Method Article
Poplar Adventitious Roots Induced by Stem Canker Pathogens: An Experimental System for Studying Roots Biology and Light Response-Related Processes
* These authors contributed equally
In This Article
Summary
Here, we present a protocol to induce the production of adventitious roots (ARs) through the phloem- or epidermis-girdle fungal pathogen inoculation pathway, which is suitable for the study of root biology and the light response-related physiological processes in poplar.
Abstract
Valsa sordida and Botryosphaeria dothidea are two crucial necrotrophic fungal pathogens that damage many plant hosts, particularly species in the genus Populus. These two fungal pathogens occur mainly in poplar branches, stems, and twigs, causing classic symptoms such as canker lesions, canopy dieback, and wilting. Pathogen inoculation is the most efficient pathway to study the mechanism of plant disease. Besides the canker lesions around the inoculation sites on the stems, a novel developmental phenomenon, copious adventitious roots (ARs) with bright red color, were observed in poplar species after stem canker pathogen inoculations. In this study, we described the method for inducing ARs using fungal pathogens in poplar trees. The crucial step of this method is the pathogen inoculation after (phloem or epidermis) girdling manipulation. The second crucial step is the application of the moisturizing material. Compared to the moisturizing manipulation with Parafilm, wrapping the inoculated sites with household polyethylene (PE) plastic wrap can produce colorful, numerous, and robust ARs in 20 days after girdling-inoculation. Finally, white ARs sprouted from the inoculated rings in the poplar stems after shading treatment (wrapping the stems with aluminum foil). This method introduces a novel experimental system for studying root development and morphogenesis, which is crucial for understanding the biology of root development, morphogenesis, and response under disease stress. Furthermore, when combined with shading treatment, this study can provide a convenient experimental system for investigating light response-related processes, for example, the biosynthesis of flavonoids, anthocyanins, or other related metabolites, and genes or transcription factors involved in these processes.
Introduction
Poplar stem canker diseases caused by necrotrophic fungal pathogens, Valsa sordida and Botryosphaeria dothidea, are the two crucial tree diseases in north China that severely damaged the development of ecological and economic plantations of poplar species. Poplar canker diseases always occur on the bark of the trunks and branches, while canker lesions are their typical symptoms. After the onset of diseases, the expanding canker lesions progressively damaged the phloem, cambium, and xylem of hosts. Further, they affected the transport of assimilated products and water through the vascular system. However, how the canker pathogens impede the phloem and xylem transport remains unclear.
To reveal the transport mechanisms of carbohydrates and water in poplars infected by canker pathogens, we proposed the phloem or epidermis girdling inoculation methods1,2, which combined classic garden girdle manipulation and pathogen inoculation method (mycelia block wounding inoculation). These methods can simulate the infestation process and the blockage of water and carbohydrates induced by canker pathogens.
Our research illustrated that fungal pathogens caused poplar canopy dieback by initially inducing carbon starvation, not hydraulic failure1,3,4,5. Surprisingly, we have observed a special rhizogenesis on poplar stems that were associated with the inoculation of stem canker pathogens: copious red adventitious roots (ARs) grow from the low end of the upper stems (opposite to the upper edge of the phloem or epidermis girdling rings). Moreover, our experiments illustrated that the production of ARs is universal in poplar-canker pathogen interaction. They can be produced from kinds of poplar species or clones at different ages (1-, 2- or even 6-years old) and can be induced by different canker pathogens (V. sordida and B. dothidea) or their isolates. In addition, we have studied the color mechanisms of poplar ARs, and results showed it is associated with the biosynthesis of flavonoids and anthocyanins, as well as gene expression regulation of light-related genes (or gene modules) under lighting conditions6. Therefore, these poplar ARs induced by pathogens can be used as a stable and ideal experimental system for the study of plant-pathogen interaction, root biology, and the function and expression of light-related genes.
In this study, we will introduce and provide the protocol to establish a poplar ARs experimental system through girdling inoculation pathway; moreover, we point out the crucial factors that affect the formation of ARs and expound on the potential application of the girdling inoculation in the study of poplar root biology and other light response-related physiological processes.
Protocol
1. Induction of poplar ARs through girdling inoculation
- Culture of fungal canker pathogen
- Dissolve 6 g of potato extract, 20 g of dextrose, and 20 g of agar in 1000 mL water to prepare the potato dextrose agar (PDA) medium. Sterilize the medium at 121.1 °C for 30 min and pour the medium into Petri dishes (9cm in diameter), each dish containing about 20 mL of PDA medium.
- Inoculate the activated fungal mycelium cube (~0.5 cm) in the center of the PDA Petri dish.
- Incubate the inoculated PDA plates in the dark at 28 °C for 7-10 days.
- Cut the incubated PDA medium with fungal mycelium into straps (1.2 cm in width; about 3-6 cm in length).
- Preparation of poplar materials
- Select 1-2 years-old, vigorous-growing poplar saplings.
- Select mature stems/branches from poplar saplings (1-2 cm in diameter, free of diseases and pest infestation).
- Wash the inoculation regions (about 30 cm high above the ground or the base of the branches) of poplar stems/branches; sterilize the stems/branches with 75% alcohol solution.
- Induction of ARs through phloem girdling-inoculation
- Carefully girdle the epidermis and phloem of the sterilized poplar stems/branches, remove the girdled phloem rings (1 cm in width, including partial cambium), and expose the white, inner cambium/xylem tissues.
NOTE: Steps 1.3.2-1.3.4 detail alternative methods of inoculation. - Completely cover the girdling region with mycelia straps (1.2 cm in width) as phloem girdling inoculation treatment (GP). The fungal hyphae face the exposed xylem.
- Scrape and damage the exposed inner cambium tissue with sterilized knives, and then inoculate the PDA straps (1.2 cm in width) on the girdling regions as girdling cambium-removal treatment (GR).
- Inoculate the girdling regions directly with the uncultured PDA medium straps (1.2 cm in width) as a girdling control (GC).
- Wrap the inoculated stems/branches with stretchable, colorless, and transparent grafting tape (or PE plastic film). Wrap 4 layers to keep moisture in.
- Tie the inoculated stems/branches with (metal, plastic, or woody) sticks (over 50 cm) to keep them from windbreak.
- Cultivate the girdled poplar materials with regular irrigation during the experiment.
- Observe from outside and record the formation of poplar ARs 14-30 days after inoculation.
- Carefully girdle the epidermis and phloem of the sterilized poplar stems/branches, remove the girdled phloem rings (1 cm in width, including partial cambium), and expose the white, inner cambium/xylem tissues.
- Induction of ARs through epidermis girdling-inoculation
- Select and prepare inoculated materials as described in step 1.2.
- Carefully girdle the epidermis of poplar stems/branches.
- Remove the epidermis rings (1.0 cm in width) and expose the green phloem tissue.
- Slightly and vertically scrape the phloem tissue four times and expose the inner structure of the phloem.
- Inoculate the girdled epidermis region with the fungal mycelium (eGP) and PDA straps (eGC). Perform inoculation manipulations similar to steps 1.3.2-1.3.4.
- Wrap the inoculated stems with grafting tape (or PE plastic film) as described in step 1.3.5.
- Manageme poplars and observe ARs as described in steps 1.3.6-1.3.8.
2. Establishment of an experimental system for the research of light-related genes through girdling inoculation method
- Induce poplar ARs using the phloem girdling inoculation method (steps 1.3.2-1.3.4).
- Alternatively, induce poplar ARs using the epidermis girdling inoculation method as described in step 1.4.5.
- Wrap the pathogen-inoculated regions of poplar stems/branches (15 cm in height) with aluminum foil (shading treatment, S) or without aluminum foil wrap (lighting treatment, L).
- Tie the stems/branches to >50 cm long sticks to keep them from windbreak. Cultivate and manage the poplars regularly and keep them well irrigated during the experiment as described in steps 1.3.6-1.3.7.
- Remove the aluminum foil from the stems at ~20 days after inoculation.
- Observe and photograph the aluminum foil shaded (S) and unshaded poplar ARs (L) immediately.
- Cultivate the shaded poplar ARs in sunlight or other artificial light sources/conditions.
- Remove the grafting tapes (or PE plastic films) and harvest the poplar ARs at 1-5 days after light exposure.
- Wrap all the AR samples with aluminum foil. For the poplar ARs that underwent shading treatment, harvest the samples in the dark.
- Soak the AR samples in liquid nitrogen and store them at -80 °C for further investigation.
- Harvest the light-exposed or unexposed poplar ARs (conducted in the dark) after wrapping them with aluminum foil, and store them at 4 °C for morphological and other in situ assays.
Results
The workflow of stem canker pathogens inducing adventitious roots through girdling inoculation is shown in Figure 1. The experiments conducted here showed that both stem canker pathogens, V. sordida, B. dothidea, and their isolates (from different hosts, regions, or pathogenicity) can induce the formation of ARs in poplar species. In this protocol, we used V. sordida isolate CZC, CFCC86775, and B. dothidea isolates SD50 and SD81 to produce ARs in P. alba var. pyramidalis. V sodida CZC is a typical pathogen used, and it has been deposited in China General Microbiological Culture Collection Center (CGMCC 40575); V. sordida isolate CFCC86775 was purchased from China Forestry Culture Collection Center, while two SD isolates were collected from the canker disease poplar trees in Shandong Province, and deposited in our laboratory. As shown in Figure 2, callus formed on the girdling region (Figure 2A, panel 1), and few ARs formed on gilding and cambium removal treatment (Figure 2A, panel 2), while red, plenty of fibrous roots were produced after the phloem-girdling inoculation treatment (Figure 2A, panels 3-6). We also observed that the ARs were induced in different poplar species or clones, such as P. alba var. pyramidalis, Populus × beijingensis, P. alba × P. tremula var. glandulosa clone 84K, P. euramericana cv. 'Bofeng 3', or other hybrid poplars). However, in this protocol, the ARs are produced only on the stems of 1-year-old P. alba var. pyramidalis. Finally, as shown in Figure 2 panel 7, ARs structures were also induced in the trunks of 6-year-old P. alba var. pyramidalis after phloem-girdling inoculation by pathogen V. sordida. The diameters of the poplar trunk are around 6-7 cm and the length of the longest fibrous roots was longer than 7.0 cm.
As shown in Figure 2, the red segments were produced in the shading poplar ARs after being exposed to the sunlight condition, from white milky or light red ARs produced on the aluminum foil-wrapped poplars (Figure 2B, panel 1) to the red ARs 3 days later (Figure 2B, panel 4). Moreover, we also noticed that red pigments were produced in a short period after light exposure, and the gene expression analysis illustrated that the biosynthesis of pigments (mainly cyanidin-3-O-glucoside) was directly transformed from their anthocyanidin substrates, not the de novel biosynthesis from the beginning of the phenylpropane metabolic pathway6.
In this protocol, both the two girdling methods (phloem and epidermis girdling) can induce the formation of ARs on poplar stems after pathogen inoculation. Less windbreaks occurred and less influence on the physiology (such as transport of water and carbohydrate) in epidermis-girdling-inoculated poplars; however, it seems that the production of ARs is a little fast and efficient in phloem girdling. Therefore, the phloem girdling inoculation method is recommended in the research of poplar ARs.

Figure 1: The schematic workflow of adventitious roots (ARs) formation induced by stem canker pathogen through girdling inoculation methods in poplars. Firstly, the stem canker pathogen (cultured at 28 °C in darkness for 7-10 days) was cut into mycelium straps (1.2 cm in width). One-year-old poplar saplings or the one-year-old branches were girdled on the stems (at ~30 cm above the grounds or the base of the branches, indicated by the blue arrows) through two methods: phloem girdling (girdling and discarding the girdled rings which including epidermis, phloem and partial cambium tissues on the stems) or epidermis girdling (girdling and discarding the girdled rings which only including epidermis of the stems). The width of girdled phloem or phloem rings is 1.0 cm. Then, the girdled regions were covered entirely with mycelium straps and wrapped with PE plastic tape immediately; the mycelium sides of the strap faced the girdled region. For the research of root biology, such as rhizogenesis and root development, the inoculated poplar saplings/branches were cultivated for 14-30 days under regular management (Pathway I, highlighted in yellow arrows). For the research of biosynthesis of segments, photomorphogenesis, or expression regulation of the light-related genes, additional aluminum foil wrapping was adopted, then the inoculated saplings/branches were cultivated ~20 days to produce the ARs. When the ARs were produced, the aluminum foil was removed from the stems/branches, and the poplar ARs were exposed under sunlight or other light condition (varied in the light spectrum, intensity, period, or their combinations) (Pathway II, highlighted in red arrows). Please click here to view a larger version of this figure.
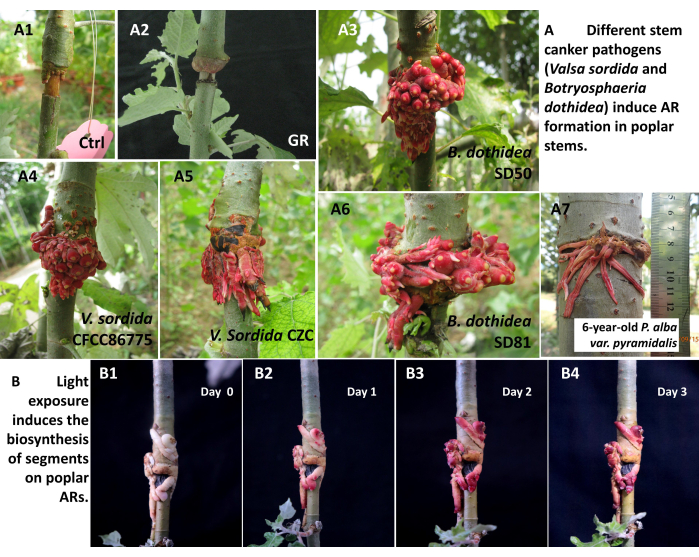
Figure 2: Stem canker pathogens induced the formation of adventitious roots (ARs) and segments biosynthesis in the light conditions of ARs in poplar. (A) Different stem canker pathogens induce AR formation in poplar stems. A1: girdling control; A2: girdling and cambium removal (GR); A3-6: Different stem canker pathogens (V. sordida and B. dothidea) induce AR formation in 1-year-old P. alba var. pyramidalis saplings; A7: ARs produced on the branch of 6-year-old P. alba var. pyramidalis.(B) Light exposure induces the biosynthesis of segments on poplar ARs. B1-B4 represent poplar ARs exposed in light conditions at 0, 1, 2, and 3 days after exposure. Please click here to view a larger version of this figure.
Discussion
Poplar species are apt to produce ARs or lateral Roots (LRs) from stem cuttings, which contributes to their reproduction and as the model for root biology studying in wood plants7,8. Moreover, research indicated that inoculation of specific microorganisms, such as beneficial bacteria (Agrobacterium rhizogenes9,10; Plant growth-promoting rhizobacteria [PGPR]11), endophytes bacteria12,13, arbuscular mycorrhizal fungi (AMF)14,15 or ectomycorrhizal fungi (EMF)16,17 could promote the growth or development of plant roots. However, no report about pathogens (neither bacteria nor fungal pathogens) that induce or promote the ARs structure on the host plants was reported.
The experiments here have shown that different poplar canker pathogens (such as V. sordida, B. dothidea) could induce the formation of ARs in poplar stems/branches (Figure 2A, panels 3-6). Moreover, experiments showed ARs could be induced on different poplar species/clones, for example, 1-2 years old saplings of P. alba var. pyramidalis, Populus × beijingensis, P. alba × P. tremula var. glandulosa clone 84K, P. euramericana cv. 'Bofeng 3', and even 6-year-old poplar branches (Figure 2A, panel 7). However, no ARs structure were produced on the 1-year-old saplings/branches of Malus spp., Prunus spp., Cedrus deodara, and Pinus massoniana. Then, this protocol provided a new pathway of poplar ARs production: inducing ARs through girdling inoculation of tree fungal canker pathogens (V. sordida and B. dothidea) on poplar stems/branches.
Experiments also indicated that both the phloem- and epidermis-girdling methods could induce the formation of ARs on poplar stems after pathogen inoculation; however, poplar stems after phloem-girdling inoculation are easily wind-breakage at the girdling sites for the significant decrease of the toughness that caused by the girdling and removal of bark (phloem) manipulation, and pathogens invasion. Therefore, for the ARs induction, the poplar stems/branches should be well tied to sticks to prevent them from breaking, especially when the phloem-girdling method was used.
The plants themselves mainly determine the formation of ARs. However, it is also affected by some environmental factors. For example, ARs can be induced in waterlogging or flooding conditions in some dicot species18,19. Moisture keeping was the crucial step of ARs induction on the aboveground stems/branches. In this protocol, both the Parafilm and household PE film were used for moisture preservation. However, the Parafilm wrappage can be penetrated by the newly formed ARs, then causing water loss, growth retard, browning, and lignifications of poplar ARs. On the contrary, abundant, tender, and water-rich ARs were harvested in the PE-wrapped poplar stems. Therefore, household PE film, not Parafilm film, was recommended in this protocol.
Roots are crucial organs of plants, playing important roles in the reproduction, growth, nutrients, and water absorption of plants. Then the methods should be used in the study of rhizogenesis20, morphology and development, and root system architecture (RSA)21 in poplar species. Moreover, research indicated that associations between the root system and the rhizosphere bacteria could improve the disease resistance of host plants22; then, girdling-canker pathogens inoculation should induce some molecular and epigenetic changes in poplar ARs, which have a potential application in the cultivation of disease-resistance seedlings.
Light is a critical environmental factor that impacts plant growth, development, and reproduction. The shading-exposure experiment (Figure 2B) indicated that the pathogen-induced poplar ARs are an ideal experiment system for the biosynthesis of plant segments (flavonoids, anthocyanins23, etc.). Previous research also illustrated that light conditions (intensity, quality, duration, and quantity exposed to the parent plant) affect root formation or rhizogenesis and the development of both adventitious and lateral roots24,25,26. Therefore, through the fine-tuning of lighting conditions, the pathogen-induced poplar ARs system in this protocol can be used in the research of root biology and other light response-related processes of poplar plants.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was jointly funded by the Central Public-interest Scientific Institution Basal Research Fund of State Key Laboratory of Tree Genetics and Breeding (grant number CAFYBB2020ZY001-2) and the National Natural Science Foundation of China (grant number 32171776) to Jiaping Zhao.
Materials
| Name | Company | Catalog Number | Comments |
| Agar | Solarbio | A8190 | Provide nutrition for fungal growth |
| Aluminum foil | biosharp | BS-QT-027B | To provide shading for the infected area |
| Girdling knife | MoGong Hardware tool firms | Girdle the epidermis of poplar stems/branches | |
| Grafting tape | CAPI | 5cm | To fix fungi on the plants |
| PD (Potato extraction, Dextrose) | Solarbio | P7360 | Provide nutrition for fungal growth |
| PE plastic film | MiaoJie | 413703 | To fix fungi on the plants |
| Petri dishes | Bkman biological Co.,Ltd | 90mm | Prepare the PDA medium |
| Thermostatic incubator | Shanghai Kuntian Laboratory Instrument Co., Ltd | KTD-6000 | Provide an environment for fungal growth |
References
- Xing, J., et al. Fungal pathogens of canker disease trigger canopy dieback in poplar saplings by inducing functional failure of the phloem and cambium and carbon starvation in the xylem. Physiol Mol Plant Pathol. 101523, 112(2020).
- Xing, J., et al. Stem canker pathogen Botryosphaeria dothidea inhibits poplar leaf photosynthesis in the early stage of inoculation. Front Plant Sci. 13, 1008834(2022).
- Li, P., et al. Fungal canker pathogens trigger carbon starvation by inhibiting carbon metabolism in poplar stems. Sci Rep. 9, 10111(2019).
- Li, J., et al. Effects of Valsa sordida infection on photosynthetic characteristics and carbon-water metabolism in Populus alba var. Pyramidalis. For Res. 34 (05), 58-68 (2021).
- Xing, J., et al. Comparisons of photosythetic response and characteristics in leaves of Populus alba var. pyramidalis infected by the stem canker pathogen Valsa sordida and Botryosphaeria dothidea at Early Stage. Scientia Silvae Sinicae. 57 (09), 121-129 (2021).
- Li, M. Physiological and molecular mechanisms of adventitious root formation in poplar induced by stem canker pathogens [Master's Thesis]. , Chinese Academy of Forestry. (2023).
- Ahkami, A. H. Systems biology of root development in Populus: Review and perspectives. Plant Sci. 335, 111818(2023).
- Dickmann, D. I. Silviculture and biology of short-rotation woody crops in temperate regions: then and now. Biomass Bioenergy. 30, 696-705 (2006).
- De Almeida, M. R., et al. Environmental control of adventitious rooting in Eucalyptus and Populus cuttings. Trees. 31, 1377-1390 (2017).
- Zavattieri, M. A., Ragonezi, C., Klimaszewska, K. Adventitious rooting of conifers: influence of biological factors. Trees. 30, 1021-1032 (2016).
- Bhattacharyya, P. N., Jha, D. K. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol. 28, 1327-1350 (2012).
- Khan, Z., et al. Growth enhancement and drought tolerance of hybrid poplar upon inoculation with endophyte consortia. Curr Plant Biol. 6, 38-47 (2016).
- Paz, I. C., et al. Eucalyptus growth promotion by endophytic Bacillus spp. Genet Mol Res. 11, 3711-3720 (2012).
- Olah, B., Briere, C., Becard, G., Denarie, J., Gough, C. Nod factors and a diffusible factor from arbuscular mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. Plant J. 44, 195-207 (2005).
- Maillet, F., et al. Fungal lipochitooligosaccharide symbiotic signals in arbuscular mycorrhiza. Nature. 469, 58-63 (2011).
- Felten, J., et al. The ectomycorrhizal fungus Laccaria bicolor stimulates lateral root formation in poplar and Arabidopsis through auxin transport and signaling. Plant Physiol. 151, 1991-2005 (1991).
- Splivallo, R., Fischer, U., Gobel, C., Feussner, I., Karlovsky, P. Truffles regulate plant root morphogenesis via the production of auxin and ethylene. Plant Physiol. 150, 2018-2029 (2009).
- Druege, U., et al. Molecular and physiological control of adventitious rooting in cuttings: phytohormone action meets resource allocation. Ann Bot. 123, 929-949 (2019).
- Steffens, B., Rasmussen, A. The physiology of adventitious roots. Plant Physiol. 170, 603-617 (2016).
- Bannoud, F., Bellini, C. Adventitious rooting in Populus species: update and perspectives. Front Plant Sci. 12, 668837(2021).
- Li, Y., et al. Signal communication during microbial modulation of root system architecture. J Exp Bot. 75 (2), 526-537 (2024).
- Pascale, A., Proietti, S., Pantelides, I. S., Stringlis, I. A. Modulation of the root microbiome by plant molecules: the basis for targeted disease suppression and plant growth promotion. Front Plant Sci. 10, 1741(2020).
- Zoratti, L., Karppinen, K., Luengo Escobar, A., Häggman, H., Jaakola, L. Light-controlled flavonoid biosynthesis in fruits. Front Plant Sci. 5, 534(2014).
- Bellini, C., Pacurar, D. I., Perrone, I. Adventitious roots and lateral roots: similarities and differences. Annu Rev Plant Biol. 65, 639-666 (2014).
- Sorin, C., et al. Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell. 17 (5), 1343-1359 (2005).
- Jung, J. K., McCouch, S. Getting to the roots of it: Genetic and hormonal control of root architecture. Front Plant Sci. 4, 186(2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved