Method Article
Detection of Helicobacter pylori Infection and Antibiotic Resistance via Stool Quantitative Polymerase Chain Reaction Analysis
* These authors contributed equally
In This Article
Summary
The protocol employs a non-invasive stool sampling combined with a quantitative polymerase chain reaction to offer a convenient and rapid diagnostic method for Helicobacter pylori infection and its resistance to clarithromycin and quinolones.
Abstract
Helicobacter pylori (H. pylori) is widely prevalent worldwide, with approximately 50% of the global population having a history of H. pylori infection. In China, the infection rate ranges from 40% to 70%. H. pylori is primarily associated with gastrointestinal diseases such as chronic gastritis, gastric ulcers, and duodenal ulcers. Currently, the clinical treatment for H. pylori infection involves either triple or quadruple therapy. However, the extensive use of antibiotics has led to the development of antibiotic resistance in H. pylori. Therefore, detecting both H. pylori and its antibiotic resistance is crucial for guiding clinical treatment.
Diagnostic methods for H. pylori include urea breath test (UBT), antigen test, serological antibody test, endoscopy, rapid urease test (RUT), and bacterial culture. While the first three methods are non-invasive, they do not allow for bacterial recovery and, thus, cannot be used for resistance testing. The latter three methods are invasive, expensive, require high technical expertise, and may cause harm to patients.
Therefore, a non-invasive, rapid method for simultaneous detection of H. pylori infection and antibiotic resistance is of utmost clinical importance for the effective eradication of H. pylori. This paper aims to introduce a specific protocol that combines quantitative polymerase chain reaction (qPCR) with TaqMan fluorescent probe technology to rapidly detect H. pylori infection and antibiotic resistance. This method provides a convenient, rapid, and non-invasive way to diagnose H. pylori infection and resistance, unlike traditional bacterial culture and other techniques. qPCR is used to identify the infection and detect mutations in the 23S rRNA and gyrA genes, which are linked to resistance to clarithromycin and quinolones, respectively. Compared to conventional culture techniques, this approach offers a non-invasive, cost-effective, and time-efficient method for detecting Helicobacter pylori infection and determining its antibiotic resistance.
Introduction
Helicobacter pylori is a gram-negative, spiral-shaped bacterium that can persistently infect the human gastric epithelium1. In 1994, the World Health Organization classified H. pylori as a Group 1 carcinogen for gastric cancer, with ~3% of infected individuals ultimately developing the disease2. A recently published systematic review has indicated that a general trend of increased antibiotic resistance rate of H. pylori was observed in the last decade, which has reached alarming levels worldwide, especially clarithromycin resistance3. In China, the latest survey data showed that the aggregate prevalence of H. pylori among urban Chinese people is 27.08%; the resistance rates for clarithromycin and levofloxacin were 50.83% and 47.17%4, which are higher than the United States (levofloxacin 37.6%, clarithromycin 31.5%)5and Europe (levofloxacin 15.8% and clarithromycin 21.4%)6. Consequently, early and accurate diagnosis and treatment are crucial. However, the increasing antibiotic resistance of H. pylori considerably reduces treatment efficacy, highlighting the urgent need for ongoing research into its diagnosis and treatment.
H. pylori diagnostic methods are categorized into invasive and non-invasive techniques7. Invasive methods include histopathological examination, rapid urease test (RUT), and bacterial culture8. Histopathological examination relies on the processing and microscopic observation of biopsy samples, with accuracy limited by histological preparation and pathology expertise9. RUT detects H. pylori through the activity of urease, which hydrolyzes urea to produce ammonia, resulting in an alkaline shift in the reagent, thus indicating the presence of the bacterium9. It is simple and cost-effective. Bacterial culture, considered the "gold standard," often has a success rate below 50% due to the unique physiological characteristics of H. pylori10.
Non-invasive methods include the 13C/14C urea breath test (UBT), serological diagnosis, and stool antigen testing (SAT)11. The breath test operates on a principle similar to that of RUT, detecting isotopically labeled CO2 in exhaled air to confirm infection12. Serological diagnosis detects antibodies, which may persist after bacterial eradication, making it difficult to assess treatment effectiveness13. Stool antigen testing detects H. pylori-specific antigens in fecal samples, providing a reliable non-invasive method with a lower rate of false positives and good accuracy for diagnosing active infections14.
Each diagnostic method has its advantages and limitations15. Apart from bacterial culture, other methods struggle with detecting antibiotic resistance, while culture is hampered by low success rates16. Recently, molecular diagnostic methods such as real-time quantitative PCR (qPCR) have been widely applied to microbial detection17. qPCR can accurately detect H. pylori and analyze resistance gene mutations, offering a more comprehensive view of the infection18.
Fluorescent probes are designed to bind specifically to a target sequence in the DNA during the qPCR process. These probes are typically labeled with a fluorescent dye, which emits a signal when the probe binds to its target and is cleaved by the polymerase during amplification. This provides a real-time measurement of the PCR process. Fluorescent probes are known for their high specificity due to the unique design of the probe, which ensures that only the target DNA sequence is detected, reducing the chances of non-specific binding and improving assay specificity. This makes fluorescent probe technology especially useful for detecting low-abundance targets, such as Helicobacter pylori DNA in stool samples. While SYBR Green is a commonly used DNA-binding dye in PCR, it can bind to any double-stranded DNA, which may result in non-specific amplification and false positives.
Culturing Helicobacter pylori from clinical samples does not provide antibiotic resistance data directly. In contrast, fluorescent probe-based qPCR is faster, more convenient, and can be adapted for simultaneous detection of H. pylori and antibiotic resistance markers. This method not only delivers faster results than traditional culturing methods but also allows for the simultaneous detection of antibiotic resistance genes, greatly enhancing detection efficiency and convenience. This method provides a convenient, rapid, and non-invasive way to diagnose H. pylori infection and resistance, unlike traditional bacterial culture and other techniques.
There are two crucial issues that need to be considered for H. pylori antibiotic-resistance: i) the consistency of genotype and phenotype, ii) the multidrug-resistance. A large-scale multi-center study in China found that the dual-resistance patterns for clarithromycin/levofloxacin were 26.1%. Clarithromycin- and levofloxacin-resistant H pylori phenotypes and genotypes showed satisfactory agreement (kappa coefficient = 0.810 and 0.782, respectively)19. Therefore, it is feasible to use qPCR method to detect H. pylori infection and drug resistance. Stool antigen test (SAT) and qPCR methods are increasingly used due to the convenience of sampling. The sensitivity, specificity, and accuracy of the methods for H. pylori detection are as follows: qPCR > UBT > SAT > RUT> CagA IgG > culture20. All non-invasive methods are suitable for primary screening. Since qPCR can additionally detect drug resistance genes, qPCR and culture will be more suitable for guiding treatment.
Other innovative approaches, such as artificial intelligence (AI) algorithms combined with endoscopic images21, digital microfluidics22, and RPA-CRISPR/Cas12a23, theoretically show good diagnostic efficiency and promising application prospects. However, qPCR is currently the most sophisticated molecular biology technique with the potential to provide a superior solution for the diagnosis and treatment of H. pylori infections.
Protocol
This study adheres to the ethical guidelines established by the Ethics Committee of Guangdong Provincial People's Hospital, Southern Medical University, Guangzhou, China (Approval No: KY2024-445-01). Detailed information regarding the materials used in this research (reagents, chemicals, equipment, and software) can be found in the Table of Materials.
1. Participant selection
- Choose participants in the age range of 18 to 60 years.
- Choose participants who may undergo Helicobacter pylori infection screening, such as patients presenting with symptoms like gastritis, peptic ulcers, or dyspepsia, as well as asymptomatic individuals.
- Exclude from the study patients who have used antibiotics, bismuth-containing agents (such as potassium bismuth citrate capsules, colloidal bismuth pectin capsules, or bismuth-aluminum compound tablets), or antimicrobial Chinese medicines within the past month, or those who have used proton pump inhibitors or H2 receptor antagonists (such as omeprazole, pantoprazole, rabeprazole, cimetidine, or nizatidine) within the last 2 weeks. Also exclude women during their menstrual period from the study.
2. Collection of fecal samples
NOTE: Provide section 2 as instructions to the participants.
- Confirm that the outer packaging of the sampling kit is undamaged and within the expiration date. Remove the materials from the kit.
- Place a white fecal collection paper in the toilet bowl and defecate (to avoid contamination with urine).
- Using a spatula, collect a portion of feces approximately the size of a broad bean (~5 g) and transfer it into the sample container.
- Place the sampling spatula, along with the fecal sample, into the collection tube, ensuring that the liquid level rises to near the sampling line indicated on the tube.
- Securely tighten the tube cap. Invert the tube several times to ensure thorough mixing of the sample with the preservative solution.
- Store the sample under refrigeration until delivery to the clinical laboratory for the following identification of H. pylori and assessment of antibiotic resistance profiles via qPCR.
3. Nucleic acid extraction
NOTE: Complete all procedures within a biosafety cabinet to avoid contamination.
- Invert the specimen preservation tube and mix thoroughly. Transfer 1.0 mL of the fecal specimen solution into a microcentrifuge tube.
- After mixing the centrifuge tubes, place them in a metal bath at 80 °C for 10 min, with intermittent mixing for 30 s at the fifth minute. Once the tubes have cooled to room temperature, centrifuge at 10,000 × g for 5 min and collect the supernatant for further processing.
- Invert the 96-well plate multiple times to resuspend the magnetic beads. Carefully remove the aluminum foil seal, taking care to avoid shaking to prevent spillage.
- Add 200 µL of the prepared sample (from step 3.2) to each well of the 96-well plate containing lysis buffer, ensuring each well corresponds to a single sample. Place the 96-well plate into the designated sample compartment of the nucleic acid extraction instrument for automated extraction.
- Store any remaining specimens and the extracted nucleic acid samples at -20 °C for long-term preservation and future use.
4. qPCR Detection of Helicobacter pylori nucleic acids and resistance mutations to clarithromycin and quinolones
- Take the reagents from the reagent storage area and thaw them at room temperature.
NOTE: This kit was used for the qualitative detection of H. pylori nucleic acid, including mutations in the 23S rRNA gene (A2143G, A2143C, A2144G) and the gyrA gene (261A, 261G, 260T, 271A, 271T, 272G). The primers, the probes, the Taq enzyme, the UNG enzyme, and the dNTPs were available in the kit. The target genes of H. pylori and human β-actin were included in the positive quality control. The humanβ-actin gene was chosen as the internal quality control. - Based on the number of samples to be tested, use N + 2 copies of the lyophilized reagent, where N represents the number of samples to be tested and 2 represents the negative and positive QCs.
- Centrifuge briefly to ensure that the lyophilized powder settles at the bottom of the tubes. Open the lid of the lyophilized reagent (taking care to avoid any spillage of the powder) and add 25 µL per well of the extracted nucleic acids from the samples to be tested, as well as the positive and negative controls. Close the tubes tightly.
- Vortex the PCR reagents for 8-10 s, then briefly centrifuge for 3-5 s to avoid bubble formation.
- Place the 96-well qPCR plate on the qPCR machine. Set the cycling program as follows: first, incubate the reaction mixture (containing primers, probes, Taq polymerase, UNG enzyme, dNTPs, etc.) at 42 °C and 95 °C (both steps in one cycle) for 2 min; then, cycle 10x with 10 s at 95 °C and 45 s at 65 °C; finally, cycle 35x with 10 s at 95 °C and 45 s at 58 °C for denaturation, annealing, and extension.
- Set the fluorescence signal detection parameters as follows: ROX fluorescent labeling of H. pylori conserved genes; FAM fluorescent labeling of H. pylori clarithromycin resistance genes; HEX fluorescent labeling of H. pylori quinolone resistance genes; and CY5 fluorescent labeling of human β-actin gene were used as the kit's internal control. Collect data at 58 °C. Upon completion of the reaction, ensure that the data is saved for future analysis.
- Analyze the data using qPCR-specific software, as the instrument automatically selects the baseline threshold. Set all diagnostic criteria, including the presence of Helicobacter pylori infection or resistance, to a CT value ≤30; confirm that they exhibit a characteristic S-shaped curve.
Results
Application of qPCR for detection of Helicobacter pylori infection and antibiotic resistance in fecal samples
We designed primers and probes based on point mutations in the conserved genes of Helicobacter pylori, as well as in the 23S rRNA gene and the gyrA gene. These primers and probes were labeled with different fluorescent dyes and then used for qPCR detection. The quality control results for the qPCR experiments were within the recommended range, indicating reliable detection results (Figure 1). In this study, we selected five representative samples (S1-S5) to characterize the reliability of the experimental protocol (Figure 2 and Table 1). S1 is a sample without H. pylori infection, while S2-S5 are samples with different drug resistance profiles.
For samples positive for Helicobacter pylori infection, sample S2 showed CT values within the detection range only for Helicobacter pylori, indicating that this sample is positive for Helicobacter pylori and is sensitive to both clarithromycin and quinolones, allowing treatment with either drug. Sample S3 showed CT values within the detection range for both Helicobacter pylori infection and clarithromycin resistance, with no CT values detected for quinolone resistance, suggesting that sample S3 is from a patient resistant to clarithromycin. Similarly, sample S4 had CT values within the detection range for Helicobacter pylori infection and quinolone resistance, with no CT values detected for clarithromycin resistance, indicating resistance to quinolones and recommending clarithromycin for treatment. Finally, sample S5 had detectable CT values across all tests, indicating that the sample is positive for Helicobacter pylori and exhibits dual resistance to both clarithromycin and quinolones; thus, alternative treatment options should be considered by clinicians. Compared to other Helicobacter pylori detection methods, this approach not only facilitates sample acquisition but also allows for simultaneous detection of Helicobacter pylori and its resistance profile, providing reliable results to guide appropriate treatment decisions.

Figure 1: Quality control results of negative and positive controls. Negative controls should display an S-shaped growth curve for the CY5 detection channel with a CT value ≤ 30.00; fluorescence signals for FAM, HEX, and ROX channels should not show significant increases, with CT values > 30.00 or no notable signal; positive controls should present S-shaped curves in all detection channels with CT values ≤ 30.00. Please click here to view a larger version of this figure.
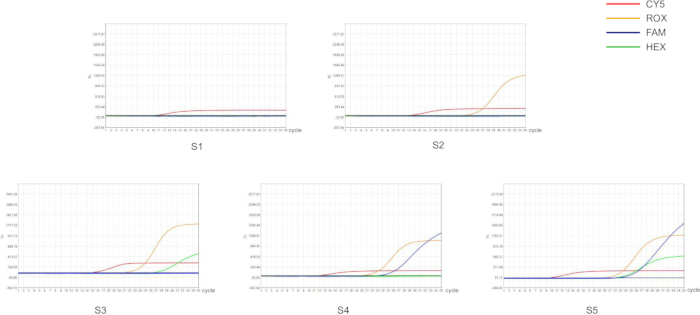
Figure 2: Detection of Helicobacter pylori and its antibiotic resistance in fecal samples by qPCR. Each color represents a fluorescent signal, and different fluorescent signals represent different genes. Fluorescence channels: ROX fluorescent labeling (orange) of H. pylori conserved genes; FAM fluorescent labeling (blue) of H. pylori clarithromycin resistance genes; HEX fluorescent labeling (green) of H. pylori quinolone resistance genes; CY5 fluorescent labeling (red) of the internal control gene. Sample phenotypes: S1 is a negative sample for H. pylori infection (no signal above threshold); S2 is an H. pylori-positive sample without antibiotic resistance (ROX signal above threshold); S3 is an H. pylori-positive sample with resistance to clarithromycin (FAM signal above threshold); S4 is an H. pylori-positive sample with resistance to quinolones (HEX signal above threshold); S5 is an H. pylori-positive sample resistant to both clarithromycin and quinolones (FAM and HEX signal above threshold). Please click here to view a larger version of this figure.
| Sample | H.Pylori (ROX) | Clarithromycin (FAM) | Quinolones (HEX) | CY5 | |||
| +/- | CT | +/- | CT | +/- | CT | CT | |
| S1 | - | - | - | - | - | - | 9.96 |
| S2 | + | 23.08 | - | - | - | - | 12.66 |
| S3 | + | 19.58 | + | 22.41 | - | - | 11.4 |
| S4 | + | 20.65 | - | - | + | 26.06 | 14.59 |
| S5 | + | 19.82 | + | 22.49 | + | 21.65 | 9.48 |
| negetive | - | - | - | - | - | - | 14.73 |
| positive | + | 12.7 | + | 14.47 | + | 13.07 | 14.78 |
Table 1: Results of H. pylori infection detection and qPCR for clarithromycin and quinolone resistance. The table provides the qualitative results of H. pylori infection. Detection of 23S rRNA gene mutations indicates clarithromycin resistance, while gyrA gene mutations indicate quinolone resistance. Symbols: +/−, qualitative result; +, positive result; −, negative result.
Discussion
In recent years, molecular detection methods have been extensively applied in the field of microbiology, significantly altering the clinical management of several infectious diseases. These methods operate at the genetic level, allowing for not only the confirmation of bacterial presence but also gene typing and antibiotic resistance testing. Real-time fluorescence quantitative PCR (qPCR) is increasingly favored due to its short processing time, high sensitivity and accuracy, and low risk of cross-contamination. It has become widely used in clinical laboratories for the diagnosis of Helicobacter pylori infections.
Combining fecal sample collection preservation solutions with gene detection methods allows for the rapid and efficient screening of H. pylori and further antibiotic resistance testing through the analysis of mutations in the clarithromycin 23S rRNA gene and the quinolone gyrA gene. This method can assess H. pylori sensitivity and resistance to drugs. Previous fecal antigen detection methods have notable limitations, such as reduced accuracy and stability due to low antigen concentrations and antigen degradation in samples. Additionally, traditional antigen detection methods may lack sufficient sensitivity and specificity in complex samples, affecting the reliability of the results.
To address these issues, we propose a method combining fecal samples with quantitative polymerase chain reaction (qPCR) technology. This approach significantly enhances detection sensitivity and accuracy, making fecal testing more reliable and efficient, and providing higher quality results for clinical use.
Quality control standards are crucial for qPCR detection. Our quality control includes the periodic calibration of the instrument, the performance verification of the detection system, the evaluation of nucleic acid extraction efficiency, and the quality control of each batch of experiments. First, our sampling instruments are calibrated every 6 months, and the extraction equipment and amplification equipment are calibrated annually. Additionally, calibration is done for each batch of reagents. After calibration, we can validate our performance for the detection system, including the Macrostone 96S fluorescence quantitative PCR instrument, the H. pylori and resistance mutation detection kits, the extraction reagents, as well as the corresponding sampling tubes, pipettes, and tips. The conformity rate, detection limit, cross-reaction, anti-interference ability, and precision meet our needs. The concentrations of DNA products were determined by a DNA concentration tester to meet the acceptance criteria for the nucleic acid extraction efficiency. The DNA concentration was above 10 ng/µL. The A260/A280 was between 1.6 and 2.0. Unqualified samples must be re-extracted. The results are only valid when the daily quality control is under control. For H. pylori and antibiotic resistance testing (clarithromycin and quinolones), the quality control criteria are as follows: negative controls should display an S-shaped growth curve for the CY5 detection channel with a CT value ≤ 30.00; fluorescence signals for FAM, HEX, and ROX channels should not show significant increases, with CT values >30.00 or no notable signal; positive controls should present S-shaped curves in all detection channels with CT values ≤ 30.00. These conditions must be met within the same experiment; otherwise, the test will be considered invalid and must be retested. Note that due to commercial proprietary constraints, primer and probe sequence information cannot be provided in this paper.
In a previous study, researchers employed a methodology identical to that utilized in this paper, demonstrating that RT-PCR testing for H. pylori infection in fecal samples exhibited exceptional diagnostic performance24. Specifically, the test achieved a sensitivity of 99.1%, specificity of 100%, and diagnostic accuracy of 99.1%, which aligned closely with results from gastric biopsy samples (93.9%) (Kappa = 0.929, p < 0.001). This remarkable agreement underscored that RT-PCR testing can be regarded as a highly reliable non-invasive method.
Furthermore, the study also evaluated the capability of fecal RT-PCR in detecting antibiotic resistance in H. pylori, particularly focusing on clarithromycin and levofloxacin resistance. For clarithromycin resistance, 43 cases (37.3%) were identified in fecal samples, with a sensitivity of 79.6%, specificity of 98.4%, and diagnostic accuracy of 78.0% (Kappa = 0.788, p < 0.001). Similarly, for levofloxacin resistance, 37 cases (32.1%) were detected, yielding a sensitivity of 86.3%, specificity of 91.1%, and diagnostic accuracy of 74.4% (Kappa = 0.739, p < 0.001). These findings were highly consistent with those derived from gastric biopsy samples (clarithromycin resistance in 54 cases, levofloxacin resistance in 36 cases), indicating that fecal RT-PCR testing is effective in detecting antibiotic resistance, possessing high specificity and acceptable sensitivity for both antibiotics. The validation of this methodology in the previous study further supports its reliability and applicability in the context of this paper.
While the fecal detection protocol based on qPCR represents a notable advancement in the field of non-invasive diagnostics for H. pylori, several limitations necessitate meticulous consideration. First, using fecal samples makes the method affected by sample quality. False negatives may result from low bacterial loads in feces, particularly in asymptomatic carriers or patients with early-stage infections, where the shedding of H. pylori into the gastrointestinal tract is intermittent25. Moreover, when taking fecal samples, the amount of bacteria can vary depending on where the sample is taken. This contrasts markedly with invasive methods, such as gastric biopsy culture, which directly sample the gastric mucosa and are thus less influenced by fluctuations in bacterial load. Second, although the protocol's targeted amplification approach is specific, it may fail to detect rare or emerging resistance mutations that are not incorporated into the primer design, potentially leading to incomplete resistance profiles26.
Future research should focus on methodological advancements to enhance mutation detection capabilities, including the adoption of digital PCR (dPCR) for improved identification of low-abundance resistance mutations27. Probe panels require strategic expansion to encompass clinically relevant polymorphisms. Conducting multicenter studies to comparatively evaluate diagnostic methodologies-including UBT, gastric mucosal or fecal PCR testing, and antigen detection-provides comprehensive diagnostic performance data for clinical practice, thus advancing the standardization of stool PCR testing protocols. These combined improvements would strengthen fecal qPCR's usefulness as a non-invasive diagnostic tool, while still being important for guiding precise antibiotic treatment.
Disclosures
The authors declare that they have no competing interests.
Acknowledgements
This study was funded by the Research Foundation for Advanced Talents of Guangdong Provincial People's Hospital [Grant No. KY012023293]. This work was supported by Jiangsu Mole Bioscience Co. The funders had no role in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| BSC-1500IIA2-X | BIOBASE | SEDA 20143222263 | Biosafety cabinet |
| Disposable fecal collection and storage tube | Mole | Collect fecal specimens | |
| E-Centrifuge | WEALTEC | Centrifuge the residual liquid off the wall of the tube | |
| Helicobacter pylori nucleic acid, clarithromycin, and quinolone resistance mutation detection kit | Mole | Detection of Helicobacter pylori infection and antibiotic resistance; freeze-dried H. pylori reagent (containing primers, probes, Taq polymerase, UNG enzyme, dNTPs, etc.), a positive control for H. pylori (containing Helicobacter pylori and human β-actin target genes), and a negative control for H. pylori (containing human β-actin target genes) | |
| Mole 96M automated nucleic acid extractor | Mole | For DNA extraction | |
| Nucleic acid extraction kit | Mole | To extract nucleic acid; contains lysis buffer (guanidine salt, tris hydroxymethyl aminomethane, Tween-20, sodium chloride), Wash Buffer 1 (sodium chloride), Wash Buffer 2 (tris hydroxymethyl aminomethane, Tween-20), Wash Buffer 3 (magnetic beads, Tween-20), Wash Buffer 4 (nuclease-free water), and Elution Buffer (tris hydroxymethyl aminomethane), along with a magnetic rack | |
| SLAN Fully automatic medical PCR analysis system | HONGSHI | Data Analysis | |
| SLAN-96S Real-Time PCR machine | HONGSHI | Fluorescent quantitative PCR amplification | |
| Ultra-low temperature freezers (DW-YL450) | MELING | SEDA 20172220091 | -20 °C for storing reagents |
| Vortex-5 | Kylin-bell | For mixing reagent |
References
- Yuan, C., et al. The global prevalence of and factors associated with Helicobacter pylori infection in children: a systematic review and meta-analysis. Lancet Child Adolesc Health. 6 (3), 185-194 (2022).
- Watari, J., et al. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol. 20 (18), 5461-5473 (2014).
- Yu, Y., et al. Global primary antibiotic resistance rate of Helicobacter pylori in recent 10 years: A systematic review and meta-analysis. Helicobacter. 29 (3), e13103 (2024).
- Wang, L., et al. cross-sectional surveillance of Helicobacter pylori prevalence and antibiotic resistance to clarithromycin and levofloxacin in urban China using the string test coupled with quantitative PCR. Lancet Microbe. 5 (6), e512-e513 (2024).
- Ho, J. J. C., Navarro, M., Sawyer, K., Elfanagely, Y., Moss, S. F. Helicobacter pylori antibiotic resistance in the United States between 2011 and 2021: A systematic review and meta-analysis. American J Gastroenterol. 117 (8), 1221-1230 (2022).
- Megraud, F., et al. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut. 70 (10), 1815-1822 (2021).
- Guevara, B., Cogdill, A. G. Helicobacter pylori: A review of current diagnostic and management strategies. Dig Dis Sci. 65 (7), 1917-1931 (2020).
- Rotimi, O., Cairns, A., Gray, S., Moayyedi, P., Dixon, M. F. Histological identification of Helicobacter pylori: Comparison of staining methods. J Clin Pathol. 53 (10), 756-759 (2000).
- Lim, L. L., Ho, K. Y., Ho, B., Salto-Tellez, M. Effect of biopsies on sensitivity and specificity of ultra-rapid urease test for detection of Helicobacter pylori infection: A prospective evaluation. World J Gastroenterol. 10 (13), 1907-1910 (2004).
- Hortelano, I., Moreno, Y., Vesga, F. J., Ferrús, M. A. Evaluation of different culture media for detection and quantification of H. pylori in environmental and clinical samples. Int Microbiol. 23 (4), 481-487 (2020).
- Wang, Y. K., et al. Diagnosis of Helicobacter pylori infection: Current options and developments. World J Gastroenterol. 21 (40), 11221-11235 (2015).
- Gisbert, J. P., Pajares, J. M. Review article: 13C-Urea breath test in the diagnosis of Helicobacter pylori infection -- a critical review. Aliment Pharmacol Ther. 20 (10), 1001-1017 (2004).
- Laheij, R. J., Straatman, H., Jansen, J. B., Verbeek, A. L. Evaluation of commercially available Helicobacter pylori serology kits: A review. J Clin Microbiol. 36 (10), 2803-2809 (1998).
- Gisbert, J. P., Pajares, J. M. Stool antigen test for the diagnosis of Helicobacter pylori infection: A systematic review. Helicobacter. 9 (4), 347-368 (2004).
- Benigno, T. G. D. S., et al. pylori primary strains and virulence genotypes in the Northeastern region of Brazil. Rev Inst Med Trop Sao Paulo. 64, e47 (2022).
- Savoldi, A., Carrara, E., Graham, D. Y., Conti, M., Tacconelli, E. Prevalence of antibiotic resistance in Helicobacter pylori: A systematic review and meta-analysis in World Health Organization regions. Gastroenterology. 155 (5), 1372-1382.e17 (2018).
- Kalach, N., et al. Usefulness of gastric biopsy-based real-time polymerase chain reaction for the diagnosis of Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr. 61 (3), 307-312 (2015).
- Han, X., et al. Quantitative PCR of string-test collected gastric material: A feasible approach to detect Helicobacter pylori and its resistance against clarithromycin and levofloxacin for susceptibility-guided therapy. Helicobacter. 28 (4), e12985 (2023).
- Zhong, Z., et al. A retrospective study of the antibiotic-resistant phenotypes and genotypes of Helicobacter pylori strains in China. Am J Cancer Res. 11 (10), 5027-5037 (2021).
- Hussein, R. A., Al-Ouqaili, M. T. S., Majeed, Y. H. Detection of Helicobacter pylori infection by invasive and non-invasive techniques in patients with gastrointestinal diseases from Iraq: A validation study. PloS One. 16 (8), e0256393 (2021).
- Bang, C. S., Lee, J. J., Baik, G. H. Artificial intelligence for the prediction of Helicobacter pylori infection in endoscopic images: Systematic review and meta-analysis of diagnostic test accuracy. J Med Internet Res. 22 (9), e21983 (2020).
- Liu, J., et al. Rapid and multi-target genotyping of Helicobacter pylori with digital microfluidics. Biosens Bioelectron. 256, 116282 (2024).
- Liu, H., Wang, J., Hu, X., Tang, X., Zhang, C. A rapid and high-throughput Helicobacter pylori RPA-CRISPR/Cas12a-based nucleic acid detection system. Clin Chim Acta. 540, 117201 (2023).
- Fan, C. J., et al. Diagnostic accuracy of a real-time PCR assay for detection of Helicobacter pylori and resistance to clarithromycin and levofloxacin directly from stool. Eur Rev Med Pharmacol Sci. 28 (12), 3836-3840 (2024).
- Patel, S. K., Pratap, C. B., Jain, A. K., Gulati, A. K., Nath, G. Diagnosis of Helicobacter pylori: what should be the gold standard. World J Gastroenterol. 20 (36), 12847-12859 (2014).
- Celiberto, F., et al. The state of the art of molecular fecal investigations for Helicobacter pylori (H. pylori) antibiotic resistances. Int J Mol Sci. 24 (5), 4361 (2023).
- Yang, H., Hu, B. Diagnosis of Helicobacter pylori infection and recent advances. Diagnostics. 11 (8), 1305 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved