Method Article
Generation and Characterization of Rat Uterus Organoids from Rat Endometrial Epithelial Stem Cells
In This Article
Summary
Here, we present a protocol to isolate and culture rat endometrial epithelial stem cells (reESCs), generating rat endometrial organoids. This method facilitates in vitro studies of endometrial diseases, enabling gene editing and other cellular manipulations.
Abstract
Endometrial organoids offer valuable insights into the development and pathophysiology of endometrial diseases and serve as platforms for drug testing. While human and mouse endometrial organoids have been developed, research on rat endometrial organoids remains limited. Given that rats can better simulate certain endometrial pathologies, such as intrauterine adhesions, this study aimed to establish rat endometrial organoids. We present a detailed protocol for the isolation and culture of rat endometrial epithelial stem cells (reESCs) and the generation of rat endometrial organoids. Using a refined reESCs expansion medium, we successfully isolated and stably expanded reESCs, demonstrating their long-term culture potential. The reESC-generated organoids exhibited typical structural and functional characteristics of the endometrium, including hormone responsiveness. Our results showed that rat endometrial organoids could be cultured over a long term with stable proliferation, maintaining the glandular structure, cell polarity, and functional characteristics of the endometrial epithelium. This novel rat-derived endometrial organoid model provides a valuable platform for studying endometrial diseases and testing therapeutic interventions, with potential applications across various mammalian species.
Introduction
The endometrium, a versatile and regenerative tissue in the human body, undergoes periodic shedding, regeneration, and differentiation under the influence of ovarian hormones1. Abnormalities in the endometrium are linked to various female reproductive system diseases, such as endometriosis, endometrial cancer, and infertility2. The lack of reliable research models for the endometrium hampers in-depth studies of the pathogenesis, clinical diagnosis, and treatment of these diseases. While cell lines and animal models are commonly utilized for endometrial research, challenges such as phenotypic instability in cell lines, interspecies differences in animal models, and other limitations make it difficult to replicate the complex physiological structure and dynamic functional changes in the human endometrium3.
Organoids are three-dimensional structures formed by culturing stem cells in an extracellular environment, possessing self-renewal and self-organization capabilities. They can mimic the structure and function of physiological and pathological tissues and are recognized as preclinical models of human diseases4. In 2017, successful construction of mouse and human endometrial organoids was achieved by embedding fragmented free endometrial tissue obtained through enzymatic digestion into an extracellular matrix scaffold, followed by adding a mixture of specific growth factors and signaling factors for cultivation5. The results demonstrated that ex vivo endometrial organoids exhibit long-term and stable proliferative capacity, maintaining the glandular structure, cell polarity, and functional characteristics of the endometrial epithelium, including mucus secretion and hormone response6. However, the ex vivo culture of adult stem cells forming the endometrial organoids requires gland-like structural support, leading to challenges such as loss of stemness and difficulties in passaging7.
Currently, the cultivation of endometrial organoids relies on the tissue block digestion culture method. In a previous study, our research team cultured human endometrial epithelial stem cells for an extended period in vitro using a primary culture medium primarily composed of Y27632, which we employed to construct endometrial organoids8. Building on this success, we isolated and cultured endometrial epithelial stem cells from rat endometrial tissue using a small molecule compound culture medium, establishing a long-term in vitro culture system. Furthermore, we utilized rat endometrial epithelial stem cells (reESCs) to generate rat endometrial organoids. The development of this model will enhance future in vitro and in vivo studies of endometrial-related diseases in conjunction with rat models.
Protocol
Six 7/8-week-old female Sprague-Dawley rats weighing 200-250 g were used in this work. The rats were housed in a climate-controlled animal facility with ad libitum access to food and water. All experimental procedures involving animals were conducted in adherence to the Institutional Guidelines for the Care and Use of Laboratory Animals and were approved by the institutional review board for animal experiments at the Research Ethics Committee of the Meizhou People's Hospital.
The following section outlines the process for isolating, passaging, freezing, and thawing rat endometrial epithelial stem cells using a refined reESCs expansion medium (REEM) comprised mainly of Y27632, A8301, and CHIR9902178,9. The REEM formulation is based on serum-free DMEM/F12 enriched with specific concentrations of key components: 10 µM Y27632, 3 µM CHIR99021, and 0.5 µM A8301. Comprehensive details regarding the components can be found in the Table of Materials. Once the rats have been anesthetized with isoflurane inhalation, the subsequent procedures depicted in Figure 1 should be implemented.
1. Surgical procedure
- Anesthetize the rat by isoflurane inhalation.
- Place the rats in an induction chamber filled with 3-5% isoflurane mixed with oxygen. Confirm proper anesthetization by the absence of a response to a toe pinch and the loss of the righting reflex.
- Once anesthetized, maintain the rats under 1-3% isoflurane via a nose cone during the procedure. Apply vet ointment to the eyes to prevent dryness while the rat is under anesthesia.
- Make a low abdominal midline incision to expose the uterus horns.
NOTE: All surgical instruments must be sterilized before use. The surgical area on the rat is shaved and disinfected with an antiseptic solution. The surgeon wears sterile gloves, a mask, and a gown. The surgical procedure is performed on a sterile drape. - Perform longitudinal incisions on both sides of the uterus to expose the inner endometrium. Use a T10 scalpel blade to scrape the endometrium until the surface is rough.
- After the surgical procedure, transfer the rats to a sterile and warm recovery area with regulated temperature and humidity levels and monitor them closely until they regain full consciousness and can support themselves in a sternal recumbent position. House groups of three post-operative rats separately in separate cages. To alleviate postsurgical pain, administer analgesics like buprenorphine (0.05-0.1 mg/kg, subcutaneously); repeat every 8-12 h for up to 48-72 h as required.
2. Tissue processing
- Place all uterine endometrium fragments in a 3.5 cm dish and wash them 3x with PBS.
- Mix the uterine endometrium fragments with 3 mL of 1% collagenase type I (diluted in 0.25% trypsin/1 mM EDTA) at 37 °C for 60 min. Filter the undigested tissue using a 100 µm cell strainer to isolate and collect the filtrate for further culturing.
- Seed the collected cells into 12-well plates in REEM medium for further culturing. Calculate the volume of the cell suspension needed to seed the cells in each well of the 12-well plate to achieve the desired cell density (typically ~1-2 × 105 cells/well in 1.5 mL of REEM).
- Wash the cells collected in step 2.2 for 3x with PBS. After the initial wash, centrifuge the cells at 300 × g for 5 min. Discard the supernatant; resuspend the cell pellet in PBS containing 1% BSA (bovine serum albumin) to minimize non-specific binding and incubate for 15-30 min on ice.
- Incubate the cells with primary antibodies specific to different markers (see the Table of Materials) for 30 min to 1 h at 4 °C. Dilute the antibodies in PBS with 1% BSA as per the manufacturer's instructions.
- After incubation, wash the cells 2x with PBS to remove any unbound antibodies. After the final wash, resuspend the cells in 600 µL of PBS with 1% BSA for flow cytometry analysis.
- Configure the instrument with lasers and filters appropriate for the fluorophores used in the experiment (e.g., FITC, PE, APC). Use the forward scatter (FSC) and side scatter (SSC) settings to gate on the live, single-cell population, excluding debris and cell aggregates. Calibrate the flow cytometer using an unstained control to ensure proper alignment. Set specific gates based on the unstained control to define positive populations for each marker of interest (the threshold is 0.02%).
3. Long-term culture of rat endometrial epithelial stem cells
- Colony formation assay
NOTE: This assay helped determine the optimal culture medium for reESCs.- Seed P0 reESCs into 6-well plates at a density of 1,000 cells/well and culture them for 14 days in either REEM or REEM lacking A8301, Y27632, or CHIR99021.
- After the culture period, fix the cells with 4% paraformaldehyde for 20 min at room temperature and subsequently stain them with crystal violet solution for another 20 min.
- Quantify the number of cell clusters containing more than 50 cells. Select the culture medium based on which medium supports the growth of cell clusters.
NOTE: The omission of specific factors from the REEM resulted in a decreased proliferative capacity of reESCs (Figure 1B,C). Based on these findings, REEM was selected as the preferred medium for the culture of reESCs to optimize the culturing conditions.
- Change the REEM every 2 days. When the cells reach 80-90% confluence, digest them with 0.25% trypsin/1 mM EDTA for 5-6 min.
- Passage the cells at a ratio of 1:3 in REEM.
- Proliferation assay
- Seed the P1, P7, and P14 reESCs into 96-well plates at a density of 4 × 103 cells/well in 150 µL of REEM.
- Add 10 µL of CCK-8 reagent to each well after 24, 48, 72, and 96 h of culture, followed by a 2 h incubation at 37 °C.
- Determine the proliferation rate by measuring the absorbance at 450 nm using a microplate reader.
- Preparing and using frozen stocks
- Prepare the frozen stock solution using REEM with 10% DMSO.
- Harvest the reESCs with 0.25% trypsin/1 mM EDTA.
- Resuspend the cell pellet in frozen stock solution at a concentration of 1 × 106 cells/mL. Transfer the cell suspension into tubes.
- Freeze the tubes first at -80 °C and then, transfer them to liquid nitrogen within 24 h.
NOTE: If intending to thaw cells soon, it is recommended to store the frozen cells at -80 °C for a maximum duration of 6 months. - Prewarm the REEM to 37 °C.
- Retrieve the frozen reESCs and submerge them in a 37 °C water bath for 1-2 min.
- Transfer the thawed reESCs into 6-well plates with a seeding density of 1 × 105 cells/well in REEM.
- Immunofluorescence staining
- Seed reESCs in the logarithmic growth phase onto u-Slide 8-well plates at a density of 2 × 103 cells per well, and culture them in REEM.
- When the reESCs reach 90% confluence, fix them with 4% paraformaldehyde for 20 min. Wash the reESCs with PBS 2x before and after fixation.
- Permeabilize the cells with 0.2% Triton X-100 for 10 min.
- Block the cells with 30% goat serum diluted in 5% bovine serum albumin (BSA) for 30 min at room temperature. Wash the reESCs with PBS 2x before and after blocking.
- Incubate the cells with primary antibodies (anti-SSEA-1 and anti-Cytokeratin) for 12 h at 4 °C. Wash the reESCs with PBS 2x after incubation.
- Incubate the cells with secondary antibodies for 1 h at room temperature. Wash the reESCs with PBS 2x after incubation.
- Counterstain the cells with 150 µL of DAPI to label all nuclei.
- Capture images of the stained cells using an inverted confocal microscope.
4. Establishing rat endometrial organoids from reESCs
- Generation of the organoids
- When reESCs reach 70% confluence, digest the cells with 0.25% trypsin/1 mM EDTA for 5-6 min.
- Resuspend 5 × 105 reESCs in 500 µL of thawed Matrigel and place in 12-well plates. Incubate the plates at 37 °C for 20 min to allow gelation.
- Once the cell-Matrigel mixture solidifies, add 1.5 mL/well REEM to cover the Matrigel. Change the REEM every 2-3 days.
- Immunofluorescence staining
- Aspirate the REEM and add 1 mL/well of precooled Organoid Harvesting Solution in the 12-well plates. Incubate on ice for 40-60 min.
- Collect the organoids with a Pasteur pipette and centrifuge at 300 × g for 3 min at 4 °C.
- Rinse the organoids 2-3x with PBS.
- Fix the organoids with 4% paraformaldehyde for 20 min and block using 5% BSA for 60 min.
NOTE: Wash 2x with PBS before and after each step. - Incubate the organoids overnight at 4 °C with primary antibodies diluted in 1% BSA.
- Incubate the organoids with secondary antibodies for 1 h at room temperature.
- Counterstain the organoids with 200 µL of DAPI.
- Capture images using an inverted confocal microscope.
- HE staining
- Dehydrate the organoids through a graded ethanol series: 70% ethanol for 30 min; 80% ethanol for 30 min; 95% ethanol for 30 min; 100% ethanol for 2 x 30 min. Clear the organoids by immersing them in xylene for 2 x 30 min.
- Immerse the cleared organoids in melted paraffin at 60 °C for 1 h. Transfer the paraffin-infiltrated organoids into embedding molds, fill the molds with melted paraffin, and place the molds in a cold plate for the paraffin to solidify. Remove the paraffin blocks from the molds after the paraffin has solidified and trim the excess paraffin.
- Mount the paraffin blocks onto the microtome. Cut 5 µm thick sections and float them on a warm water bath (42-45 °C) to flatten. Carefully transfer the sections to glass slides and dry them on a slide warmer.
- Deparaffinize the sections by immersing slides in xylene for 2 x 5 min. Rehydrate through a series of graded ethanol: 100% ethanol for 2 x 3 min; 95% ethanol for 3 min; 70% ethanol for 3 min; rinse in distilled water.
- Stain with hematoxylin for 5 min; then, rinse in running tap water for 5 min. Differentiate in 1% acid alcohol for a few seconds; rinse in running tap water for 5 min; place in blue in 0.2% ammonia water for 30 s; rinse in running tap water for 5 min; stain with eosin for 2 min. Dehydrate through graded ethanol: 70% ethanol for 1 min; 95% ethanol for 1 min; 100% ethanol for 2 x 1 min. Clear in xylene for 2 x 1 min.
- Add a drop of mounting medium to the stained sections. Then, place a coverslip over the tissue section, avoiding air bubbles. Observe the stained sections under a light microscope.
- Fluorescence staining
- Perform deparaffinization and rehydration as described in steps 4.3.1. to 4.3.4.
- Preheat the antigen retrieval solution in a microwave oven. Place the slides in the heated antigen retrieval solution and maintain them at sub-boiling temperature for 20 min. Allow the slides to cool in the antigen retrieval solution for 40 min. Rinse slides in deionized water for 5 min.
- Incubate the slides in 0.3% hydrogen peroxide in methanol for 10 min to quench endogenous peroxidase activity. Rinse slides for 3 x 5 min in PBS and incubate them in blocking solution for 1 h at room temperature in a humidified chamber. Tap off the excess blocking solution but do not rinse.
- Dilute the primary antibody in blocking solution according to the manufacturer's instructions. Apply the diluted primary antibody to the tissue sections and incubate overnight at 4 °C in a humidified chamber.
- Wash the slides for 3 x 5 min in PBS. Dilute the HRP-conjugated secondary antibody in blocking solution according to the manufacturer's instructions. Apply the diluted secondary antibody to the tissue sections and incubate for 1 h at room temperature in a humidified chamber. Wash slides for 3 x 5 min in PBS to remove unbound secondary antibody.
- Prepare the Fluorescein Tyramide working solution according to the kit's instructions. Apply the working solution to the tissue sections and incubate for 15 min at room temperature in a humidified chamber. Rinse the slides for 3 x 5 min in PBS.
- Repeat step 4.4.1. to 4.4.5 until staining has been completed with all three antibodies. Apply a drop of fluorescent mounting medium with DAPI to each tissue section. Carefully place a coverslip over the tissue section to avoid air bubbles. Examine the slides inverted confocal microscope.
- Quantitative PCR
- Collect the organoids as described in steps 4.2.1. to 4.2.3. Aspirate the PBS and extract the total RNAs from the organoids following the manufacturer's instructions.
- Use any commercially available reverse transcription kit to synthesize cDNA from 1 µg of total RNA. Set up a q-PCR reaction according to the kit manufacturer's protocol. See Table of Materials for primer sequences used for q-PCR.
5. Long-term culture of rat endometrial organoids
- Aspirate the REEM and add 1 mL of Organoid Harvesting Solution for 40 min to dissociate the organoids. Incubate on ice to maintain organoid integrity.
- After incubation, collect the organoids using a Pasteur pipette and centrifuge at 300 × g for 3 min at 4 °C to pellet them for further processing.
NOTE: Trimming of the pipette tip can be helpful for handling larger organoids (over 500 µm) without causing damage. - Resuspend the pelleted organoids in Matrigel, in a ratio of 1:2 or 1:3, for embedding and subsequent culture in 12-well plates.
- Prepare the frozen stock solution: REEM with 10% DMSO.
- Harvest the organoids, resuspend them in the frozen stock solution, and then store in a freezer at -80 °C or in liquid nitrogen for long-term preservation.
- To thaw the frozen organoids, prewarm the REEM in a 37 °C water bath, and immerse the frozen stock tube in the water bath to gently thaw the organoids.
- After thawing, resuspend the organoids in 500 µL of Matrigel for re-embedding and culture in a 12-well plate. Follow steps 4.1.2. to 4.1.3.
NOTE: It is advised to store frozen organoids at -80 °C for shorter durations or transfer to liquid nitrogen for longer-term storage.
6. Optional: Transitioning organoids to adherent culture
NOTE: The protocol mentions an alternative method for obtaining flat-cultured reESCs by transitioning organoids to adherent culture, allowing for the restructuring of organoid morphology.
- Aspirate the REEM and add 1 mL of Organoid Harvesting Solution to dissociate the organoids. Incubate on ice to maintain organoid integrity.
- After incubation, collect the organoids using a Pasteur pipette and centrifuge to pellet them at 300 × g for 3 min at 4 °C.
- Resuspend the organoids in 3 mL of prewarmed REEM and transfer the resuspended organoids to a 3.5 cm dish. Change the REEM media every 2 days.
- When the cells reach 70% confluency, digest them with 0.25% trypsin/1 mM EDTA for 5-6 min.
- Reconstruct the organoids with reESCs by following steps 4.1.1 to 4.1.3.
7. Sequential culturing of organoids with estradiol (E2) and progesterone (P4)
- Prepare stock solutions of E2 and P4 in DMSO. Dilute the stock solutions to desired working concentrations (E2: 10 nM; P4: 1 µM) in REEM, ensuring the final DMSO concentration does not exceed 0.1% to avoid cytotoxic effects.
- Culture organoids in REEM as steps 4.1.1. to 4.1.3. for 4 days. Replace the medium with REEM supplemented with E2 and culture for 7 days. Monitor organoid growth and morphology daily. Change the medium every 2-3 days.
- Replace the medium with REEM supplemented with E2 and P4. Monitor organoid growth and morphology daily. Change the medium every 2-3 days. Continue to culture the organoids for 7 days.
Results
The reESCs and rat uterus organoids were established from six female Sprague-Dawley rats weighing between 200 g and 250 g following the protocol outlined in Figure 1. Drawing on the success of the long-term culture of human endometrial epithelial stem cells, the REEM formulation predominantly consisted of Y27632, A8301, and CHIR99021. To stabilize the reESCs in vitro, we initially isolated endometrial cells from rat endometrium using enzymatic and mechanical techniques. Flow cytometry analysis in Figure 2A revealed that the primary endometrial cells comprised approximately 50% epithelial cells expressing CD9 (49.8%), with low levels of EpCAM (4.49%) and CD24 (2.64%). The SSEA-1 marker for reESCs was present at 6.58%, while endothelial markers CD31 (30.9%) and CD45 (70.3%) were relatively high.
However, culturing in REEM resulted in P1 reESCs displaying a uniform whorled or polyhedral morphology and forming compact clone structures (Figure 2D). Gradual removal of individual factors from REEM led to a corresponding decrease in the proliferative capacity of reESCs. The clone formation assay highlighted the critical role of Y27632 in the stable culture of reESCs (Figure 2B,C). By the third passage, reESCs exhibited consistent expression of SSEA-1 and Cytokeratin, indicating successful expansion of primary reESCs during culture (Figure 2F). Moreover, reESCs maintained robust and stable proliferative capacity even at late passages (Figure 2E), demonstrating their successful isolation and expansion in the current REEM system.
Organoid cultures were established following a previously reported system derived from human endometrial stem cells8. Within 3 days, the reESCs exhibited rapid self-organization into organoid-like structures with a hollow center, which subsequently increased in size and thickness (see Figure 3A). The typical HE staining results illustrating the formation of organoids by reESCs are presented in Figure 3B. These organoid cultures can be sustained through passaging, as evidenced by the high cell viability indicated by Ki67 staining following both direct passaging and freeze-thaw cycles. Furthermore, HE staining was performed on the 10th generation of organoids, and the results showed that the organoid structure could be maintained intact (see Figure 3E). Additionally, qPCR results indicated that Nanog, Sox2, and Oct4 were expressed at levels comparable to those of the P1 generation (Figure 3F). This finding suggests the potential for long-term culture of organoids within the current system.
Our study conducted a preliminary investigation into the response of rat uterine-like structures to E2 and P4. Immunofluorescence staining in Figure 4A revealed the presence of estrogen and progesterone receptors. Following E2 supplementation, these organoids underwent a transition from a hollow spherical form to a more densely populated internal growth pattern (Figure 4C). Subsequent exposure to P4 led to decreased permeability of the dense structures, ultimately resulting in their disintegration (Figure 4B). These findings suggest that E2 potentially drives further differentiation of rat uterine-like structures, whereas P4 may trigger apoptotic pathways.
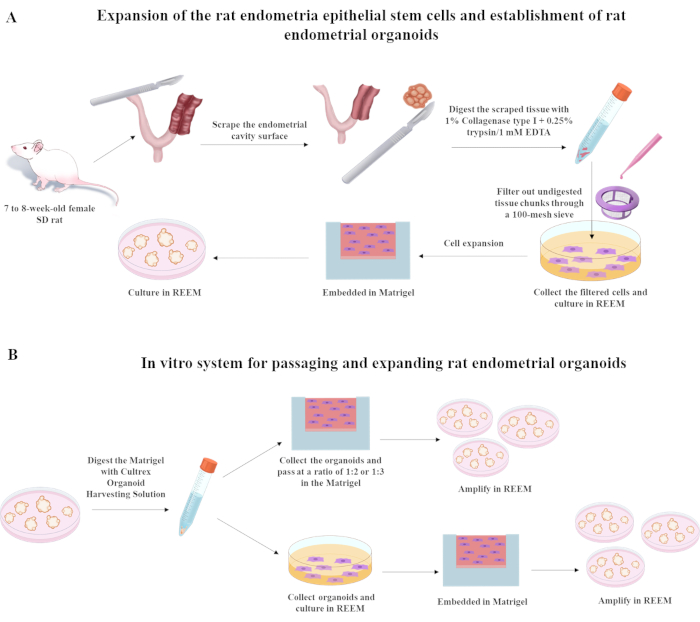
Figure 1: Procedures used to establish rat endometrial epithelial stem cells and rat uterus organoids. Abbreviation: SD = Sprague-Dawley. Please click here to view a larger version of this figure.
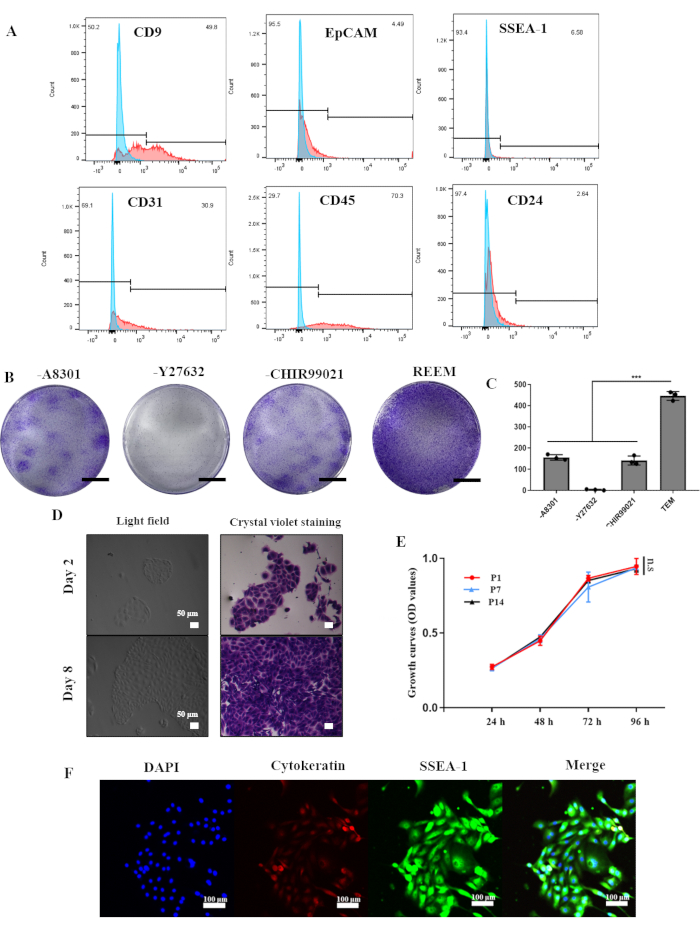
Figure 2: Generation and long-term culture of rat endometrial epithelial stem cells in vitro. (A) Flow cytometric analysis showing the proportion of positive cells in the rat endometrium. (B) Crystal violet staining of clones in REEM with or without A8301, Y27632, or CHIR99021, respectively. Scale bars = 1 cm. (C) The clone numbers in REEM with or without factors. Error bars represent standard deviation; n = 3 donors (***, p<0.001). (D) Light microscopy images of P1 rat endometrial epithelial stem cells with and without crystal violet staining. (E) CCK-8 analyses of rat endometrial epithelial stem cells at passage 1, passage 7, and passage 14. Error bars represent standard deviation, n = 3. (F) Immunofluorescence analyses demonstrating the expression of Cytokeratin and SSEA-1. Abbreviation: REEM = reESCs expansion medium. Please click here to view a larger version of this figure.

Figure 3: Generation and long-term culture of rat endometrial organoids in vitro. (A) Light microscopy images of P1 rat endometrial organoids. (B) H&E staining for P1 organoids from rat endometrial epithelial stem cells. (C) Immunofluorescence analyses demonstrating the expression of Cytokeratin and SSEA-1. (D) Immunofluorescence analyses demonstrating the expression of Ki67 and SSEA-1. (E) H&E staining for P 10 rat organoids. (F) qPCR analyses for the expression of Nanog, Sox2 and Oct-4 in P1 and P10 rat endometrial organoids. Expression normalized to GAPDH (n = 3, two-tailed unpaired t-test, n.s. = non-significant). Abbreviation: H&E = hematoxylin and eosin. Please click here to view a larger version of this figure.
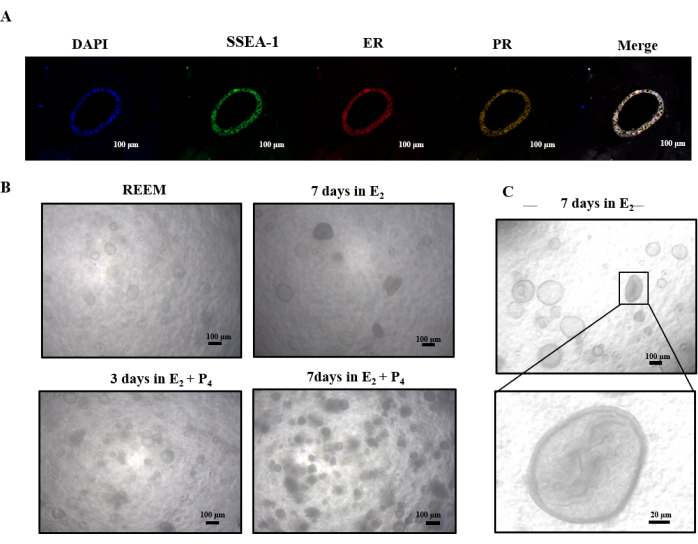
Figure 4: Sequential culturing of organoids with E2 and P4. (A) Immunofluorescence analyses demonstrating the expression of estrogen receptor, progestogen receptor, and SSEA-1. (B) Overview of light microscopy images of rat organoids cultured in E2 and P4. (C) Light microscopy images demonstrate the transformation of a hollow sphere into dense within the inner cavity of organoids after being cultured 7 days in E2. Abbreviations: ER = estrogen receptor; PR = progestogen receptor. Please click here to view a larger version of this figure.
Discussion
In this study, we have described a straightforward method for isolating and culturing rat endometrial epithelial stem cells (reESCs) and refined the previously established ex vivo system for human endometrial epithelial stem cells8. Our approach utilizes a small molecule culture medium containing Y27632, A8301, and CHIR99021 as core components to enable stable and long-term ex vivo culture. Moreover, we successfully generated rat endometrial organoids in real time using reESCs. This novel system simplifies the current endometrial organoid culturing methods, which typically rely on tissue blocks, thereby providing greater clarity regarding cell lineage10. Importantly, our system facilitates gene editing and other manipulations at the cellular level to generate organoids expressing specific genes. Furthermore, this organoid system allows for the targeted knockout or overexpression of specific genes in rat endometrium, enabling the screening and cultivation of endometrial epithelial stem cells to produce endometrial organoids. This comprehensive research approach elucidates specific gene functions from both in vivo and ex vivo perspectives, paving the way for investigating diseases related to the endometrial epithelium.
Drug screening is a critical application of organoids. However, the current sources and preparation methods of organoids are complex, leading to significant variability between batches that can impact the outcomes of drug screening studies11,12. Our system demonstrates that organoids derived from stable ex vivo cultured cell lines can effectively minimize differences among formed organoids, enhancing uniformity in experimental setups. Furthermore, rat organoids formed by reESCs have demonstrated the ability to respond to estrogen stimulation in vitro, leading to morphological changes, suggesting that they can be used as a tool for drug screening ex vivo. The reESCs can be easily isolated from endometrial tissue and expanded ex vivo with fewer small molecules compared to human endometrial epithelial stem cells. This characteristic is particularly valuable for investigating stem cell properties, precursor cells, differentiation processes, and microenvironment interactions. Importantly, this approach can be extrapolated to other mammalian species such as monkeys, pigs, and dogs, enabling the establishment of endometrial organoid culture systems that serve as more physiologically relevant models for studying endometrial biology, function, diseases, and drug responses. Despite its advantages, this technique has limitations. One major limitation is the reliance on Matrigel, a complex and variable extracellular matrix that can introduce inconsistencies between experiments. Furthermore, while rat models provide valuable insights, interspecies differences still exist, and findings may not fully translate to human endometrial physiology and pathologies. Future studies should aim to address these limitations, potentially through the development of more defined and consistent extracellular matrices and by exploring the applicability of this model across other species.
Despite its advantages, this technique has limitations. One major limitation is the reliance on Matrigel, a complex and variable extracellular matrix that can introduce inconsistencies between experiments. Therefore, a key technique in this study is the removal of Matrigel. The use of a gentle matrix gel digestant, ensuring operations on ice, and sufficient digestion time are critical for success. Due to the larger diameter of the Pasteur pipette, using Pasteur pipette aspiration can reduce damage to organoids and accelerate the digestion of the matrix gel. Subsequent research can compare different extracellular matrix gel culture methods to further optimize the culture and digestion systems of organoids. Future studies should aim to address these limitations, potentially through the development of more defined and consistent extracellular matrices and by exploring the applicability of this model across other species.
While existing literature has described techniques for ex vivo culturing of epithelial organoids, this study presents a novel, straightforward, and readily reproducible system for cultivating and expanding rat endometrial organoids sourced from clear cells. This advancement is poised to enhance the pace of research in the realm of women's reproductive biology.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
This work was supported by GuangDong Basic and Applied Basic Research Foundation (2023A1515110760).
Materials
| Name | Company | Catalog Number | Comments |
| Anti-CD15 (SSEA-1) | Abcam | ab135377 | Rabbit, 1:200 (IHC) |
| Anti-Estrogen Receptor alpha | Abcam | ab32063 | Rabbit, 1:200 (IHC) |
| Anti-pan Cytokeratin | Abcam | ab7753 | Mouse, 1:250 (IHC) |
| Anti-Progesterone Receptor | Abcam | ab101688 | Rabbit, 1:200 (IHC) |
| Anti-Ki67 | Abcam | ab279653 | Mouse, 1:250 (IHC) |
| A8301 | TargetMol | 909910-43-6 | |
| β-Estradiol | Merck | E8875 | |
| Cell Counting Kit-8 | Beyotime | C0038 | |
| CD9 | BioLegend | 109819 | 1:20 (FC), Pacific Blue |
| CD24 | BioLegend | 101806 | 1:20 (FC), FITC |
| CD31 | BioLegend | 303120 | 1:20 (FC), APC |
| CD45 | BioLegend | 301703 | 1:20 (FC), PE |
| CHIR99021 | TargetMol | CT99021 | |
| Cultrex Organoid Harvesting Solution | R&D Systems | 3700-100-01 | |
| Cy3 TSA Fluorescence System Kit | APExBIO | K1051 | |
| Cy5 TSA Fluorescence System Kit | APExBIO | K1052 | |
| DAPI | Sigma | D9542 | 1 μg/mL |
| DMEM/F-12 | Invitrogen | 11330032 | |
| EpCAM | BioLegend | 369803 | 1:20 (FC), PerCP |
| Fluorescein TSA Fluorescence System Kit | APExBIO | K1050 | |
| Goat anti-Rabbit IgG, Alexa Fluor 488 | Invitrogen | A-11008 | 1:500 |
| Goat anti-Mouse IgG, Alexa Fluor 555 | Invitrogen | A-21422 | 1:500 |
| Goat Anti-rabbit IgG/HRP antibody | APExBIO | bs-0295G-HRP | |
| Knockout serum replacement | Invitrogen | 10828028 | |
| Matrigel | Corning | 356234 | |
| PrimeScript RT Master Mix | Takara | RR063A | |
| Progesterone | Merck | 57-83-0 | |
| Sprague-Dawley rat | Shanghai JieSiJie Laboratory Animals Co., LTD, China | ||
| SSEA-1 | BioLegend | 323047 | 1:20 (FC), APC |
| TB Green Fast qPCR Mix | Takara | RR820A | |
| TriZOL | Invitrogen | 15596026CN | RNA extraction |
| u-Slide 8-well plates | Ibidi | 80827 | |
| Y27632 | TargetMol | 146986-50-7 | |
| qPCR primers of target genes | |||
| Genes | Company | Sequences | |
| rat GAPDH F | Sangon biotech | GACATGCCGCCTGGAGAAAC | |
| rat GAPDH R | Sangon biotech | AGCCCAGGATGCCCTTTAGT | |
| rat Nanog F | Sangon biotech | GACTAGCAACGGCCTGACTCA | |
| rat Nanog R | Sangon biotech | CTGCAATGGATGCTGGGATA | |
| rat Sox2 F | Sangon biotech | ATTACCCGCAGCAAAATGAC | |
| rat Sox2 R | Sangon biotech | ATCGCCCGGAGTCTAGTTCT | |
| rat Oct4 F | Sangon biotech | CCCAGCGCCGTGAAGTTGGA | |
| rat Oct4 R | Sangon biotech | ACCTTTCCAAAGAGAACGCCCA GG |
References
- Jabbour, H. N., Kelly, R. W., Fraser, H. M., Critchley, H. O. Endocrine regulation of menstruation. Endocr Rev. 27 (1), 17-46 (2006).
- Garcia-Alonso, L., et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat Genet. 53 (12), 1698-1711 (2021).
- Li, M., Izpisua Belmonte, J. C. Organoids - preclinical models of human disease. N Engl J Med. 380 (6), 569-579 (2019).
- Mulaudzi, P. E., Abrahamse, H., Crous, A. Insights on three dimensional organoid studies for stem cell therapy in regenerative medicine. Stem Cell Rev Rep. 20 (2), 509-523 (2024).
- Boretto, M., et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 144 (10), 1775-1786 (2017).
- Turco, M. Y., et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 19 (5), 568-577 (2017).
- Tempest, N., Maclean, A., Hapangama, D. K. Endometrial stem cell markers: current concepts and unresolved questions. Int J Mol Sci. 19 (10), 3240 (2018).
- He, W., et al. Long-term maintenance of human endometrial epithelial stem cells and their therapeutic effects on intrauterine adhesion. Cell Biosci. 12 (1), 175 (2022).
- Katsuda, T., et al. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell Stem Cell. 20 (1), 41-55 (2017).
- Murphy, A. R., Campo, H., Kim, J. J. Strategies for modelling endometrial diseases. Nat Rev Endocrinol. 18 (12), 727-743 (2022).
- Boretto, M., et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 21 (8), 1041-1051 (2019).
- Esfandiari, F., et al. Endometriosis organoids: prospects and challenges. Reprod Biomed Online. 45 (1), 5-9 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved