas. 20 (3), 270-276 (2000).
- Hynes, R. O. The extracellular matrix: not just pretty fibrils. Science. 326 (5957), 1216-1219 (2009).
- Frantz, C., Stewart, K. M., Weaver, V. M. The extracellular matrix at a glance. J Cell Sci. 123 (24), 4195-4200 (2010).
- Kuehn, C., Vermette, P., Fulop, T. Cross talk between the extracellular matrix and the immune system in the context of endocrine pancreatic islet transplantation. A review article. Pathologie Biologie (Paris). 62 (2), 67-78 (2014).
- Irving-Rodgers, H. F., et al. Pancreatic islet basement membrane loss and remodeling after mouse islet isolation and transplantation: impact for allograft rejection. Cell Transplant. 23 (1), 59-72 (2014).
- Tomei, A. A., et al. Device design and materials optimization of conformal coating for islets of Langerhans. Proceedings of the National Academy Science U. S. A. 111 (29), 10514-10519 (2014).
- Miao, G., et al. Basement membrane extract preserves islet viability and activity in vitro by up-regulating alpha3 integrin and its signal. Pancreas. 42 (6), 971-976 (2013).
- Peloso, A., et al. The human pancreas as a source of protolerogenic extracellular matrix scaffold for a new-generation bioartificial endocrine pancreas. Annals of Surgery. 264 (1), 169-179 (2016).
- Salvatori, M., et al. Extracellular Matrix Scaffold Technology for Bioartificial Pancreas Engineering: State of the Art and Future Challenges. Journal of Diabetes Science and Technology. 8 (1), 159-169 (2014).
- Mirmalek-Sani, S. H., et al. Porcine pancreas extracellular matrix as a platform for endocrine pancreas bioengineering. Biomaterials. 34 (22), 5488-5495 (2013).
- Chaimov, D., et al. Innovative encapsulation platform based on pancreatic extracellular matrix achieve substantial insulin delivery. Journal of Control Release. 257, 91-101 (2017).
- Sackett, S. D., et al. Extracellular matrix scaffold and hydrogel derived from decellularized and delipidized human pancreas. Science Reports. 8 (1), 10452 (2018).
- Jiang, K., et al. 3-D physiomimetic extracellular matrix hydrogels provide a supportive microenvironment for rodent and human islet culture. Biomaterials. 198, 37-48 (2019).
- Citro, A., et al. Biofabrication of a vascularized islet organ for type 1 diabetes. Biomaterials. 199, 40-51 (2019).
- Hashemi, J., et al. Application of a novel bioreactor for in vivo engineering of pancreas tissue. Journal of Cell Physiology. 233 (5), 3805-3816 (2018).
- Guruswamy Damodaran, R., Vermette, P. Decellularized pancreas as a native extracellular matrix scaffold for pancreatic islet seeding and culture. Journal of Tissue Engineering and Regeneration Medicine. 12 (5), 1230-1237 (2018).
- Thivolet, C. H., Chatelain, P., Nicoloso, H., Durand, A., Bertrand, J. Morphological and functional effects of extracellular matrix on pancreatic islet cell cultures. Experimental Cell Research. 159 (2), 313-322 (1985).
- Llacua, L. A., Faas, M. M., de Vos, P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia. 61 (6), 1261-1272 (2018).
- Orlando, G., et al. Cell replacement strategies aimed at reconstitution of the beta-cell compartment in type 1 diabetes. Diabetes. 63 (5), 1433-1444 (2014).
- Chaimov, D., et al. Innovative encapsulation platform based on pancreatic extracellular matrix achieve substantial insulin delivery. Journal of Control Release. 257, 91-101 (2016).
- Hadavi, E., et al. Microwell Scaffolds Using Collagen-IV and Laminin-111 Lead to Improved Insulin Secretion of Human Islets. Tissue Engineering Part C Methods. 25 (2), 71-81 (2019).
- Crisostomo, J., et al. ECM-enriched alginate hydrogels for bioartificial pancreas: an ideal niche to improve insulin secretion and diabetic glucose profile. Journal of Applied Biomaterial and Functional Mater. 17 (4), 2280800019848923 (2019).
- Davis, N. E., et al. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials. 33 (28), 6691-6697 (2012).
- Carlo, D. Pancreatic acellular matrix supports islet survival and function in a synthetic tubular device: In vitro and in vivo studies. International Journal of Molecular Medicine. 25 (2), (2009).
- Freytes, D. O., Martin, J., Velankar, S. S., Lee, A. S., Badylak, S. F. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 29 (11), 1630-1637 (2008).
- Orlando, G., et al. Discarded human kidneys as a source of ECM scaffold for kidney regeneration technologies. Biomaterials. 34 (24), 5915-5925 (2013).
- Afara, I. O., Prasadam, I., Arabshahi, Z., Xiao, Y., Oloyede, A. Monitoring osteoarthritis progression using near infrared (NIR) spectroscopy. Science Reports. 7 (1), 11463 (2017).
- Tendulkar, S., et al. A scalable microfluidic device for the mass production of microencapsulated islets. Transplantation Protocol. 43 (9), 3184-3187 (2011).
- Fraker, C. A. The Role of Oxygen During In Vitro Culture and Immunoisolation of Islets of Langerhans. University of Miami. , (2011).
- Keane, T. J., Londono, R., Turner, N. J., Badylak, S. F. Consequences of ineffective decellularization of biologic scaffolds on the host response. Biomaterials. 33 (6), 1771-1781 (2012).
- Edgar, L., et al. Utility of extracellular matrix powders in tissue engineering. Organogenesis. , 1-15 (2018).
- Katari, R., et al. . Current Developments in Biotechnology and Bioengineering. , 325-347 (2017).
- Peloso, A., Katari, R., Tamburrini, R., Duisit, J., Orlando, G. Glycosaminoglycans as a measure of outcome of cell-on-scaffold seeding (decellularization) technology. Expert Reviews on Medical Devices. 13 (12), 1067-1068 (2016).
- Pineda Molina, C., Lee, Y. C., Badylak, S. F. . Transplantation, Bioengineering, and Regeneration of the Endocrine Pancreas. , 527-536 (2020).
- Thomas, F. T., et al. Anoikis, extracellular matrix, and apoptosis factors in isolated cell transplantation. Surgery. 126 (2), 299-304 (1999).
- Kenmochi, T., et al. Improved quality and yield of islets isolated from human pancreata using a two-step digestion method. Pancreas. 20 (2), 184-190 (2000).
- Lamb, M., et al. In vitro maturation of viable islets from partially digested young pig pancreas. Cell Transplant. 23 (3), 263-272 (2014).
- Stendahl, J. C., Kaufman, D. B., Stupp, S. I. Extracellular matrix in pancreatic islets: relevance to scaffold design and transplantation. Cell Transplant. 18 (1), 1-12 (2009).
- de Vos, P., et al. Enzymes for Pancreatic Islet Isolation Impact Chemokine-Production and Polarization of Insulin-Producing beta-Cells with Reduced Functional Survival of Immunoisolated Rat Islet-Allografts as a Consequence. PLoS One. 11 (1), 0147992 (2016).
- Smink, A. M., de Vos, P. Therapeutic Strategies for Modulating the Extracellular Matrix to Improve Pancreatic Islet Function and Survival After Transplantation. Current Diabetes Report. 18 (7), 39 (2018).
- Orlando, G., Soker, S., Stratta, R. J. Organ bioengineering and regeneration as the new Holy Grail for organ transplantation. Annals of Surgery. 258 (2), 221-232 (2013).
- Orlando, G., et al. Rethinking Regenerative Medicine From a Transplant Perspective (and Vice Versa). Transplantation. 103 (2), 237-249 (2019).
Erratum
Formal Correction: Erratum: Detergent-Free Decellularization of the Human Pancreas for Soluble Extracellular Matrix (ECM) Production
Posted by JoVE Editors on 12/10/2020. Citeable Link.
An erratum was issued for: Detergent-Free Decellularization of the Human Pancreas for Soluble Extracellular Matrix (ECM) Production. The author list was updated.
The author list was updated from:
Riccardo Tamburrini1,2,3, Deborah Chaimov1,3, Amish Asthana1,3, Kevin Enck3, Sean M. Muir4, Justine Mariam Aziz5, Sandrine Lablanche6, Emily Tubbs6, Alice A. Tomei7,8, Mark Van Dyke9, Shay Soker3, Emmanuel C. Opara3, Giuseppe Orlando1,3
1Department of Surgery, Wake Forest Baptist Medical Center,
2Department of General Surgery, PhD Program in Experimental Medicine, University of Pavia,
3Wake Forest Institute for Regenerative Medicine, Wake Forest School of Medicine,
4Wake Forest University College of Arts and Science,
5Wake Forest University School of Medicine,
6Laboratory of Fundamental and Applied Bioenergetics (LBFA), and Environmental and System Biology (BEeSy), Grenoble Alps University,
7Department of Biomedical Engineering, University of Miami,
8Diabetes Research Institute, University of Miami Miller School of Medicine,
9Department of Biomedical Engineering and Mechanics, School of Biomedical Engineering and Sciences, Virginia Polytechnic Institute and State University
to:
Riccardo Tamburrini1,2,3, Deborah Chaimov1,3, Amish Asthana1,3, Carlo Gazia3,4, Kevin Enck3, Sean M. Muir5, Justine Mariam Aziz6, Sandrine Lablanche7, Emily Tubbs7, Alice A. Tomei8,9, Mark Van Dyke10, Shay Soker3, Emmanuel C. Opara3, Giuseppe Orlando1,3
1Department of Surgery, Wake Forest Baptist Medical Center,
2Department of General Surgery, PhD Program in Experimental Medicine, University of Pavia,
3Wake Forest Institute for Regenerative Medicine, Wake Forest School of Medicine,
4Department of Surgery, Tor Vergata University of Rome
5Wake Forest University College of Arts and Science,
6Wake Forest University School of Medicine,
7Laboratory of Fundamental and Applied Bioenergetics (LBFA), and Environmental and System Biology (BEeSy), Grenoble Alps University,
8Department of Biomedical Engineering, University of Miami,
9Diabetes Research Institute, University of Miami Miller School of Medicine,
10Department of Biomedical Engineering and Mechanics, School of Biomedical Engineering and Sciences, Virginia Polytechnic Institute and State University
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved