Method Article
Highly-Multiplexed Tissue Imaging with Raman Dyes
In This Article
Summary
Electronic pre-resonance stimulated Raman scattering (epr-SRS) imaging of rainbow-like Raman dyes is a new platform for highly multiplexed epitope-based protein imaging. Here, we present a practical guide including antibody preparation, tissue sample staining, SRS microscope assembly, and epr-SRS tissue imaging.
Abstract
Visualizing a vast scope of specific biomarkers in tissues plays a vital role in exploring the intricate organizations of complex biological systems. Hence, highly multiplexed imaging technologies have been increasingly appreciated. Here, we describe an emerging platform of highly-multiplexed vibrational imaging of specific proteins with comparable sensitivity to standard immunofluorescence via electronic pre-resonance stimulated Raman scattering (epr-SRS) imaging of rainbow-like Raman dyes. This method circumvents the limit of spectrally-resolvable channels in conventional immunofluorescence and provides a one-shot optical approach to interrogate multiple markers in tissues with subcellular resolution. It is generally compatible with standard tissue preparations, including paraformaldehyde-fixed tissues, frozen tissues, and formalin-fixed paraffin-embedded (FFPE) human tissues. We envisage this platform will provide a more comprehensive picture of protein interactions of biological specimens, particularly for thick intact tissues. This protocol provides the workflow from antibody preparation to tissue sample staining, to SRS microscope assembly, to epr-SRS tissue imaging.
Introduction
Complex tissue systems are composed of distinct cellular subpopulations whose spatial locations and interaction networks are deeply intertwined with their functions and dysfunctions1,2. To reveal the tissue architecture and interrogate its complexity, knowledge of the spatial locations of proteins at single-cell resolution is essential. Hence, highly multiplexed protein-imaging technologies have been increasingly appreciated and could become a cornerstone for studying tissue biology3,4,5. Current common multiplexed protein imaging methods can be classified into two main categories. One is serial immunofluorescence imaging relying on multiple rounds of tissue staining and imaging, and the other is imaging mass cytometry coupled with heavy metal tagged antibodies6,7,8,9,10,11,12.
Here, an alternative strategy for multiplexed antibody-based protein imaging is introduced. Unlike the prevalent fluorescence imaging modality, which can only visualize 4-5 channels simultaneously due to the broad excitation and emission spectra (full width at half maximum (FWHM) ~500 cm-1), Raman microscopy exhibits much narrower spectral linewidth (FWHM ~10 cm-1) and hence provides scalable multiplexity. Recently, by harnessing the narrow spectrum, a novel scheme of Raman microscopy named electronic pre-resonance stimulated Raman scattering (epr-SRS) microscopy has been developed, providing a powerful strategy for multiplexed imaging13. By probing the electronically coupled vibrational modes of Raman dyes, epr-SRS achieves a drastic enhancement effect of 1013-fold on Raman cross-sections and overcomes the sensitivity bottleneck of conventional Raman microscopy (Figure 1A)13,14,15. As a result, the detection limit of epr-SRS has been pushed to sub-µM, which enables Raman detection of interesting molecular markers such as specific proteins and organelles inside cells13,16. In particular, utilizing Raman dye-conjugated antibodies, epr-SRS imaging of specific proteins in cells and tissues (called immuno-eprSRS) was demonstrated with comparable sensitivity to standard immunofluorescence (Figure 1B)13,17. By tuning the pump wavelength by only 2 nm, the epr-SRS signal will be completely off (Figure 1B), which showcases high vibrational contrast.
On the probe side, a set of rainbow-like Raman probes called Manhattan Raman scattering (MARS) dyes has been developed for antibody conjugation13,18,19,20. This unique Raman palette consists of novel dyes bearing π-conjugated triple bonds (Supplementary Material), each displaying a single and narrow epr-SRS peak in the bioorthogonal Raman spectral range (Figure 1C). By modifying the structure of the core chromophore and isotopically editing both atoms of the triple bond (Supplementary Material), spectrally separated Raman probes have been developed. Leveraging the scalable multiplexity, epr-SRS microscopy coupled with the MARS dye palette offers an optical strategy for one-shot multiplex protein imaging in cells and tissues.
Immuno-eprSRS provides an alternative strategy to current multiplex protein imaging methods with unique strengths. Compared to fluorescence approaches with cyclic staining, imaging, and signal removal, this Raman-based platform ensures single-round staining and imaging. Therefore, it circumvents practical complexity in cyclic procedures and largely simplifies the protocol, hence opening new territories of multiplexed protein imaging. For instance, harnessing a Raman-dye-tailored tissue clearing protocol, immuno-eprSRS has been extended to three dimensions for highly multiplexed protein mapping in thick intact tissues17. Over 10 protein targets were visualized along millimeter-thick mouse brain tissues17. More recently, coupling immuno-eprSRS with an optimized biomolecule-retention expansion microscopy (ExM) protocol21, one-shot nanoscale imaging of multiple targets has also been demonstrated22. Compared to imaging mass spectroscopy4,9, epr-SRS is nondestructive and has intrinsically optical sectioning ability. Furthermore, epr-SRS is more time-efficient on tissue scanning. Typically, a tissue region of 0.25 mm2 with a pixel size of 0.5 µm takes merely a few minutes to image for a single epr-SRS channel. For example, the total imaging time of four SRS channels plus four fluorescence channels in Figure 4 is about 10 min.
Protocol
The protocol was conducted in accordance with the animal experimental protocol (AC-AABD1552) approved by the Institutional Animal Care and Use Committee at Columbia University.
1. Preparation of Raman-dye-conjugated antibodies
- Prepare the conjugation buffer as ~0.1 M NaHCO3 in PBS buffer, pH = 8.3, store at 4 °C.
- Prepare N-hydroxysuccinimidy (NHS) ester-functionated MARS probe (Supplementary Material) solution as 3 mM in anhydrous DMSO. Synthesis of MARS probes can be referred to prior reports13,17,18.
NOTE: For storage purposes, the dye NHS ester solution must be protected from light and kept at -20 °C. - Dissolve antibody solids in the conjugation buffer to a concentration of 2 mg/mL. For antibodies that are dissolved in other buffers, exchange them into the conjugation buffer to a concentration of 1-2 mg/mL with centrifugal filters.
NOTE: Highly cross-adsorbed secondary antibodies are preferred for multiplex staining to minimize cross-species reactivity. The secondary antibodies used are listed in Table 1 and Table of Materials. - Perform the conjugation reaction.
- For secondary antibodies, add 15-fold molar excess of dye solution to the antibody solution in a glass vial slowly with stirring. For example, in 0.5 mL 2 mg/mL antibody solution, add 35 µL 3 mM dye solution.
- Incubate the reaction mixture at room temperature (RT) with stirring for 1 h. Protect the reaction from light.
- Purification.
- Prepare the slurry of gel filtration resin (Table of Materials) in PBS buffer, following steps 1.5.2-1.5.4.
- Add 10 mL of gel filtration resin powder into 40 mL of PBS buffer inside a 50-mL tube.
- Keep the solution in a 90 °C water bath for 1 h.
- Decant the supernatant and re-add PBS to 40 mL. Store the slurry at 4 °C.
- Pack the size exclusion column (1-cm diameter, gravity-flow columns) with the slurry solution to the height of 10-15 cm.
- Rinse and wash the column with ~10 mL of PBS buffer to further pack the resin.
- Pipet the conjugation reaction mixture (~0.5 mL) to the column. Immediately add 1 mL of PBS buffer as the elution buffer when all reaction mixture is loaded. Constantly refill the elution buffer (PBS) to the column.
- Collect the eluate of the conjugate solution by looking at the color on the column (MARS dyes have light green to blue colors) or measuring the absorbance at 280 nm (A280).
- Concentrate the collected solution to 1-2 mg/mL with a centrifugal filter.
- Determine the concentration and the average degree of labeling (DOL, dye-to-protein ratio) by measuring the ultraviolet-visible (UV-Vis) spectrum of the conjugate solution with a Nano plate reader.
NOTE: Supplementary Material provides properties of MARS-dye NHS-esters for calculation. The normal DOL for the secondary antibody is around 3.
2. Tissue sample preparation
- Paraformaldehyde fixed mouse brain tissues.
- Anesthetize the mice (C57BL/6J, Female, 25 d postnatal) with isoflurane. Assess the proper anesthetization with a toe pinch test.
- Kill the mice by cervical displacement. Perfuse the mice immediately with 4% paraformaldehyde (PFA) in PBS transcardially.
- Collect the mouse brain, following steps 2.1.4-2.1.5
- Cut upward from the brain stem along the sagittal suture. Peel the two halves of the skull away to the side and scoop out the brain with a tweezer.
- Fix the collected brain in 4% PFA in PBS at 4 °C for 24 h. Then, wash the brain in PBS buffer at 4 °C for 24 h to remove excess PFA.
- Place solid agarose into water to a final concentration of 7% (w/v) in a beaker, with a loose lid. Stir the solution with a glass stirring rod. Heat the slurry in microwave until the solution is clear.
- Allow the agarose to cool to 45-55 °C.
- Pour the liquid agarose into a small chamber, then transfer the brain from PBS to liquid agarose and orient it with a spatula to embed the brain. Wait for the tissue-agarose block to harden.
- Section the tissue-agrose into 40-µm-thick coronal slices using a vibratome.
- Transfer the tissue to a 4-well plate for the following staining. Remove the agarose with a tweezer. Wash the tissue with 1 mL of PBS, three times.
- Fixed frozen mouse pancreas tissues.
- Fix the mouse pancreata in 4% PFA in PBS at 4 °C with rocking for 16-20 h.
- Wash the sample in 1 mL of PBS (4 °C) three times to remove PFA.
- Embed the sample (~0.3-0.5 cm in size) in optimal cutting temperature (OCT) compound blocks. Put 2 drops of OCT into a plastic cryomold. Place the tissue in correct orientation and pour OCT on top of tissues until none of the tissue remains exposed.
- Section the pancreata to 8-µm-thick slices and immobilize them onto a tissue binding glass slide, store them in -80 °C.
- Before staining, equilibrate the specimen to RT. Wash the tissue with PBS to remove OCT blocks.
- FFPE samples.
- Bake the FFPE tissue slide at 60 °C for 10 min.
- Deparaffinization and rehydration: Place samples sequentially in the following solutions in a 50-mL tube at RT for 3 min each time with mild shaking:
xylene two times,
ethanol two times,
95% (vol/vol) ethanol in deionized water two times,
70% (vol/vol) ethanol in deionized water two times,
50% (vol/vol) ethanol in deionized water one time,
deionized water one time. - Transfer the sample into 20 mM sodium citrate (pH 8.0) at 100 °C in a glass jar. Make sure the tissues are immersed in the solution.
- Put the jar in a 60 °C water bath for 45 min.
- Wash the sample with deionized water at RT for 5 min.
3. Tissue immuno-eprSRS staining
- Use a hydrophobic pen to draw a boundary around the tissue sections on the slide.
NOTE: A slide staining jar is used for following incubation steps of tissue on the slide. Floating tissues (40-µm-thick mouse brain sections) are stained in well plates. - Incubate the tissues with 0.3-0.5% PBST (Triton X-100 in PBS) for 10 min.
- Incubate the tissues with blocking buffer (5% donkey serum, 0.5% Triton X-100 in PBS) for 30 min.
- Prepare the primary staining solution: add all primary antibodies to 200-500 µL of staining buffer (2% donkey serum, 0.5% Triton X-100 in PBS) at desired concentrations. Centrifuge the primary staining solution at 13,000 x g for 5 min. Only use the supernatant if precipitates form.
- Incubate the tissue in the primary antibody solution at 4 °C for 1-2 days.
NOTE: For staining tissue sections on the slide, put the sample in a staining box with a wet wipe to maintain humidity. - Wash the slides three times with 0.3-0.5% PBST at RT for 5 min each. Use 1 mL PBST for floating tissues. For tissues on the slide, wash the slides in a slide staining jar and make sure tissues are all immersed in the solution.
- Incubate the tissue in 200-500 µL of blocking buffer for 30 min.
- Prepare the secondary staining solution: add all secondary antibodies (and lectins if required) to 200-500 µL of staining buffer with desired concentrations (normally 10 µg/mL). Centrifuge the secondary staining solution at 13,000 x g for 5 min. Only use the supernatant if precipitates form.
- Incubate the tissues in 200-500 µL of secondary antibody solution at 4 °C for 1-2 days.
- Wash the slides twice with 0.3-0.5% PBST at RT for 5 min each.
- Incubate with 200-500 µL of DAPI solution for 30 min.
- Wash the slides three times with PBS at RT for 5 min each.
- For floating tissue sections, transfer them to glass slides with a glass-dropping pipette. Spread the tissue with a tissue brush and clean the surrounding with wipes if needed.
- Mount the tissue in a drop of antifade reagents with a glass coverslip and secure it with nail polish.
4. SRS microscope assembly
NOTE: A commercial confocal fluorescence system is used in tandem SRS-fluorescence imaging. More descriptions can be found in a prior report17. This protocol will focus on the SRS imaging side using narrowband excitation.
- Prepare a vibration-isolated optical table in a room with temperature control.
- Place a synchronized dual-laser system (pump and Stokes) on the optical table (Figure 2A) with a chiller connected.
NOTE: The fundamental laser in the dual-laser system provides an output pulse train at 1064 nm with 6-ps pulse width and 80-MHz repetition rate. The Stokes beam is from the fundamental laser. The intensity of the Stokes beam was modulated sinusoidally by a built-in electro-optic amplitude modulator (EOM) at 8 MHz with a modulation depth of more than 90%. The other portion of the fundamental laser is frequency-doubled to 532 nm, which is further used to synchronously seed a picosecond optical parametric oscillator (OPO) to produce a mode-locked pulse train with 5-6 ps pulse width (the idler beam of the OPO is blocked with an interferometric filter). The output wavelength of the OPO is tunable from 720-950 nm, which serves as the pump beam. - Mount the mirrors (wavelength range: 750-1100 nm), dichroic beam splitters (DBS, 980 nm long-pass filter, rectangular), and lens (achromatic, AR coating for 650-1050 nm) to their respective mounts. Use very stablekinematic mirror mounts for the mirrors and dichroic beam splitters.
- Measure the height of the laser output and the beam sizes of the pump and the Stokes beams. Adjust the height of mirrors and lenses to ensure the light will hit the center of all the optical elements.
- Place mirror M1 on the optical table and make it ~45° to the laser output (Figure 2B). Use the knobs on the kinematic mount to make tip and tilt adjustments. Ensure the light travels at the same height along the length of the table and a straight line with respect to the table.
- Place and align the dichroic beam splitters (980 nm long-pass filters) and mirrors to split the pump and Stokes beams (Figure 2A-B).
- Place and align lens pairs (L1, L2, and L3, L4) on each of the beam paths to collimate the beams and expand the beam diameters to match the back pupil of the objective (Figure 2A-B).
- Use M7 and M8 mirrors to align combined laser beams into the microscope (Figure 2C). Align one beam into the microscope first and use the mirror pairs on the other beam to ensure the spatial overlap of the two beams.
- Set up the detection part.
- Put on an infrared-coated oil condenser (1.4-NA) to collect the forward-going pump and Stokes beams after passing through the specimens (Figure 2C).
- Mount a large-area Si photodiode onto a shielded box with BNC connectors (Figure 2E). Add a 64-V DC power supply to the mounted photodiode to increase its saturation threshold and response bandwidth.
- Reflect the forward-going light with a 2-inch mirror. Refocus the light onto the photodiode after an optical filter to block the modulating Stokes beam (Figure 2D).
- Send the output current of the photodiode to a fast lock-in amplifier terminated with 50 for signal demodulation. Send an 8-MHz trigger to the lock-in amplifier as the reference signal.
- Send the in-phase X-component of the lock-in amplifier into the analog interface box of the microscope.
- Optimize the temporal overlap with the built-in motorized delay stage by measuring the SRS signal of pure D2O liquid at the microscope.
5. Image acquisition and analysis
- Perform multi-channel epr-SRS imaging with sequential pump wavelength tuning.
- Set laser power to Ppump = 10-40 mW and PStokes = 40-80 mW on the laser control panel.
- Set pixel dwell time to 2-4 µs and use multiple frames averaging typically 10-20 frames on the microscopy software.
NOTE: Avoid a combinatorial use of high laser power (Ppump> 40 mW, PStokes> 80 mW) and small pixel size (<0.2 µm), which likely cause 'bleaching effect' of Raman dyes due to multiphoton excitation. - Set the time constants of the lock-in amplifier to half of the pixel dwell time.
- Linear spectral unmixing.
NOTE: epr-SRS follows a strict linear signal-to-concentration dependence over the entire concentration range; thus, linear spectral unmixing is effective to remove any potential cross-talks between channels. For N-channel epr-SRS measurement with N MARS probes, measured signals (S) can be expressed as S = MC, where C is the MARS probe concentrations, and M is an N x N matrix determined by Raman cross-sections of MARS probes.- Measure matrix M on single-color immuno-eprSRS samples labeled with different MARS probes.
- Use equation C = M−1·S to determine the concentration matrix of the MARS probe with multiplex sample signal measurement S.
Results
Figure 3 shows example images of epr-SRS in different samples, including fixed cells (Figure 3A), paraformaldehyde (PFA)-fixed mouse tissues (Figure 3B), and formalin-fixed paraffin-embedded (FFPE) human specimens (Figure 3C). The spatial resolution of SRS microscopy is diffraction-limited, the typical lateral resolution is ~300 nm, and the axial resolution is 1-2 µm using near-infrared light for excitation. As a result, fine subcellular structures such as microtubules in HeLa cells were faithfully revealed with immuno-eprSRS imaging of α-tubulin (Figure 3A). Moreover, epr-SRS is generally compatible with FFPE tissues (Figure 3C), which is a common form of biopsy specimens for clinical diagnosis and pathology research. Similar to two-photon fluorescence microscopy, as a nonlinear process, epr-SRS has optical sectioning capability for visualizing three-dimensional patterns with subcellular resolution (Figure 3D-E).
We first showcased the multiplex protein imaging utility of epr-SRS on fixed frozen tissue samples of mouse islets of Langerhans in the pancreas. Several interested targets are selected, including hormone expression (e.g., insulin, glucose agonist (glucagon), pancreatic polypeptide (PP), and somatostatin) for cell-type classification (β-cells and non-β-cells (α-, δ-cells)) and transcription factors which are known to be related with β-cell heterogeneity23. Of note, since fluorescence detection is orthogonal to SRS detection, epr-SRS is fully compatible with confocal fluorescence and two-photon fluorescence. As a proof-of-concept, 7-color SRS-fluorescence tandem imaging on a single islet was readily achieved (Figure 4) with good contrast and correct patterns. Low-expression targets such as the transcription factor Pdx1 were imaged with sufficient contrast.
We also demonstrated an eight-color SRS-fluorescence tandem imaging in PFA-fixed mouse cerebellum tissues (Figure 5). Through established biomarkers, different cell types, such as cerebellar granule neurons (NeuN), Purkinje neurons (Calbindin), astrocytes (GFAP), oligodendrocytes (MBP), and GABAergic neurons (GABA B2 receptor) can be identified.

Figure 1: Epr-SRS microscopy for highly multiplexed protein imaging. (A) Energy diagram for spontaneous Raman, non-resonant SRS, and electronic pre-resonant SRS (epr-SRS). Vibrational transition rate of chromophores will be enhanced in epr-SRS by up to 1013-fold. (B) Epitope-based immuno-imaging of α-tubulin was demonstrated in COS-7 cells stained by ATTO740 with high vibrational contrast by epr-SRS. The epr-SRS signal completely disappears when the pump laser wavelength is off-resonance by only 2 nm (right). Scale bars, 20 µm. (C) Epr-SRS spectra of NHS ester-conjugated MARS probes as listed in Supplementary Material. Please click here to view a larger version of this figure.
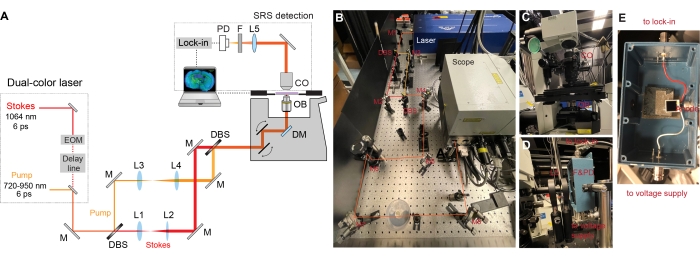
Figure 2: The design of SRS microscope set-up. (A) Schematic diagram of the SRS set-up. EOM = electro-optic modulator, M = mirror, L = lens, DBS = dichroic beam splitter, DM = dichroic mirror, OB = objective lens, CO = condenser, F = filter, PD = photodiode. (B) This panel shows the laser excitation part. Dual-color beam from laser output is first separated with each beam being collimated and expanded and later combined and directed into the microscope body. (C) This panel shows the transmitted collection with a condenser. (D) This panel shows the SRS detection part. Photodiode and filter are mounted to shielded box with two BNC female connectors. Lower BNC connector is for reverse bias voltage, and the higher BNC connector is for current signal output to lock-in amplifier terminated with 50 Ω. (E) This panel shows how the Si photodiode is mounted inside the shielded box. Please click here to view a larger version of this figure.

Figure 3: Raman dye imaging of distinct protein markers via immunolabeling. (A) Immuno-eprSRS imaging of α-tubulin in HeLa cells. (B) Immuno-eprSRS imaging of NeuN in PFA-fixed mouse brain cortex. (C) Immuno-eprSRS imaging of Vimentin in human kidney FFPE tissue. (D) Volume-rendered image ofMARS2145 stained GFAP in 100-µm thick mouse brain tissue. The step size in z was 2 µm. (E) Volume-rendered image ofMARS2228 stained NeuN in 40-µm thick mouse brain tissue. The step size in z was 1 µm. Scale bars, 20 µm in (A), 50 µm in (B-C), 30 µm in (D-E). Please click here to view a larger version of this figure.

Figure 4: Representative results of 7-color tandem imaging of hormones and transcription factors on frozen mouse islet tissue. Epr-SRS: Insulin (detected by Cy5, β-cell marker, green), Pdx1 (detected by MARS2228, transcription factor, red), Glucagon (detected by MARS2216, α-cell marker, yellow), PP (detected by MARS2147, PP-cell marker, blue). Fluorescence: Somatostatin (Alexa488, δ-cell marker, orange), Nkx2.2 (Cy3, transcription factor, magenta), DAPI (nucleus, dark blue). Scale bar, 20 µm. Please click here to view a larger version of this figure.
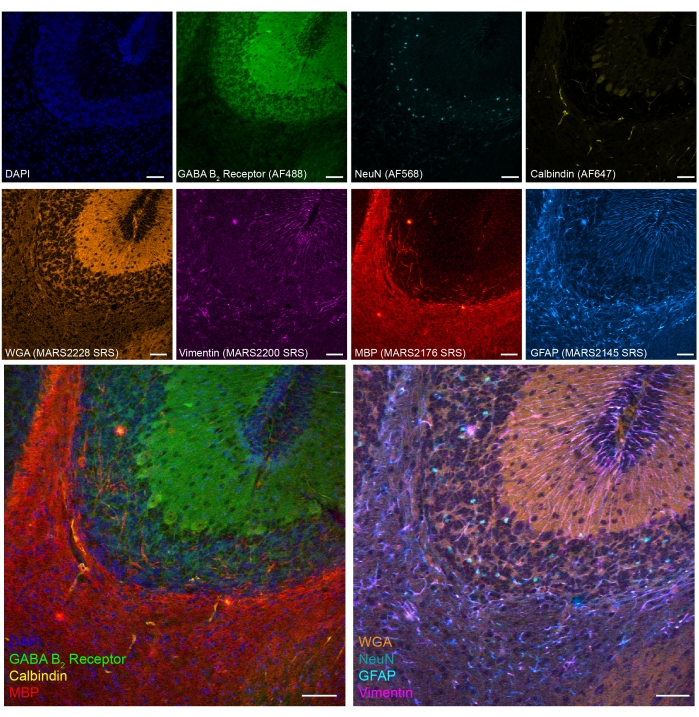
Figure 5: Representative results of 8-color tandem imaging of cell type markers on PFA-fixed mouse brain section. Fluorescence: DNA (DAPI), GABA (γ-aminobutyric acid) B receptor 2 (GABAergic neurons, Alexa Fluor 488), neuronal nuclei (NeuN; neurons, Alexa Fluor 568) and Calbindin (Purkinje neurons, Alexa Fluor 647); epr-SRS: wheat germ agglutinin (WGA; MARS2228), Vimentin (MARS2200), myelin basic protein (MBP; oligodendrocytes, MARS2176) and GFAP (astrocytes and neural stem cells, MARS2145).Scale bar, 50 µm. Please click here to view a larger version of this figure.
Table 1: Validated antibodies for immuno-eprSRS. Refer to the Table of Materials for more details. Please click here to download this Table.
Supplementary Material: Properties of 8 utilized NHS-ester-functionalized MARS probes. λabs and excitation coefficients of MARS dyes were measured in DMSO solution on UV-Vis spectrometer using 1-cm glass cuvette as the container. The absolute Raman cross-sections of MARS dyes were determined in DMSO by comparing the epr-SRS signal of MARS dyes with the standard C−O stretch mode (1030 cm-1) of methanol. The absolute Raman cross-section for the standard C−O stretch mode (1030 cm-1) of methanol was reported as 2.1 x 10-30 cm2 at 785 nm. A cross-section of 0.9 x 10-30 cm2 was estimated under 860-nm pump wavelength by extrapolation. Please click here to download this File.
Discussion
Here, we present the immuno-eprSRS protocol which is broadly applicable to common tissue types, including freshly-preserved mouse tissues, FFPE human tissues, and frozen mouse tissues. Immuno-eprSRS has been validated for a panel of epitopes in cells and tissues, as listed in Table 1. This one-shot platform is particularly suitable for applications where cyclic strategies do not function well. For example, cyclic fluorescence is demanding for thick tissues as multiple rounds of 3D immunolabeling are unpractical lengthy17. It is also very likely to introduce registration errors due to nonlinear 3D histological changes11,17. Immuno-eprSRS overcomes practical barriers of cyclic fluorescence in such a scenario and brings opportunities to reveal protein interaction networks across a large volume17.
Current multiplexity is mainly restricted by the availability of secondary antibodies. While in this protocol, we focused on indirect immunolabeling, in which MARS probes are conjugated to secondary antibodies, direct immunolabeling and lectin staining are feasible17. After more primary antibody validation with Raman dyes, 20 channels are expected with currently developed Raman dyes13,18,24. Moreover, imaging very low-abundant targets could be challenging for epr-SRS due to its slightly compromised sensitivity compared to the confocal fluorescence system. In this regard, we recommend assigning relatively low-abundant targets to brighter MARS dyes and low-expression targets to fluorescence channels.
A critical aspect of the protocol is the accessibility of instruments and probes. Instrumentation-wise, an SRS microscope is generally composed of a dual-color laser source with an optical modulator, a microscope, a photodiode detector, and a lock-in amplifier for demodulation25. Each component is commercially available with a slightly higher total cost than a two-photon laser scanning fluorescence microscope. A fully integrated multimodal SRS/fluorescence research microscope has been commercialized26 using a similar picosecond laser as here for SRS excitation and continuous-wave (CW) laser sets for fluorescence. This system is readily applicable for multiplex vibrational imaging in everyday biological research. Probe-wise, MARS probes haven't been commercialized yet and require some synthesis capabilities. Alternatively, many commercial far-red fluorophores (refer to Extended Data Table 1 in L. Wei et al. Nature 201713) can be used for epr-SRS. Yet, the multiplexity might be compromised. Moreover, since MARS probes by nature are small organic molecules, immuno-eprSRS is similar to immunofluorescence in terms of tissue staining. Therefore, the archive of validated affinity reagents such as antibodies in immunofluorescence can be readily transferred into immuno-eprSRS applications.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Ruth A. Singer and Richard K.P. Benninger for providing mouse pancreas tissues. W.M. acknowledges support from NIH R01 (GM128214), R01 (GM132860), R01 (EB029523) and US Army (W911NF-19-1-0214).
Materials
| Name | Company | Catalog Number | Comments |
| 16% Paraformaldehyde, EM Grade | Electron Microscopy Sciences | 15710 | |
| α-tubulin | Abcam | ab18251 | Primary antibodies |
| α-tubulin | BioLegend | 625902 | Primary antibodies |
| β-III-tubulin | BioLegend | 657402 | Primary antibodies |
| β-III-tubulin | Abcam | ab41489 | Primary antibodies |
| β-tubulin | Abcam | ab131205 | Primary antibodies |
| Agarose, low gellling temperature | Sigma Aldrich | A9414 | For brain embedding |
| Anti-a-tubulin antibody produced in rabbit (α-tubulin) | Abcam | ab52866 | Primary antibodies |
| Anti-Calbindin antibody produced in mouse (Calbindin) | Abcam | ab82812 | Primary antibodies |
| Anti-GABA B receptor R2 antibody produced in guinea pig (GABA B receptor R2) | Millipore Sigma | AB2255 | Primary antibodies |
| Anti-GFAP antibody produced in goat (GFAP) | Thermo Scientific | PA5-18598 | Primary antibodies |
| Anti-Glucagon antibody produced in mouse (Glucagon) | Santa Cruz Biotechnology | sc-514592 | Primary antibodies |
| Anti-insulin antibody produced in guinea pig (insulin) | DAKO | IR00261-2 | Primary antibodies |
| Anti-MBP antibody produced in rat (MBP) | Abcam | ab7349 | Primary antibodies |
| Anti-NeuN antibody produced in rabbit (NeuN) | Thermo Scientific | PA5-78639 | Primary antibodies |
| Anti-Pancreatic polypeptide (PP) antibody produced in goat- Pancreatic polypeptide (PP) | Sigma Aldrich | SAB2500747 | Primary antibodies |
| Anti-Pdx1 antibody produced in rabbit (Pdx1) | Milipore | 06-1379 | Primary antibodies |
| Anti-Somatostatin antibody produced in rat (Somatostatin) | Abcam | ab30788 | Primary antibodies |
| Anti-Vimentin antibody produced in chicken (Vimentin) | Abcam | ab24525 | Primary antibodies |
| Band-pass filter | KR Electronics | KR2724 | 8 MHz |
| BNC 50 Ohm Terminator | Mini Circuits | STRM-50 | |
| BNC cable | Thorlabs | 2249-C | Coaxial Cable, BNC Male / Male |
| Broadband dielectric mirror | Thorlabs | BB1-E03 | 750 - 1100 nm |
| C57BL/6J mice | Jackson Laboratory | 000664 | |
| Centrifuge | |||
| Condenser | Olympus | oil immersion, 1.4 N.A. | |
| Cytokeratin 18 | Abcam | ab7797 | Primary antibodies |
| Cytokeratin 18 | Abcam | ab24561 | Primary antibodies |
| DC power supply | TopWard | 6302D | Bias voltage is 64 V |
| Dichroic mount | Thorlabs | KM100CL | Kinematic Mount for up to 1.3" (33 mm) Tall Rectangular Optics, Left Handed |
| Donkey anti-Chicken IgY (H+L) | Jackson ImmunoResearch | 703-005-155 | Secondary antibodies for MARS conjugation |
| Donkey anti-Goat IgG (H+L) | Jackson ImmunoResearch | 705-005-147 | Secondary antibodies for MARS conjugation |
| Donkey anti-Guinea Pig IgG (H+L) | Jackson ImmunoResearch | 706-005-148 | Secondary antibodies for MARS conjugation |
| Donkey anti-Mouse IgG (H+L) | Jackson ImmunoResearch | 715-005-151 | Secondary antibodies for MARS conjugation |
| Donkey anti-Rabbit IgG (H+L) | Jackson ImmunoResearch | 711-005-152 | Secondary antibodies for MARS conjugation |
| Donkey anti-Rat IgG (H+L) | Jackson ImmunoResearch | 712-005-153 | Secondary antibodies for MARS conjugation |
| Donkey anti-Sheep IgG (H+L) | Jackson ImmunoResearch | 713-005-147 | Secondary antibodies for MARS conjugation |
| DPBS | Fisher Scientific | 14-190-250 | |
| EpCAM | Abcam | ab71916 | Primary antibodies |
| Ethanol | Sigma Aldrich | 443611 | |
| Fast-speed look-in amplifier | Zurich Instruments | HF2LI | DC - 50 MHz |
| FFPE Kidney Sample | USBiomax | HuFPT072 | |
| Fibrillarin | Abcam | ab5821 | Primary antibodies |
| Giantin | Abcam | ab24586 | Primary antibodies |
| Glucagon | Santa Cruz Biotechnology | sc-514592 | Primary antibodies |
| H2B | Abcam | ab1790 | Primary antibodies |
| HeLa | ATCC | ATCC CCL-2 | |
| High O.D. bandpass filter | Chroma Technology | ET890/220m | Filter the Stokes beam and transmit the pump beam |
| Hydrophobic pen | Fisher Scientific | NC1384846 | |
| Insulin | ThermoFisher | 701265 | Primary antibodies |
| Integrated SRS laser system | Applied Physics & Electronics, Inc. | picoEMERALD | picoEMERALD provides an output pulse train at 1,064 nm with 6-ps pulse width and 80-MHz repetition rate, which serves as the Stokes beam. The frequency doubled beam at 532 nm is used to synchronously seed a picosecond optical parametric oscillator (OPO) to produce a mode-locked pulse train with five~6 ps pulse width (the idler beam of the OPO is blocked with an interferometric filter). The output wavelength of the OPO is tunable from 720–950 nm, which serves as the pump beam. The intensity of the 1,064-nm Stokes beam is modulated sinusoidally by a built-in EOM at 8 MHz with a modulation depth of more than 90%. The pump beam is spatially overlapped with the Stokes beam by using a dichroic mirror inside picoEMERALD. The temporal overlap between pump and Stokes pulse trains is achieved with a built-in delay stage and optimized by the SRS signal of pure D2O at the microscope. |
| Inverted laser-scanning microscope | Olympus | FV1200MPE | |
| Kinematic mirror mount | Thorlabs | POLARIS-K1-2AH | 2 Low-Profile Hex Adjusters |
| Lectin from Triticum vulgaris (wheat) | Sigma Aldrich | L0636-5 mg | |
| Long-pass dichroic beam splitter | Semrock | Di02-R980-25x36 | 980 nm laser BrightLine single-edge laser-flat dichroic beamsplitter |
| MAP2 | BioLegend | 801810 | Primary antibodies |
| Microscopy imaging software | Olympus | FluoView | |
| NanoQuant Plate | Tecan | For absorbance-based, small volume analyses in a plate reader. | |
| Normal donkey serum | Jackson ImmunoResearch | 017-000-121 | |
| NucBlue Fixed Cell ReadyProbes Reagent (DAPI) | Thermo Scientific | R37606 | |
| Nunc 4-Well Dishes | Fisher Scientific | 12-566-300 | |
| Objective lens | Olympus | XLPlan N | x25, 1.05-NA, MP, working distance = 2 mm |
| Paint brush | |||
| Periscope assembly | Thorlabs | RS99 | includes the top and bottom units, Ø1" post, and clamping fork. |
| pH meter | |||
| Plate reader | Tecan | Infinite 200 PRO | An easy-to-use multimode plate reader. Absorbance measurement capabilities over a spectral range of 230–1000 nm. |
| ProLong Gold antifade reagent | Thermo Scientific | P36930 | |
| PSD95 | Invitrogen | 51-6900 | Primary antibodies |
| Sephadex G-25 Medium | GE Life Sciences | 17-0033-01 | gel filtration resin for desalting and buffer exchange |
| Shielded box with BNC connectors | Pomona Electronics | 2902 | Aluminum Box With Cover, BNC Female/Female |
| Si photodiode | Thorlabs | FDS1010 | 350–1100 nm, 10 mm x 10 mm Active Area |
| Synapsin 2 | ThermoFisher | OSS00073G | Primary antibodies |
| Tissue Path Superfrost Plus Gold Slides | Fisher Scientific | 22-035813 | Adhesive slide to attract and chemically bond fresh or formalin-fixed tissue sections firmly to the slide surface (tiisue bindling glass slides) |
| Triton X-100 | Fisher Scientific | BP151-500 | |
| Vibratome | Leica | VT1000 | |
| Vimentin | Abcam | ab8069 | Primary antibodies |
| Xylenes | Sigma Aldrich | 214736 |
References
- Goltsev, Y., et al. Deep profiling of mouse splenic architecture with CODEX multiplexed imaging. Cell. 174 (4), 968-981 (2018).
- Taube, J. M., et al. The Society for Immunotherapy of Cancer statement on best practices for multiplex immunohistochemistry (IHC) and immunofluorescence (IF) staining and validation. Journal for Immunotherapy of Cancer. 8 (1), 000155 (2020).
- Lewis, S. M., et al. Spatial omics and multiplexed imaging to explore cancer biology. Nature Methods. 18 (9), 997-1012 (2021).
- Bodenmiller, B. Multiplexed epitope-based tissue imaging for discovery and healthcare applications. Cell Systems. 2 (4), 225-238 (2016).
- Hickey, J. W., et al. Spatial mapping of protein composition and tissue organization: a primer for multiplexed antibody-based imaging. Nature Methods. , (2021).
- Lin, J. -. R., et al. Highly multiplexed immunofluorescence imaging of human tissues and tumors using t-CyCIF and conventional optical microscopes. eLife. 7, 31657 (2018).
- Black, S., et al. CODEX multiplexed tissue imaging with DNA-conjugated antibodies. Nature Protocols. 16 (8), 3802-3835 (2021).
- Giesen, C., et al. Highly multiplexed imaging of tumor tissues with subcellular resolution by mass cytometry. Nature Methods. 11 (4), 417-422 (2014).
- Keren, L., et al. MIBI-TOF: A multiplexed imaging platform relates cellular phenotypes and tissue structure. Science Advances. 5 (10), 5851 (2019).
- Gerdes, M. J., et al. Highly multiplexed single-cell analysis of formalin-fixed, paraffin-embedded cancer tissue. Proceedings of the National Academy of Sciences of the United States of America. 110 (29), 11982 (2013).
- Angelo, M., et al. Multiplexed ion beam imaging of human breast tumors. Nature Medicine. 20 (4), 436-442 (2014).
- Radtke, A. J., et al. IBEX: A versatile multiplex optical imaging approach for deep phenotyping and spatial analysis of cells in complex tissues. Proceedings of the National Academy of Sciences of the United States of America. 117 (52), 33455 (2020).
- Wei, L., et al. Super-multiplex vibrational imaging. Nature. 544, 465 (2017).
- Wei, L., Min, W. Electronic preresonance stimulated Raman scattering microscopy. The Journal of Physical Chemistry Letters. 9 (15), 4294-4301 (2018).
- Shi, L., et al. Electronic resonant stimulated Raman scattering micro-spectroscopy. The Journal of Physical Chemistry B. 122 (39), 9218-9224 (2018).
- Fujioka, H., et al. Multicolor activatable Raman probes for simultaneous detection of plural enzyme activities. Journal of the American Chemical Society. 142 (49), 20701-20707 (2020).
- Shi, L., et al. Highly-multiplexed volumetric mapping with Raman dye imaging and tissue clearing. Nature Biotechnology. , (2021).
- Miao, Y., Qian, N., Shi, L., Hu, F., Min, W. 9-Cyanopyronin probe palette for super-multiplexed vibrational imaging. Nature Communications. 12 (1), 4518 (2021).
- Miao, Y., Shi, L., Hu, F., Min, W. Probe design for super-multiplexed vibrational imaging. Physical Biology. 16 (4), 041003 (2019).
- Qian, N., Min, W. Super-multiplexed vibrational probes: Being colorful makes a difference. Current Opinion in Chemical Biology. 67, 102115 (2022).
- Klimas, A., et al. Nanoscale imaging of biomolecules using molecule anchorable gel-enabled nanoscale in-situ fluorescence microscopy. Nature Portfolio. , (2021).
- Shi, L., et al. Super-resolution vibrational imaging using expansion stimulated Raman scattering microscopy. bioRxiv. , (2021).
- Benninger, R. K. P., Hodson, D. J. New understanding of β-cell heterogeneity and in situ islet function. Diabetes. 67 (4), 537 (2018).
- Hu, F., et al. Supermultiplexed optical imaging and barcoding with engineered polyynes. Nature Methods. 15 (3), 194-200 (2018).
- Hu, F., Shi, L., Min, W. Biological imaging of chemical bonds by stimulated Raman scattering microscopy. Nature Methods. 16 (9), 830-842 (2019).
- . Coherent Raman Scattering Microscope Available from: https://www.leica-microsystems.com/products/confocal-microscopes/p/leica-tcs-sp8-cars/ (2022)
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved