Method Article
Using 16α-[18F]-Fluoro-17β-Estradiol PET to Visualize Estrogen Receptor α Expression in Human Breast Cancer Xenografts in Female Ovariectomized Mice
In This Article
Summary
Here, we demonstrate a protocol to use 16α-[18F]-fluoro-17β-estradiol (18F-FES) positron emission tomography (PET) as a tool to visualize ERα expression in ERα-positive breast xenografts.
Abstract
To demonstrate how estrogen receptor alpha (ERα) positive breast cancer xenografts may be visualized in BALB/c nude mice using 16α-[18F]-fluoro-17β-estradiol (18F-FES) positron emission tomography (PET), ovariectomized BALB/c nude mice were injected with ERα-positive breast cancer cells (MCF-7, 3 × 106 cells; shoulder [n = 10] or 4th inguinal mammary fat pad [n = 10]) or ERα-negative breast cancer cells (MDA-MB-231, 1 × 106 cells; mammary fat pad [n = 5]). Mice harboring MCF-7 cells received subcutaneous injections of 20 µg of 17β-estradiol (20 µg/20 µL; corn oil:ethanol, 9:1) in the nape of their necks 2 days prior to cell injection, followed by daily injections five times per week for 5 weeks. Tumor volumes were measured according to the formula: (L*W2)/2 (L; length, W; width). Once tumor volumes reached approximately 100 mm3, 17β-estradiol injections were halted 2 days prior to mice receiving 18F-FES for PET imaging to avoid competitive binding with ERα. Upon 18F-FES administration via the lateral tail vein, PET/MRI was performed for 15 min at 1 h to 1.5 h post-injection. 18F-FES uptake was not observed in ERα-negative, MDA-MB-231 tumor-bearing mice. 18F-FES uptake was most pronounced in mice harboring MCF-7 tumors in the shoulder. In MCF-7 tumors grown in the inguinal mammary fat pad, 18F-FES uptake was less visible, as the intestinal excretion pattern of 18F-FES obscured the radioactivity detectable in these tumors. To use 18F-FES PET as a tool to visualize ERα expression in ERα-positive breast xenografts, we demonstrate that the visibility of 18F-FES uptake is clear in tumors located away from the abdominal region of mice, such as in the shoulder.
Introduction
Breast cancers (BC) can be stratified into different molecular subtypes1. Breast tumors that are classified as the luminal subtype overexpress estrogen receptor alpha (ERα). As such, this subtype of BC is also referred to as ERα-positive (ERα+). Fortunately, those diagnosed with ERα+ BC experience the highest 10-year survival coupled with low rates of distant metastasis2,3. Due to ERα expression, such patients have access to a collection of hormone therapy options, including selective estrogen receptor modulators (SERMs), anti-estrogen drugs, and aromatase inhibitors4.
To assess whether a breast cancer patient is eligible for hormone therapy, the expression levels of ERα within breast tumors must be determined5,6,7. While the gold standard of testing is conducted using immunohistochemistry (IHC) methods, many reports highlight the issue of both the reproducibility and reliability of the results obtained6,8,9. IHC can give rise to result discordance as the technique is semi-quantitative in nature, where differences in tissue processing and subsequent interpretation can lead to variability6. To rectify this recurring problem, guidelines were set in 2010 and updated in 2020 by the American Society of Clinical Oncology with the intention of reducing interobserver variation10. Currently, the clinically validated cut-off sits at ≥1%, with ERα expression even at very small expression levels demonstrating clinically meaningful benefits using endocrine therapy11.
In advanced BC, ERα expression may differ between metastases and the primary tumor. Some observations report an 18%-55% discrepancy in ERα expression levels between metastatic lesions and the primary tumor, pointing to the importance of determining the ERα status of BC metastases12. To address this, guidelines highlight the importance of confirming hormone receptor status in metastatic lesions to make informed treatment plans13,14. However, the feasibility of this is questionable, particularly through IHC methods, considering that metastases may exist in places that are difficult to take biopsies from.
Molecular imaging methods have emerged to become essential tools for the detection and visualization of tumor lesions within cancer patients. In particular, positron emission tomography (PET) imaging requires the use of tracers or, more specifically, radiopharmaceuticals, which are designed to exploit certain features of tumors with the intention of visualizing these lesions non-invasively. The most common PET tracer used in oncology is 18F-fluorodeoxyglucose (18F-FDG)15. In this study, we explore the use of a radiolabeled form of estradiol, 18F-fluoroestradiol (18F-FES). Estradiol - a ligand for ERα - is a hormone predominantly produced by the ovaries in females16. 18F-FES received recent approval from the Food and Drug Administration (FDA) and is marketed as CeriannaTM. This imaging agent is designed to be used as an adjunct to biopsies in patients with recurrent or metastatic BC17. Whole-body PET imaging with 18F-FES can be used as a non-invasive method to detect ERα levels both in the primary tumor and in distant metastases in regions from which biopsies are difficult to obtain18. The prediction of ERα levels using 18F-FES PET imaging correlates with IHC results, and moreover, little to no detection of ERα using 18F-FES PET imaging is a reliable predictor of tumors that are unlikely to respond to hormone therapy18. To ensure the appropriate use of 18F-FES in the clinic, guidelines have been formulated through consensus by experts in the field19. In this study, we evaluate the use of 18F-FES PET in preclinical models of breast cancer in mice.
Protocol
All animal studies were approved by the Austin Hospital Animal Ethics Committee (A2023/05812) and conducted in compliance with the Australian Code for the care and use of animals for scientific purposes.
1. Cell preparation
- Using routine cell culture procedures, maintain MDA-MB-231 and MCF-7 cells in DMEM/F-12 supplemented with penicillin/streptomycin and 10% fetal bovine serum (FBS) in T175 tissue culture flasks. Prior to preparing cells for injection, passage cells at least 3x following revival from liquid nitrogen stores. Grow the cells in a humidified incubator at 37 °C with 5% CO2.
- Propagate cells such that on the day of preparing cells for injection, there are 3 × 106 MCF-7 cells per injection per mouse for 15 mice (i.e., at least 45 × 106 MCF-7 cells) and 1 × 106 MDA-MB-231 cells per injection per mouse for 5 mice (i.e., at least 5 × 106 MDA-MB-231 cells).
NOTE: To allow an equal number of cells in each mouse, prepare enough cells for five extra injections (e.g., prepare enough cells for 10 injections for injecting five mice). - On the day of cell injection, remove the media from all flasks and place approximately 5 mL of trypsin-EDTA (0.25% trypsin and 0.05% EDTA) in 1x phosphate buffered saline (PBS) (pH 7.4) onto cells to facilitate cell detachment.
- Once cells have detached, place approximately 10 mL of complete medium into flasks to inhibit trypsin activity. Resuspend cells and transfer the single-cell suspension into a 50 mL tube. Centrifuge at ~300 × g for 2 min to pellet cells.
- Resuspend the cell pellet in an appropriate volume of media and count cells to determine the total number of cells harvested.
NOTE: Aim to count solutions of about 1 × 106 cells per mL (50-100 cells per 16 squares if using a counting chamber). For cells that grow at 5 × 106 per full flask, harvest cells in 5 mL of media. For cell lines with higher density, use 10 mL of media per flask. - Perform this step on ice in a sterile laminar flow cabinet. Once this number is attained, centrifuge the cell suspension again at 300 × g for 2 min to pellet cells. Resuspend the pellet in cold PBS and ice-cold basement membrane matrix solution such that per 3 × 106 MCF-7 cells, there is 40 µL of PBS and 10 µL of basement membrane matrix solution.
- Resuspend the MDA-MB-231 cell pellet with the same constituents such that per 1 × 106 cells, there is 40 µL of PBS and 10 µL of basement membrane matrix solution. Transfer the cell-PBS-basement membrane matrix mix from the 50 mL tube into smaller 1.5 mL tubes with a cold pipette tip and keep the cell mixture on ice just prior to cell injection into mice.
NOTE: The basement membrane matrix used in this study is stored at -20 °C. The day before the basement membrane matrix solution is required, an aliquot of the solution is placed at 4 °C for thawing overnight. The next day, it is ready for use at cold temperatures (on ice). When pipetting the solution, ensure that the pipette tips used are cold to prevent the basement membrane matrix solution from solidifying. It is good practice to keep a box of sterile pipette tips in the fridge prior to the day of cell preparation for injection. When resuspending the cell pellet in ice-cold PBS and basement membrane matrix solution, PBS is added first, followed by the required amount of basement membrane matrix solution.
2. Cell injection into ovariectomized mice
- Allow 6-8-week-old female ovariectomized BALB/c nude mice to acclimatize at the animal housing facility for at least 1 week prior to cell injection.
NOTE: This study had access to older, 12-14-week-old mice. Ovariectomy ensures low levels of endogenous estradiol and reduces competition with the 18F-FES ligand. Depending on the model being studied, younger mice (e.g., 4-8 weeks) may not need to undergo ovariectomy due to lower levels of endogenous estradiol20,21. - Induce anesthesia in a mouse with 4% isoflurane and oxygen at a rate of 4 L/min using an anesthesia induction chamber. Observe the mouse and check for loss of righting reflex. Once they are no longer moving, transfer the mouse into a sterile laminar flow cabinet, place the snout into the nose mask, and maintain anesthesia by adjusting the isoflurane to 2% and oxygen to a rate of 2 L/min.
NOTE: Anesthetise one mouse at a time to ensure optimal care. - For intramammary fat pad (IMF) injections, swab the skin over the 4th inguinal mammary gland with alcohol. Use a pre-cooled (on ice) 27 G syringe to draw up the cold cell suspension and leave the syringe on ice to prevent warming/solidifying the basement membrane matrix solution prior to injection.
- Using the thumb and index finger of the non-dominant hand, gently lift the 4th mammary gland and insert a 27 G syringe needle with the dominant hand, ensuring the bevel of the needle faces upwards. Slowly inject 50 µL of the cell suspension containing the cell-PBS-basement membrane matrix solution mixture into the mammary fat pad.
NOTE: A bubble should be felt between the fingers as the cells are injected. Check for any leaks at the injection site. - Once the injection is completed, turn the isoflurane off and keep the snout of the mouse in the nose cone to breathe in oxygen and regain consciousness (this should take about a minute). Place the mouse back in a cage on a heat pad for optimal recovery.
- For shoulder injections, use the above procedure outlined for IMF injection, the only difference being the location of cell injection.
NOTE: Alternatively, the shoulder injection can be completed without anesthesia (see step 2.7 below). - For shoulder injection without anesthesia, hold and lift the skin over the shoulder of the mouse using the non-dominant hand between the thumb and index finger, forming a 'tent' in the skin. Slowly inject 50 µL of cell suspension containing the cell-PBS-basement membrane matrix solution using a 27 G syringe needle. Gently withdraw the needle and check for leakages.
NOTE: As an alternative, a Hamilton syringe can be used for small-volume injections to ensure the accuracy of volume delivery. Between injections, 80% v/v ethanol is used to rinse the syringe when using different cell lines, and PBS is used to rinse and remove residual ethanol from the syringe.
3. Preparation of estradiol solution
- Using a scale that can accurately measure milligrams of a substance, weigh out 7 mg of estradiol powder into a 1.5 mL tube.
- Add 700 µL of 100% ethanol into 7 mg of estradiol powder. Place the 1.5 mL tube on a gentle shaker for approximately 1 h at room temperature (RT) or until the estradiol powder has fully dissolved.
- Aliquot the solution across ten 1.5 mL tubes, each containing 70 µL ethanol-estradiol solution. Place tubes at -20 °C for future use.
NOTE: Estradiol-ethanol stocks can be kept at -20 °C for about 2 weeks.
4. Subcutaneous injection of estradiol solution
- On the day that an estradiol injection is required, make up the following mixture fresh. Combine 630 µL of corn oil as a vehicle into the 1.5 mL tube containing 70 µL of the ethanol-estradiol solution. Vortex the solution until homogenous, ensuring the ethanol does not separate and forms a layer at the top of the vehicle oil.
NOTE: This yields 700 µL of a 20 µg/20 µL corn oil:ethanol, 9:1 solution. Each mouse receives 20 µL of this solution. Always make extra solution as the oil in the mixture tends to adhere to the outside of the syringe and on the sides of a 1.5 mL tube due to its viscosity (e.g., for 20 mice, although 400 µL would suffice, making almost double the amount (700 µL) provides enough room for error). - Using a 29 G needle attached to an insulin syringe, slowly draw up 20 µL of estradiol solution.
NOTE: Since the solution contains 9 parts oil to 1 part estradiol-ethanol, the mixture will take time to draw into the syringe. - Mice to be injected with estradiol solution are those that harbor tumors that express ERα and, therefore, rely on estrogen to grow (MCF-7 in this study). Mice harboring tumors that do not express ERα (MDA-MB-231 in this study) do not receive estradiol solution. Gently position the mouse to be injected in front, preferably on a towel, to prevent hurting its limbs while restraining.
- Using the non-dominant hand, restrain the mouse by holding the upper portion of its tail between the ring and little finger. Use the thumb and index finger to lift the loose skin near the neck of the mouse to form a 'tent' in the skin for subcutaneous injection of the estradiol solution.
- While keeping the mouse in this restraint, use the dominant hand to inject the mouse with the estradiol solution. With the bevel of the needle facing upwards, push the needle under the 'tent' of the skin until less than half of the needle is visible.
NOTE: As the solution is injected, the skin should visibly balloon out. - To prevent any backflow of the solution, keep the needle under the skin for a few seconds. If any backflow occurs, gently pat the area dry with a tissue to remove the residual solution.
NOTE: In some experiments, estradiol combined with sesame oil was used to administer the hormone. Using sesame oil as a vehicle leads to local inflammation under the skin at the sites of estradiol injection. As such, corn oil was chosen as the vehicle for subsequent experiments, in which signs of inflammation at the sites of estradiol injection were absent or minimal22. See the discussion for further details. - After removing the needle, gently swab the site of injection with a topical antiseptic using a cotton bud. This is to prevent inflammation on the surface at the site of injection due to the insertion/removal of the needle through the skin of the mouse.
- Inject the mice harboring tumors that express ERα (MCF-7) with estradiol injections subcutaneously 3 days prior to cell injection, followed by 5 times a week for 5 weeks (Figure 1). Halt estradiol injections 2 days prior to imaging day to avoid competitive binding with the 18F-FES probe to its target, ERα.
NOTE: In this study, mice harboring MCF-7 tumors were injected with estradiol daily from Monday to Friday. If they were to be imaged the following week on a Wednesday, estradiol injections continued on Saturday and Sunday. Estradiol was not administered on Monday and Tuesday before the mice were imaged on Wednesday.
5. 18F-FES PET and MRI imaging of ovariectomized mice
CAUTION: Use protective equipment when handling radioactivity. Follow all applicable regulatory procedures when handling radioactivity.
- Weigh the mouse and record its weight.
- For this study, 18F-FES was synthesized and formulated at a clinical grade23(obtained from the Department of Molecular Imaging and Therapy, Austin Health) for use in animal models. Dilute 18F-FES (109 min radioactive half-life) in 10% w/v ethanol-saline solution at an adjusted decay-corrected injection concentration of approximately 150-300 µCi/100 µL.
NOTE: A specific activity of 1000 Ci/mmol is sufficient for in vivo PET imaging of ERα24, and the 18F-FES received had a specific activity above this baseline value. - Draw 100 µL with an insulin syringe with a 29 G needle. Use a dose calibrator to measure and record the radioactivity dose and time. Place the syringe behind a lead shield until the time of injection.
NOTE: Measure the amount of 18F-FES radioactivity in each dose with a dose calibrator. The dose calibrator should be calibrated against a standard reference material, such as cesium-137, per the manufacturer's protocol. The time of the reading is important to record to determine the decay correction. Ensure that the time shown on the calibrator matches the time on the computer that is used for PET acquisition. - To dilate the tail veins for intravenous injection, place the mouse under a heating element at a 30 cm distance for 2 min. Wipe the tail with 70% w/v ethanol to disinfect the area before injection.
- Administer 100 µL of 18F-FES (the entire volume in the syringe) with a bolus injection via the lateral tail vein and record the time of injection. Press the injection site with a tissue to stop the tail vein from bleeding.
- Measure the remaining dose in the syringe plus the tissue using the dose calibrator and record the measurement and time. Inject mice staggered in batches of two to allow imaging using the multi-chamber.
NOTE: Some probe will be left in the syringe. The use of insulin syringes is preferred over syringes connected to needles via Luer locks because of the decreased dose volume trapped in the syringe/needle after administering the injection. In addition, the radioactivity in the tissue that is used to stop any bleeding that may occur following intravenous injection of the probe is measured. This is because the backflow of blood absorbed by the tissue may contain some radioactivity from 18F-FES. In addition, the time taken to warm the lateral tail veins will depend on variables such as the intensity of the heat source and how close it is to the mice. For standard heat lamps it may take up to 15 min for veins to dilate. Mice must be closely monitored for signs of overheating and removed from the heating source between injections if needed. - For mice that are allocated to sleep post-injection, place the injected mouse in the anesthesia chamber kept under a maintenance level of isoflurane (2%-2.5%) and oxygen (2 L/min) for 1 h on a heating pad to allow the probe to be distributed via the mouse's systemic circulation prior to the PET scan. Allow the other mice in the group to walk freely in their cage for 1-1.5 h until the PET scan.
- After 1-1.5 h, place the mouse in an imaging chamber under nose-cone isoflurane anesthesia. Turn on the bed heater to 32 °C, select a multi-chamber setup and turn on the respiratory monitoring system. Place the two mice to be imaged in a prone position. Place the imaging chamber in the PET/MRI machine.
- Monitor the breathing of the mouse throughout image acquisition. Use a respiration chart to ensure 60-80 breaths per minute at any given time during the scan. If the breathing rate falls below or above the desired range, adjust the isoflurane dose as required.
NOTE: The respiration of the animal can be monitored due to the presence of a sensor in the imaging chamber. - Pair a static 15 min PET acquisition with a Gradient Echo (GRE) 3D axial MRI scan. See below for acquisition parameters:
- To set up the PET protocol, open the Radiopharmaceutical Editor window in the relevant software. Specify the isotope being used, the syringe activity (MBq), injection time and applied activity (MBq), and body weight. All of these parameters are essential to help calculate and quantify the uptake of the probe in the tissue of interest.
- To ensure the mouse is correctly positioned for imaging, use a 2D Scout (front and back) sequence to determine the PET field of view. Proceed to a 15 min static PET acquisition using an energy window of 400-600 keV, coincidence mode of 1-5, 5 ns. Perform a GRE3D axial MRI scan for approximately 27 min following this.
- Reconstruct the acquired images using relevant software.
- Perform static reconstruction of the PET/MRI image and use the geometric planner to modify the area of reconstruction to ensure it covers the entirety of the mouse, ensuring optimal reconstruction time.
- Under the Results Identification window, switch random corrections, attenuation correction (AC) + scatter, position range to On. Set the decay reference time to ADMIN. Set the body-air threshold to 30% and base the attenuation correction on the material map.
- Use relevant software for the separation of multi-chamber images, co-registration and to draw regions of interest (ROIs). For co-registration, realign the MRI to fit the PET, and reconstruct the PET image.
- To separate the multi-chamber images, click on Image Processing > Segmentation > Hotel Separator. Turn on the Scientific Mode and adjust the threshold and volume parameters as needed until 2 objects are found.
- Enter the dose at the time of injection after accounting for any residual dose left in the syringe to convert the PET data to the unit of percent-injected dose per gram (%ID/g) or additionally enter the subject's weight to convert the PET data to the unit of standardized uptake value (SUV). Do this conversion by right-clicking on the PET data set and locating the %ID/g field on the Basic Info tab. Enter the %ID/g recorded earlier.
- To draw ROIs on the tumor sections, click the Measurements tab on the left-hand side of the program window. Draw the ROI with the polygon or freehand tool. Carefully draw around the circumference of the tumor, ensuring that the action is performed while the PET image is in the active window (check using Fn-A). The result gives a value of the ROI as %ID/g.
NOTE: ROI analysis gives a good estimate of the %ID/g. A well-calibrated camera should give an accuracy of ±5% compared to ex vivo biodistribution data.
6. Harvesting and fixing tumour tissue for immunohistochemistry
- At the experimental endpoint, euthanize the mouse using lethal inhalation of isoflurane at 4%-5% or over-inhalation of CO2.
- Using surgical scissors and tweezers, carefully create a small incision near the surface of the tumor and resect the tumor tissue. If the skin is attached to the tumor tissue, use a scalpel blade to gently separate the skin from the tumor.
- Place the tumor tissue in a 5 mL vial containing approximately 3 mL of 10% neutral buffered formalin. Ensure the entirety of the tissue is covered in solution to facilitate tissue fixation. Keep the tissue submerged in formalin for 24 h.
- The following day, transfer the fixed tumor tissue into a 5 mL vial containing 70% ethanol. Label a tissue cassette specific for the tumor tissue in preparation for paraffin embedding.
- Place the tumor tissue in a tissue cassette and store it in a 70% ethanol solution until paraffin embedding.
NOTE: In this study, paraffin embedding was conducted by anatomical pathologists from Austin Health.
7. Immunohistochemistry for the detection of estrogen receptor alpha (ERα)
- Using a microtome, cut 4 µm thick sections of the tumor tissue required for staining, following standard procedures.
- Place the glass microscope slides with cut tumor sections into a slide rack and place the rack into a 37 °C oven to dry overnight and to ensure tissues adhere to the slides before staining.
- The next morning, place the rack with slides containing cut tumor sections into a 60 °C oven for 30 min to facilitate the dewaxing process.
NOTE: The glass slides should face upwards while baking. This ensures tissue adherence, and any wax that melts melts onto its own slide rather than adjacent slides. - To rehydrate sections, submerge the baked sections into xylene two times, 5 min each (2x 5 min) 100% ethanol for 2x 5 min and then 70% ethanol for 1x 5 min. Wash slides in distilled water for 5 min.
- For antigen retrieval, immerse slides in a container of 1x EDTA buffer, pH 8, for 25-30 min in a 100 °C water bath. Remove slides from the water bath and allow slides to sit at RT for at least 1 h. Once slides have cooled, wash them in running tap water for 2 min and then TBST (TBS with 0.01% Tween20) for 2x 5 min.
- Using a hydrophobic barrier pen, draw a circle around the sections on the slides.
- Quench endogenous peroxidases by placing approximately 100 µL of 3% H2O2 solution (diluted in distilled water) onto sections for 10 min at RT. Wash slides in running tap water for 2 min and then TBST for 2x 5 min.
- Block sections with approximately 100 µL/section of blocking buffer (5% bovine serum albumin (BSA) in TBST) for 30 min at RT.
- Drain off the blocking buffer by gently flicking the excess solution into the incubation tray. Add approximately 100 µL of primary antibody (human anti-ERα raised in rabbit) to sections at a 1:300 dilution (or at optimal pre-determined dilution specific for the antibody used). Make up antibody dilutions in 1% BSA TBST and place slides to incubate with the antibody at 4 °C overnight.
NOTE: The incubation tray should be filled with water at the bottom to ensure sections do not dry overnight. - The next morning, wash the slides with TBST for 3x 5 min. Add approximately 100 µL of anti-rabbit secondary antibody onto sections and leave on for 45 min at RT. Wash the slides with TBST for 3x 5 min.
- In a fume hood, add approximately 100 µL of 3,3'-diaminobenzidine (DAB) reagent onto sections and keep the solution on for approximately 3 min per section. Inhibit DAB reaction by washing slides with running tap water for 10 min.
- Counterstain slides with hematoxylin for 1 min, and rinse in running tap water for 2 min. Submerge slides in Scott's water for 1 min, then rinse in running tap water for another 2 min.
- To dehydrate sections, submerge the sections in 70% ethanol for 2 min, 100% ethanol for 2x 2 min, and then xylene for 2x 2 min.
- In a fume hood, carefully mount sections in dibutyl phthalate polystyrene xylene (DPX) and place a coverslip over the sections, ensuring that bubbles are not trapped over the tissue of interest. Leave the slides to dry overnight in the fume hood.
- The following day, use a slide scanner to capture images and visualize the staining.
Results
To determine the location for which ERα positive tumors can be clearly visualized using 18F-FES PET, three cohorts of ovariectomized mice were used in this study (Figure 1). Two groups of mice were injected with MCF-7 cells - an ERα positive breast cancer cell line - either IMF or in the shoulder. As a negative control, another cohort of mice was injected with MDA-MB-231 cells, a commonly used triple-negative breast cancer cell line that does not express ERα (Figure 1). A diagram outlining the workflow of this study highlights the use of subcutaneous estradiol injections to facilitate the growth of estrogen-dependent tumors. In all models, tumor volumes reached approximately 100 mm3 in 4-5 weeks following cell injection (Figure 1 and Figure 2). MCF-7 tumors grown in the shoulder of ovariectomized mice took longer to establish (30 days) compared to tumors grown orthotopically (20 days) (Figure 2). MDA-MB-231 tumors IMF took an average of 30 days to establish.
Representative mice were selected for imaging (Figure 1) when tumors across all groups reached an average volume of 74.59 mm3 (SEM ± 6.80 mm3, n = 20; Figure 2). To assess how in vivo18F-FES distribution is best visualized in mice, mice were injected with 18F-FES via the lateral tail vein and were either put to sleep or placed back into their cage and allowed to stay active for 1 h post-injection (Figure 3). After 1 h, PET images of mice were acquired with a 15 min scan time to visualize 18F-FES distribution. Upon analysis, it was observed that in mice that were awake an hour post 18F-FES injection, clearer PET images were produced with reduced background as opposed to mice that were asleep for an hour post 18F-FES injection (Figure 3). Upon this observation, the remaining mice to be imaged were kept awake for 1-1.5 h post 18F-FES injection.
Next, mice from all three cohorts were injected with an average of 10.5 MBq of 18F-FES (Figure 4). Representative coronal and maximum intensity projection (MIP) images depict the uptake and distribution of 18F-FES in these cohorts. Uptake of 18F-FES by MCF-7 tumors was visible both in the mammary fat pad as well as in the shoulder (Figure 4A-D). Importantly, 18F-FES uptake was most obvious in mice harboring MCF-7 tumors in the shoulder (1.7-3.9 %ID/g, n = 9). The accumulation of 18F-FES in other organs did not obscure this signal, as the shoulder tumor was at a distance from such organs (Figure 4A,B). In MCF-7 tumors grown in the 4th inguinal mammary fat pad, 18F-FES uptake was visible, yet overlap with the intestinal excretion pattern of 18F-FES partially obscured the radioactivity detectable in these tumors (0.83-1.88 %ID/g, n = 4) (Figure 4C,D). 18F-FES uptake was not observed in the ERα-negative, MDA-MB-231 tumour-bearing mice (0.18 - 0.22 %ID/g, n = 2) (Figure 4E,F).
At the endpoint, tumors were resected, and immunohistochemistry was performed to assess expression levels of 18F-FES target, ERα. The expression of ERα was comparable in MCF-7 tumor tissue grown in either the mammary fat pad or the shoulder (Figure 5). ERα expression was expectedly void in triple-negative MDA-MB-231 tumor tissue (Figure 5).

Figure 1: Workflow for establishing tumors in female ovariectomized BALB/c nude mice prior to 18F-FES PET/MRI imaging. Three cohorts of mice were used in this study. A diagram of the cell lines injected, ERα status of the cell line, site of injection, and number of estradiol (E2) injections received is shown. In mice that were to harbor tumors dependent on estrogen for growth, subcutaneous injections of E2 (blue arrow) commenced on day 0, three days prior to cell injection. On day 4, cells were injected either IMF or in the shoulder of mice. Tumors take approximately 4 to 5 weeks to establish. During this period, E2 injections were administered 5 times over 7-day intervals (5 injections per week). In the week prior to imaging, E2 injections were given for a total of 7 times that week. E2 injections were halted 2 days prior to PET imaging. Abbreviations: E2 = estradiol; IMF = intramammary fat pad. Please click here to view a larger version of this figure.
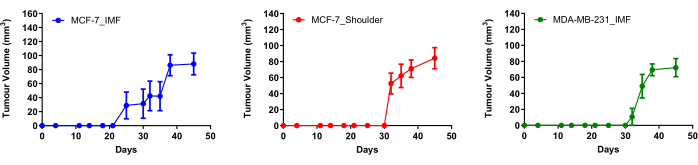
Figure 2: Tumor growth curves of MCF-7 and MDA-MB-231 cells injected into female ovariectomized BALB/c nude mice. MCF-7 cells (3 million) were injected either in the 4th inguinal mammary fat pad (n = 10) or the shoulder of mice (n = 5), and 1 million MDA-MB-231 cells were injected IMF (n = 5)). Mice harboring MCF-7 cells received subcutaneous E2 injections five times per week for 5 weeks. The average tumor volume of mice that were imaged was 101.03 mm3 (SEM ± 11.77 mm3, n = 6). Data is mean, and error bars represent ± SEM. Abbreviations: E2 = estradiol; IMF = intramammary fat pad. Please click here to view a larger version of this figure.

Figure 3. In vivo 18F-FES distribution is better visualized in mice that are active for 1 h following 18F-FES administration. On the day of imaging, mice harboring tumors that were approximately 100 mm3 were injected with the 18F-FES probe via the lateral tail vein. Selected mice were either put to sleep or were awake for 1 h following probe administration. PET images of mice were acquired with a 15 min scan time. Representative images demonstrate in vivo 18F-FES distribution in the two different groups. Abbreviations: h p.i. = hours post-injection. Please click here to view a larger version of this figure.
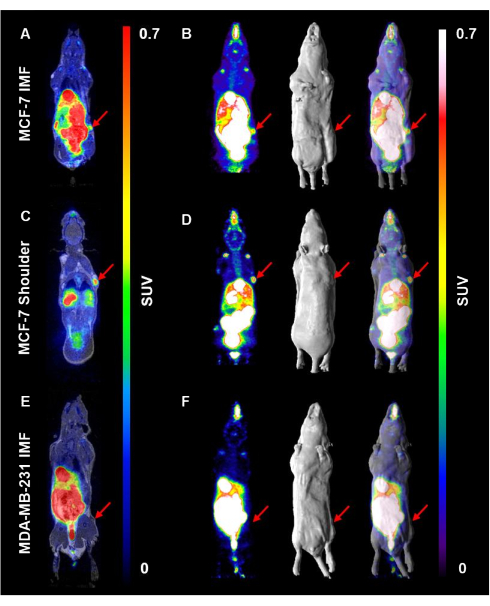
Figure 4. 18F-FES uptake is better visualized in the shoulder compared to the inguinal mammary fat pad in ERα positive tumors. Mice were injected subcutaneously with E2 to support the growth of ERα positive tumors. E2 injections were halted for 48 h prior to injection of an average of 10.5 MBq 18F-FES probe/mouse. PET images were acquired with a 15 min scan time 1 h post-injection,. (A) A representative coronal section of a mouse harboring MCF-7 tumors IMF is shown, in addition to (B) a maximum intensity projection image, surface rendered MRI, and PET/MRI overlay to visualize 18F-FES uptake. The same series of images are displayed for mice harboring (C,D) MCF-7 tumors in the shoulder and (E,F) in MDA-MB-231 tumor-bearing mice. Red arrows represent tumor location. Please click here to view a larger version of this figure.

Figure 5. MCF-7 tumors resected from ovariectomized BALB/c nude mice express 18F-FES target, ERα. Tumors were resected from mice harboring either MCF-7 or MDA-MB-231 tumors at the endpoint following 18F-FES imaging. Tumor tissue was stained for ERα using immunohistochemistry. ERα expression is visualized in the nucleus. The scale bar represents 50 µm. Please click here to view a larger version of this figure.
Discussion
Here, we describe the utility of 18F-FES PET/MRI in the detection of breast tumors characterized by ERα expression. As an example, we demonstrate that one location at which ERα positive tumors can be visualized is in the shoulder of mice - these tumors can be clearly identified by 18F-FES uptake, compared to tumors located within the 4th inguinal mammary fat pad (Figure 4). 18F-FES uptake was not visible in MDA-MB-231 tumors, confirming its lack of ERα expression, which is observed using immunohistochemistry (Figure 4 and Figure 5).
As the excretion pattern of 18F-FES occurs through the hepatobiliary system and intestinal tract, it becomes difficult to accurately detect the uptake of 18F-FES breast tumors situated in the inguinal mammary fat pads of mice25. This is because the anatomical location of these glands in mice is in close proximity to their liver and digestive tract. Although the visibility of tumors in the 4th mammary fat pad via 18F-FES uptake may be possible, this relies on the tumor to grow away from the intestinal tract of the mouse and to protrude outwards. From a practical perspective, the direction in which a tumor will grow following cell injection in mice is unpredictable. In contrast, although we find that breast tumors take longer to establish in the shoulder of mice (Figure 2), the tumor location is conveniently situated away from the anatomical region through which 18F-FES is excreted. As such, the success rate of detecting 18F-FES uptake in ERα positive tumors located in the shoulder is increased (Figure 4). In this study, we show that establishing ERα positive tumors in the shoulder is one location where 18F-FES uptake in ectopic breast cancer models can be visualized clearly. In addition, we show that establishing tumors in the 4th inguinal mammary fat pad is not ideal due to the excretion pattern of 18F-FES. Many in vivo studies of breast cancers may prefer to make use of orthotopic breast cancer models, ensuring the growth of breast tumors within the mammary fat pad in the vicinity of supporting breast tissue26. In such cases, research groups interested in visualizing ERα expression in the context of breast cancer may attempt to use the 2nd and 3rd thoracic mammary fat pads27. From an anatomical perspective, the thoracic mammary fat pads are located further from the hepatobiliary system and intestinal tract. Therefore, 18F-FES uptake by tumors in this region is expected to be clearer compared to tumors grown in the inguinal mammary fat pads of mice27. While we do not demonstrate the advantages of establishing tumors in the thoracic mammary fat pad in this study, other groups have demonstrated the utility of this location as an orthotopic breast cancer model that can also be coupled with 18F-FES PET imaging20,27. Undeniably, studying cancer in orthotopic mouse models best represents how cancers arise and present in humans28.
A critical step in the methodology described here involves the use of ovariectomized mice and regular estradiol supplementation to promote the growth of breast tumors that rely on the hormone for development. In this study, we chose MCF-7 - a luminal breast cancer cell line that expresses high levels of ERα29. As the detection of ERα tumors relies on receptor occupancy, the use of ovariectomized mice in this study was vital30. This is because estrogens are largely produced by the ovaries, and if present, endogenous estrogens can compete with the injected probe, limiting the binding opportunity of 18F-FES to ERα16. There are a variety of methods that can be employed for estrogen supplementation, including the use of silastic capsules, slow-release pellets, osmotic pumps, and subcutaneous injections using oil vehicles31. For our experimental needs, daily subcutaneous injections using corn oil were used as it easily allowed for the withdrawal of estradiol at the time of imaging, mitigating the issue of target receptor (ERα) saturation. Interestingly, multiple research groups report 'bubbles' or 'pockets' of oil-estradiol solution forming below the skin following subcutaneous injections of estradiol, and they observe that these 'bubbles' or 'pockets' often take weeks to disappear - we also observed this in our study when using sesame oil as a vehicle, but less apparent with corn oil22,32. Therefore, to minimize signs of inflammation under the skin at the site of estradiol injections, we prefer that corn oil rather than sesame oil be used as a vehicle for administration of the hormone. We also detail in this methodology the use of a topical antiseptic at the site of injection immediately after estradiol is administered subcutaneously. In experiments conducted in the study here, this appeared to mitigate irritation to the surface of the skin of the mice following injection, thus improving their overall wellbeing. The use of slow-release estrogen pellet implantation to facilitate the growth of estrogen-dependent tumours has been reported by some groups20,33. To limit competition between circulating estradiol and 18F-FES for receptor binding, the pellet is removed 3 days prior to imaging. It should be considered however, that long-term in vivo exposure to estrogen can occupy ERα, which can reduce binding opportunity of the 18F-FES probe34. The peroral route is another method that could have been employed for the intermittent delivery of estradiol35. This method can successfully deliver estradiol through nut butter mixtures and although the technique is non-invasive, the practicality of this method is questionable as it can be a laborious process and especially difficult if large cohorts of mice are used35.
Immunohistochemistry was used as a tool to verify target expression in representative tumors from mice that were selected for PET imaging. The expression of ERα was comparable in MCF-7 tumors grown either in the shoulder or the inguinal mammary fat pad (Figure 5). This observation suggests that lower uptake of 18F-FES by IMF tumors (0.83-1.88 %ID/g, n = 4) compared to shoulder tumors (1.7-3.9 %ID/g, n = 9) is not due to the differential expression of ERα at the two sites. This reiterates the idea that the intestinal excretion of 18F-FES interferes with accurately quantifying the uptake of the probe by IMF tumors using ROI analysis.
Multiple factors can influence the uptake of 18F-FES by ERα positive tumors, one such being the specific activity (the measurement of radioactivity per mass of cold and radioactive pharmaceutical) of the radiotracer36,37. If the specific activity of the 18F-FES preparation is low, higher amounts of 'unlabeled' or cold FES could affect receptor occupancy, limiting the amount of available ERα binding sites for 'labeled' FES. In this study, the 18F-FES used was synthesized and reformulated to clinical grade prior to preclinical application23. Furthermore, it is important to acknowledge that the size of the tumors in this study was relatively small. It is possible that some of the tumors that grew were at different stages of vascular development. This is emphasized by the standard error of the mean (SEM) in the tumor growth curves (Figure 2), exemplifying that not all tumors in each cohort grew to reach 100 mm3. To circumvent this issue, we intend to inject a larger number of cells in future experiments to increase the success rate of establishing tumors.
18F-FES PET imaging is undoubtedly a powerful tool that can help inform appropriate treatment options for patients, in particular those that harbor breast tumors that are ERα positive38. Where biopsies of breast metastases are not possible, 18F-FES PET could be used to detect ERα expression in such lesions non-invasively. 18F-FES PET can also be used to detect the loss/gain of ERα expression. For example, the loss of ERα expression in response to fulvestrant, an ERα degrader, has previously been shown to be concordant with the magnitude of reduction in 18F-FES uptake39. In addition, we are currently in the process of imaging the possible gain of ERα expression in breast cancer models that were previously void of the receptor40. From our findings, we demonstrate that one location in which ERα expressing tumors may be detected by 18F-FES uptake is those that are established within the shoulder of ovariectomized mice.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Breast Cancer Foundation (IIRS-22-071). We acknowledge the Operational Infrastructure Support program of the Victorian State Government. This research was also undertaken using the Solid Target Laboratory, an ANSTO-Austin-LICR Partnership, also supported by the National Imaging Facility and the Victorian Government. The authors acknowledge the scientific and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the La Trobe-ONJCRI node, Olivia Newton-John Cancer Research Institute (ONJCRI). Figures 1 and 3 have been made with BioRender.
Materials
| Name | Company | Catalog Number | Comments |
| 2.5% Trypsin (10x) | Gibco | 15090-046 | |
| 27 G x 13 mm 0.5 mL insulin syringe | Terumo | SS*05M2713KA | For cell injections |
| 29 G x 13 mm 0.5 mL insulin syringe | Terumo | SS*05M2913KA | For estradiol injections |
| 30% H2O2 | Chem-Supply | HA154 | Diluted to a 3% working solution with distilled water |
| Corn oil | Sigma | C8267 | |
| DAB Substrate Kit | Abcam | ab64238 | |
| Dako anti-rabbit-HRP, 110 mL | Aligent-Dako | K4003 | Secondary antibody used for IHC |
| DMEM/F-12 Medium | Gibco | 11320033 | |
| Dose calibrator | Capintec | 5130-3216 | |
| Estradiol | Sigma | E2758 | |
| Estrogen Receptor α (D8H8) Rabbit mAb | Cell Signalling Technology | #8644 | Primary antibody used for IHC |
| FBS | Bovogen | SFBS | |
| Heat element (Infra Red Lamp) | Amcal | 12400 | For tail vein dilation |
| Matrigel | Corning | 356225 | |
| MultiCell 4 Channel Monitoring kit for triple- or quadruple-mouse imaging chamber | Mediso | PR-MC900200 | For monitoring of mouse respiration |
| NanoScan PET/MRI 3T System | Mediso | PR-RD000000 | For PET/MRI acquistion |
| PBS (1x) | Gibco | 14190-144 | |
| TBST | ThermoFisher | #28360 | Wash buffer for IHC |
| Three mice imaging chamber | Mediso | PR-MC407300 | For PET/MRI acquistion |
References
- Perou, C. M., et al. Molecular portraits of human breast tumours. Nature. 406 (6797), 747-752 (2000).
- Ignatov, A., Eggemann, H., Burger, E., Ignatov, T. Patterns of breast cancer relapse in accordance to biological subtype. J Cancer Res Clin Oncol. 144 (7), 1347-1355 (2018).
- Siegel, R. L., Giaquinto, A. N., Jemal, A. Cancer statistics. CA Cancer J Clin. 74 (1), 12-49 (2024).
- Gnant, M., Turner, N. C., Hernando, C. Managing a long and winding road: Estrogen receptor-positive breast cancer. Am Soc Clin Oncol Educ Book. 43, e390922 (2023).
- Thomsen, C., Nielsen, S., Nielsen, B. S., Pedersen, S. H., Vyberg, M. Estrogen receptor-α quantification in breast cancer: Concordance between immunohistochemical assays and mRNA-in situ hybridization for ESR1 gene. Appl Immunohistochem Mol Morphol. 28 (5), 347-353 (2020).
- Shafi, S., et al. Integrating and validating automated digital imaging analysis of estrogen receptor immunohistochemistry in a fully digital workflow for clinical use. J Pathol Inform. 13, 100122 (2022).
- Harvey, J. M., Clark, G. M., Osborne, C. K., Allred, D. C. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 17 (5), 1474-1474 (1999).
- Caruana, D., Wei, W., Martinez-Morilla, S., Rimm, D. L., Reisenbichler, E. S. Association between low estrogen receptor positive breast cancer and staining performance. NPJ Breast Cancer. 6 (1), 5 (2020).
- Goldstein, N. S., Ferkowicz, M., Odish, E., Mani, A., Hastah, F. Minimum formalin fixation time for consistent estrogen receptor immunohistochemical staining of invasive breast carcinoma. Am J Clin Pathol. 120 (1), 86-92 (2003).
- Hammond, M. E. H., et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 134 (6), 907-922 (2010).
- Allison, K. H., et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 38 (12), 1346-1366 (2020).
- Amir, E., et al. Tissue confirmation of disease recurrence in breast cancer patients: Pooled analysis of multi-centre, multi-disciplinary prospective studies. Cancer Treat Rev. 38 (6), 708-714 (2012).
- Cardoso, F., Senkus-Konefka, E., Fallowfield, L., Costa, A., Castiglione, M. Locally recurrent or metastatic breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 21 (Suppl 5), v15-v19 (2010).
- Cardoso, F., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol. 31 (12), 1623-1649 (2020).
- James, W. F., et al. Recommendations on the use of 18F-FDG PET in oncology. J Nucl Med. 49 (3), 480-508 (2008).
- Pedersen, M. A., et al. Dynamic whole-body [18F]FES PET/CT increases lesion visibility in patients with metastatic breast cancer. EJNMMI Res. 14 (1), 24 (2024).
- Grabher, B. J. 18F-FES whole-body imaging protocol for evaluating tumor estrogen receptor status in patients with recurrent or metastatic breast cancer. J Nucl Med Technol. 51 (3), 188-193 (2023).
- Van Kruchten, M., et al. PET imaging of estrogen receptors as a diagnostic tool for breast cancer patients presenting with a clinical dilemma. J Nucl Med. 53 (2), 182-190 (2012).
- Ulaner, G. A., et al. Summary: Appropriate use criteria for estrogen receptor-targeted pet imaging with 16α-18F-fluoro-17β-fluoroestradiol. J Nucl Med. 64 (3), 351-354 (2023).
- He, S., Wang, M., Zhang, Y., Luo, J., Zhang, Y. Monitoring the early response of fulvestrant plus tanshinone IIA combination therapy to estrogen receptor-positive breast cancer by longitudinal 18F-FES PET/CT. Contrast Media Mol Imaging. 2019 (1), 2374565 (2019).
- Joseph, J. D., et al. The selective estrogen receptor downregulator GDC-0810 is efficacious in diverse models of ER+ breast cancer. eLife. 5, e15828 (2016).
- Alsina-Sanchis, E., et al. Intraperitoneal oil application causes local inflammation with depletion of resident peritoneal macrophages. Mol Cancer Res. 19 (2), 288-300 (2021).
- Sluka, P., et al. Characterization of an estrogen receptor α-selective 18F-estradiol PET tracer. World J Nucl Med. 23 (03), 153-160 (2024).
- Katzenellenbogen, J. A. The quest for improving the management of breast cancer by functional imaging: The discovery and development of 16α-[18f]fluoroestradiol (fes), a pet radiotracer for the estrogen receptor, a historical review. Nucl Med Biol. 92, 24-37 (2021).
- Boers, J., et al. Image quality and interpretation of [18F]-FES-PET: Is there any effect of food intake. Diagnostics (Basel). 10 (10), 756 (2020).
- Caceres, S., et al. Tumor growth progression in ectopic and orthotopic xenografts from inflammatory breast cancer cell lines. Vet Sci. 8 (9), 194 (2021).
- Kumar, M., Salem, K., Jeffery, J. J., Fowler, A. M. PET Imaging of Estrogen Receptors Using 18F-Based Radioligands. Estrogen Receptors. , (2022).
- Zhang, W., et al. Comparative study of subcutaneous and orthotopic mouse models of prostate cancer: Vascular perfusion, vasculature density, hypoxic burden and BB2r-targeting efficacy. Sci Rep. 9 (1), 11117 (2019).
- Lee, A. V., Oesterreich, S., Davidson, N. E. MCF-7 cells-changing the course of breast cancer research and care for 45 years. J Natl Cancer Inst. 107 (7), djv073 (2015).
- Pedram, H., et al. 18F-fluoroestradiol PET guides dosing of selective estrogen receptor degraders. J Nucl Med. 55 (supplement 1), 11 (2014).
- Ingberg, E., Theodorsson, A., Theodorsson, E., Strom, J. O. Methods for long-term 17β-estradiol administration to mice. Gen Comp Endocrinol. 175 (1), 188-193 (2012).
- Luengo-Mateos, M., et al. Protocol for ovariectomy and estradiol replacement in mice. STAR Protoc. 5 (1), 102910 (2024).
- Yang, Z., et al. High specific activity is not optimal: 18F-fluoroestradio positron emission tomography-computed tomography results in a breast cancer xenograft. J Labelled Comp Radiopharm. 59 (13), 576-581 (2016).
- Aliaga, A., et al. Breast cancer models to study the expression of estrogen receptors with small animal PET imaging. Nucl Med Biol. 31 (6), 761-770 (2004).
- Ström, J. O., Theodorsson, A., Ingberg, E., Isaksson, I. -. M., Theodorsson, E. Ovariectomy and 17β-estradiol replacement in rats and mice: A visual demonstration. J Vis Exp. 64, e4013 (2012).
- Pisaneschi, F., et al. Automated, resin-based method to enhance the specific activity of fluorine-18 clicked PET radiotracers. Bioconjug Chem. 28 (2), 583-589 (2017).
- Johannes, N., et al. Variation of specific activities of 68Ga-aquibeprin and 68Ga-avebetrin enables selective PET imaging of different expression levels of integrins α5β1 and αvβ3. J Nucl Med. 57 (10), 1618-1624 (2016).
- Liu, C., Ma, G., Xu, X., Song, S., Yang, Z. Can 18F-FES PET improve the evaluation of 18F-FDG PET in patients with metastatic invasive lobular carcinoma. Clin Nucl Med. 49 (4), 301-307 (2024).
- Heidari, P., et al. Pharmacodynamic imaging guides dosing of a selective estrogen receptor degrader. Clin Cancer Res. 21 (6), 1340-1347 (2015).
- Roswall, P., et al. Microenvironmental control of breast cancer subtype elicited through paracrine platelet-derived growth factor-CC signaling. Nat Med. 24 (4), 463-473 (2018).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved