Method Article
Hyperpolarized 129Xe Lung MRI and Spectroscopy in Mechanically Ventilated Mice
In This Article
Summary
Hyperpolarized xenon MRI can quantify regional lung microstructure (air-space dimensions) and physiology (ventilation and gas exchange) in translational research and clinical care. Although challenging, it can provide comparable pulmonary insights in preclinical studies. This protocol describes the infrastructure and procedures needed to perform routine xenon lung MRI in mice.
Abstract
Hyperpolarized (HP) xenon-129 (129Xe) is an inhaled magnetic resonance imaging (MRI) contrast agent with unique spectral and physical properties that can be exploited to quantify pulmonary physiology, including ventilation, restricted diffusion (alveolar-airspace size), and gas exchange. In humans, it has been used to evaluate disease severity and progression in a variety of pulmonary disorders and is approved for clinical use in the United States and United Kingdom. Beyond its clinical applications, the ability of 129Xe MRI to noninvasively assess pulmonary pathophysiology and provide spatially resolved information is valuable for preclinical research. Among animal models, mice are the most widely used due to the accessibility of genetically modified disease models. Here, 129Xe MRI is promising as a minimally invasive, radiation-free, and sensitive technique to longitudinally monitor lung disease progression and therapy response (e.g., in drug discovery). This technique can extend to preclinical applications by incorporating an MRI-triggered, free-breathing apparatus or mechanical ventilator to deliver gas. Here, we describe the steps and provide checklists to ensure robust data collection and analysis, including creating a thermally polarized xenon gas phantom for quality control, optimizing polarization, animal handling (sedation, intubation, ventilation, and care for mice), and protocols for ventilation, restricted diffusion, and gas exchange data. While preclinical 129Xe MRI can be applied in various animal models (e.g., rats, pigs, sheep), this protocol focuses on mice due to the challenges posed by their small anatomy, which are balanced by their affordability and the availability of many disease models.
Introduction
While pulmonary disorders remain the leading causes of global morbidity and mortality1, the last decade has seen dramatic improvements in patient outcomes. These improvements are driven in part by two factors. Firstly, Phase III clinical trials now prioritize changes in lung function as endpoints rather than mortality, accelerating drug trials2,3,4,5. Secondly, advancements in improved animal models have provided insights into disease mechanisms and aided therapy development6,7. Mouse models are often favored for translational research because they offer physiological parallels to humans, affordability, and rapid disease development. Genetic engineering has expanded the range and quality of available models, with the International Mouse Strain Resource now boasting over 32,000 mouse strains8, compared to only 4,218 rat strains (Rat Genome Database9). These models have opened new avenues for investigating mechanistic drivers and therapy responses for a range of lung diseases, including chronic obstructive pulmonary disease (COPD)10, cystic fibrosis (CF)11, pulmonary fibrosis12,13, pulmonary hypertension14,15, and asthma16.
Unfortunately, lung research involving mice is limited by the techniques available to quantify disease burden. Studies often rely on terminal procedures that 1) provide whole-lung information (biochemical assays) or localized information (histology) and 2) demand cross-sectional designs and large sample sizes. Thus, they capture neither spatial nor temporal disease dynamics. In contrast, non-invasive, three-dimensional imaging can assess structure, molecular processes, and function in the lungs over time.
Lung structure (e.g., airway abnormalities and interstitial fibrosis) can be visualized with ultra-short echo-time (UTE) MRI and microcomputed tomography (µCT) at high resolution. Functional and mechanistic information (e.g., ventilation, perfusion, tumor metabolism, and inflammatory processes) can be obtained with exogenous contrast agents (e.g., xenon-enhanced CT and oxygen-enhanced UTE) and ionizing nuclear medicine approaches (i.e., positron emission tomography [PET], and single-photon emission computed tomography [SPECT]). However, functional imaging is challenging due to the modest contrast-to-noise (particularly for oxygen-enhanced UTE at the high magnetic field strengths used for preclinical MRI, where T1 is lengthened) available without employing ionizing modalities with higher than normal levels of radiation. While imaging with these modalities is well tolerated in animal models using conventional doses, cumulative radiation may confound results in studies on immunology, inflammation, and lung cancer17. However, hyperpolarized (HP) xenon-129 (129Xe) magnetic resonance imaging (MRI) provides minimally invasive, non-irradiating, and highly sensitive structural and functional information. While this technique has been employed in preclinical research to characterize conditions including emphysema18,19, fibrosis20, lung cancer21, COPD22, and radiation-induced lung injury23 at single or multiple time points, it remains underutilized in the preclinical setting.
To enable routine, preclinical 129Xe MRI, several prerequisites are required, including institutional regulatory support, a hyperpolarization device, a 129Xe-tuned radiofrequency (RF) coil, and a multi-nuclear-capable scanner. Although advanced applications24,25,26,27,28,29,30,31,32,33 require vendor-specific pulse programming that is outside of the scope of this protocol, basic applications can be achieved with modest software modifications. Therefore, we focus on quality control, magnetization handling, data collection, and animal handling procedures — including mechanical ventilation — that are unique to preclinical 129Xe MRI (Figure 1).
To date, small animal 129Xe imaging has employed three MR-safe gas delivery approaches, each with advantages and disadvantages: free-breathing, piston-driven, and pressure-drop. Free-breathing allows spontaneous inhalation without risk of injury from intubation or tracheostomy but consumes significantly more HP gas and can introduce motion artifacts34,35. Commercial piston-driven devices are self-calibrating and easy to use out-of-the-box but may be prohibitively expensive36. The pressure-drop-based approach used here is well described in the literature, modular, customizable, and run by open-source code37,38,39,40. Furthermore, it is cost-effective, typically totaling less than $10k and a few weeks of dedicated build time. The pressure-drop ventilator delivers 129Xe from a dose bag within a pressurized cannister while monitoring the airway pressure of an intubated mouse.

Figure 1: Overview of the protocol to collect routine xenon-129 (129Xe) magnetic resonance imaging (MRI) in mice. (A) Steps for initial setup. (Note: scanner programming is unique to each vendor and not described in this protocol). (B) Steps to collect daily quality assurance (QA) and animal data. (C) Steps for successful experiment conclusion and data analysis. Please click here to view a larger version of this figure.
Here, we collect and analyze the three common classes of 129Xe MRI data: ventilation, diffusion-weighted imaging (alveolar-airspace size), and gas exchange. Ventilation images depict the distribution of inhaled 129Xe gas. Regions of the lungs with reduced airflow appear dark in HP gas images, and pathology is quantified by the volume of defective ventilation. In humans, the ventilation defect percentage (VDP) has shown strong repeatability41,42 and high sensitivity to lung obstruction in diseases like COPD43,44,45 and asthma46,47.
The restricted diffusion of the 129Xe atoms in the airspace can be measured via the apparent diffusion coefficient (ADC) and serves as a surrogate for air-space size. The ADC is calculated by acquiring a baseline image (b0) without diffusion weighting and one or more images acquired in the presence of bipolar gradient-induced diffusion weighting (bN). An elevated ADC reflects an increase in airspace size due to aging or emphysematous remodeling18,48. Further, using multiple b-value images (≥4) allows more detailed morphometric information (e.g., mean linear intercept) to be calculated49,50.
Gas exchange can be characterized due to 1) the solubility of 129Xe in the capillary membrane tissue, plasma, and RBCs (red blood cells) and 2) the >200 ppm downfield chemical shift of 129Xe when dissolved in these compartments. Both spectroscopic and imaging data provide insight into cardiopulmonary diseases (e.g., pulmonary hypertension and left heart failure51,52,53). While many species (humans, canines, and rats) display unique spectral peaks originating from each compartment, mice lack a unique RBC signal due to differences in hemoglobin-xenon binding site interactions. Instead, all dissolved components are combined into a single signal in mice54. However, it is possible to observe a distinct RBC resonance in transgenic mice expressing human hemoglobin, such as those used in models of sickle cell disease54. Overall, dissolved 129Xe spectroscopy and imaging provide unique insights into cardiopulmonary pathophysiology in mice55,56.
Before attempting this protocol, it is necessary to understand background information about the MRI scanner, mechanical ventilation, and mouse handling techniques required for mouse studies. Prior to initiating animal studies, all procedures must be approved by the local Institutional Animal Care and Use Committee (IACUC)57. Because the total magnetic moment available in the mouse lung is intrinsically low (i.e., tidal volume ~250 µL), voxel size must be 1000-fold smaller than in humans to achieve anatomically equivalent resolution. The murine breathing rate is also exceedingly rapid (>100 breaths/minute). As such, the single-breath-hold procedures typically used for human imaging are not feasible. Instead, only a few RF excitations can be applied within each breath, so 129Xe images must be encoded over tens to hundreds of breaths. Pulse programming may be required to permit external triggering of acquisitions and to properly loop slices, phase encodes, and/or diffusion-weighted images while balancing signal-to-noise ratio (SNR), resolution, and scan duration. Here, the ventilator outputs a transistor-transistor logic (TTL) pulse once per breath to trigger data acquisition (Figure 2).
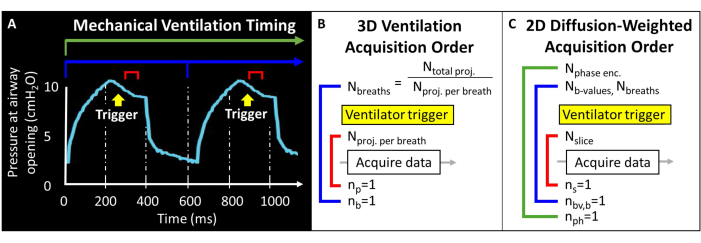
Figure 2: Representative mechanical ventilation and data acquisition timing. (A) User-controlled ventilation can trigger data acquisition at end-inspiration, during the breath hold, or at end-expiration. (B) For this 3D radial ventilation sequence, the user defines the total number of projections acquired and the number of projections per breath. (C) For a slice-selective, 2D diffusion-weighted image, the user defines the order of slices, b-value images, and phase encodes. Please click here to view a larger version of this figure.
To enable reliable ventilation and 129Xe delivery, robust sedation and intubation procedures are required. For each study, the downstream effects of each anesthetic must be considered - including changes to minute ventilation, heart rate (HR), and blood pressure58,59,60,61,62,63,64,65,66. While a variety of sedatives have been used for preclinical HP gas MRI, we employ a mixture of ketamine, xylazine, and acepromazine, due to its availability, cost-effectiveness, reliability, and duration67,68. Once sedated, animals must be intubated for effective mechanical ventilation. Intubation of mice is difficult due to the small size of their anatomy, and thus, it is important to train thoroughly in this technique. We encourage investigators to review published video protocols69,70. Because most commercial intubation cannulas contain stainless steel, we introduce a technique to craft metal-free (i.e., MRI and HP-gas compatible), wedge-shaped cannulas that can be customized to match airway diameter to create an airtight seal with the mouse tracheal wall.
Because 129Xe images are collected over many breaths, ventilator settings are critical. Protective ventilation strategies must be carefully considered to prevent lung injury71,72,73,74. In particular, the use of low tidal volume (TV), moderate positive end-expiratory pressure (PEEP), and alveolar recruitment maneuvers (RMs) reduce the risk of ventilator-induced lung injury in human patients and animal models75,76,77,78,79,80,81. Here, we recommend a simple technique that is compatible with pressure-drop 129Xe mechanical ventilation that is protective and provides sufficient 129Xe image SNR. Specifically, we apply PEEP by adding a commercial PEEP valve to the exhale line of the ventilator. To perform RMs, the exhalation line must be closed so that the animal receives multiple inhalations without exhalation until a target pressure and duration have been reached.
Throughout, we provide general ventilation settings, but it is advised to review the literature to address specific study goals82,83. In addition to monitoring the peak inspiratory pressure during mechanical ventilation, it is important to monitor the animal's temperature, which can be done using standard mouse temperature monitoring methods. While not required for imaging, monitoring heart rate via electrocardiogram (ECG) can be advantageous; ECG can indicate if an animal is waking from sedation, overdosed, or distressed, allowing the researcher to intervene.
The protocol we describe is designed to collect 129Xe 3D radial ventilation data61, 2D GRE diffusion-weighted data76, and dynamic pulse-acquire spectroscopy gas exchange data. This protocol aims to bridge the gap between preclinical research in small animal models and the potential for 129Xe MRI to advance our understanding of pulmonary disorders.
Protocol
All methods described here were approved by the Institutional Animal Care and Use Committee (IACUC) of Cincinnati Children's Hospital Medical Center.
1. Initial site preparation
- Create and test a thermally polarized 129Xe gas phantom (Figure 3).
- Obtain a borosilicate glass vessel (~60 mL), a plunger valve with a front-sealing O-ring, and a ground borosilicate glass stem, all rated up to 150 psig. Ensure there are no magnetic parts. Attach a compression fitting to the glass stem. Tighten to produce a gas-tight seal.
- Connect the vessel to a vacuum pump and oxygen reservoir according to Figure 3A. Vacuum vessel to less than 100 mTorr absolute pressure.
- Fill the vessel with oxygen to the pressure of 1.5 atm to reduce the 129Xe T1 from > 30 min to ~2 s (at 7 T magnetic field strength; For a 9.4 T scanner, use 1.6 atm oxygen). Seal vessel.
NOTE: For higher field strengths, somewhat higher oxygen partial pressure will be required to achieve T1≤ 2 s84. - Fill a gas-impermeable reservoir with 400 mL of isotopically enriched xenon (85% 129Xe).
NOTE: Natural abundance xenon (26% 129Xe) can also be used, but signal taveraging will need to be increased to mitigate the ~3-fold reduction in SNR. - Connect the vessel to the reservoir of 129Xe. Vacuum tubing to less than 100 mTorr absolute pressure.
- Fill an open-mouth, benchtop liquid N2 Dewar to ~90%. Submerge the bottom of the vessel (~5 cm) in liquid nitrogen to condense O2 and create a vacuum (Figure 3B). While submerged, open the valve to allow 129Xe from the reservoir to flow into the vessel (Figure 3C).
- Seal the plunger valve by slowly pulling the stem until the inflow hole in the plunger is pulled past the O-ring. Immediately after the hole has passed the O-ring, hand tighten to seal the vessel. Remove the vessel from liquid nitrogen and allow it to thaw.
NOTE: Once thawed, the vessel will pressurize to ~4.5 atm (2 atm O2 + 2.5 atm 129Xe). - Protect glassware (e.g., insert vessel in padded polyvinyl chloride (PVC) pipe container, Figure 3D).
NOTE: If properly maintained, the phantom can maintain pressure for a decade or longer. - Measure the T1 of the phantom (e.g., using a spectroscopic inversion recovery sequence). Confirm T1 < 2 s for 7 T scanners. Track signal and T1 over time for quality assurance (QA).

Figure 3: Creation of a thermally polarized 129Xe gas phantom guided by the protocol detailed in Step 1.1. The O2 and 129Xe partial pressures can be altered to customize the T1 to yield appropriate 129Xe T1 times and signal strength at a given field strength84. Please click here to view a larger version of this figure.
- Perform quality assurance with the thermally polarized gas phantom (Table 1 and Figure 4).
- Place the 129Xe coil at the isocenter of the magnet and center the 129Xe gas phantom within the coil. In a single pulse-and-acquire sequence ("single pulse"), set the working frequency to match the approximate 129Xe gas frequency in the phantom (~83.07 MHz at 7T).
- Center the acquisition and excitation frequencies to the 129Xe Larmor frequency, and use this frequency for all phantom calibration and quality control 129Xe scans. See Table 1 for experimental parameters for all QA scans. Confirm that the phantom is centered with a phantom localizer (Figure 4A).
- Perform nutation experiment to calibrate flip angle: Assuming SNR is sufficient, use single RF pulses with repetition time (TR) spacing > 5 x T1. For each acquisition, incrementally increase RF power until the signal nulls and begins to invert. The standard used here is: number of pulses = 65; TR = 10 s; pulse duration = 125 µs; RF power = 1-13.8 W, incremented by 0.2 W
- Fourier transform and phase the first spectrum (i.e., the spectrum acquired with the lowest RF power). Apply the same phasing for all spectra. Plot the real spectra as a function of the RF pulse power (Figure 4B).
- The power that produces a null peak (i.e., minimum peak height) corresponds to the 180° flip angle. Achieve a 90˚ flip angle by using the same power at half the pulse length needed to produce the 180˚ flip angle. Assuming the scanner software allows, set this 90° reference power and pulse length for subsequent flip angle scaling.
- Use a single pulse to shim by minimizing the full width at half maximum of the 129Xe spectrum (TR ~ 1 s). If necessary, recenter the frequency after shimming. Record the full-width half max.
- Run the 129Xe QA scan (Table 1 and Figure 4C). Record QA data: SNR, mean phantom signal, and standard deviation of the noise.
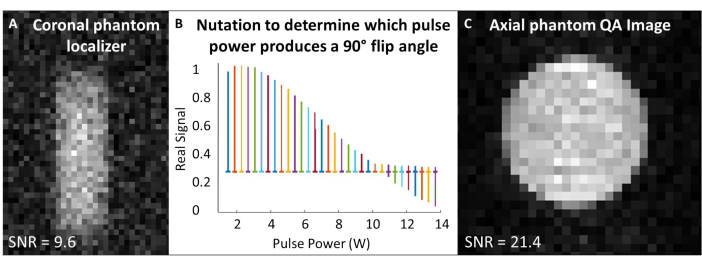
Figure 4: Pre-scan quality assurance. (A) A low-resolution 2D GRE coronal phantom localizer ensures the phantom is centered in the magnet. (B) A nutation experiment to set a 90˚ pulse shows a null peak at the 180° pulse. (C) After localizing and calibrating the flip angle, acquire a higher resolution 2D GRE QA image. Please click here to view a larger version of this figure.
| Protocol Short-Hand Name | Sequence Description | TR (ms) | TE (ms) | Averages / Repetitions | Flip Angle (˚) | Matrix Size or Npts | FOV (mm2) | RF BW (kHz) | Slice / Slab Thickness (mm) | Scan Duration |
| Single pulse | Pulse acquire | 1000 | 1 / 1 | 60 | 2048 | 10 | 1 s | |||
| Phantom localizer | 2D GRE | 200 | 3.7 | 20 / 1 | 48 | 60 × 32 | 120 × 48 | 3 | 60 | 2 min |
| Flip angle calibration | Pulse acquire | 7000 | 1 / 65 | 20 | 2048 | 5.12 | 7.5 min | |||
| 129Xe QA | 2D GRE | 5000 | 3.3 | 8 / 1 | 90 | 322 | 322 | 3 | 40 | 21 min |
Table 1: Phantom calibration quality assurance sequence parameters. TR = repetition time, TE = echo time, Npts = number of points, FOV = field of view, BW = bandwidth. Please click here to download this Table.
- Plan polarization (Figure 5A, B).
- Select polarized 129Xe volume and accumulation time: 400 mL in 15 min is optimal for this protocol (Figure 5) but can easily be adjusted for other applications and equipment.
- Assuming a known dead volume within the hyperpolarizer (e.g., Vdead vol = 80 mL), calculate the flow rate (FXe in SLM) for a dispensed volume Vdesired, 129Xe gas fraction f, and accumulation time tacc:
 (1)
(1)
NOTE: While longer production times will typically yield higher polarization, they may not be practical for in vivo imaging. Use an appropriate model for the hyperpolarizer85,86,87,88 to determine a flow rate that will balance production time and polarization. Here, the model of J. W. Plummer et al.89 (Figure 5A) was used. This applies to continuous-flow polarizers and is not applicable to stopped-flow hyperpolarizers90. - Polarize gas according to these parameters, measure the polarization with a commercial or homebuilt device and compare it to the predicted polarization for QA.
- Measure the polarization loss during transportation. If polarization decreases by a sufficiently large amount (e.g., >10%), build a magnetic carrying case to protect polarization during transportation. See Supplementary File 1: Managing polarization during transportation and Supplementary Figure 1.
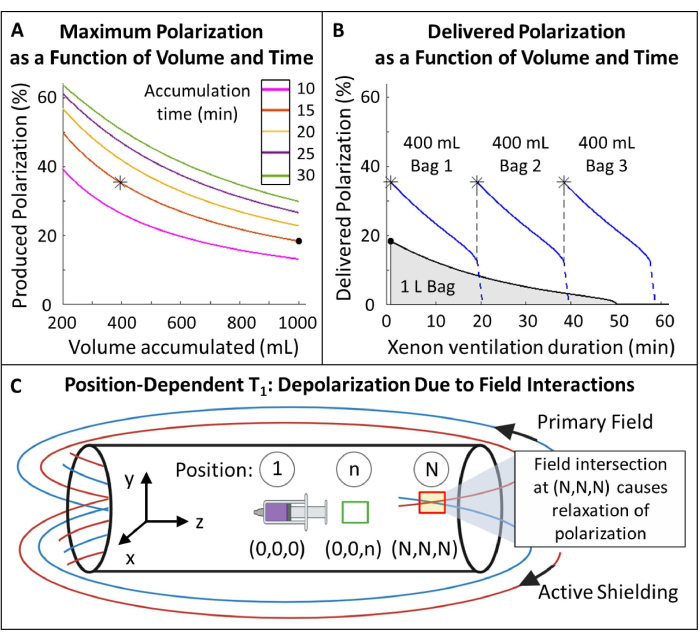
Figure 5: Polarization management. (A) Polarization and produced volume are a function of accumulation time and flow rate. A 400 mL bag of gas provides high initial polarization (~35%) over 20 min. While using 1 L of gas might seem attractive, it will have a lower initial polarization (~20%). (B) After ~15 minutes of ventilation, a 1 liter batch of HP 129Xe would deplete to <10% polarization while 600 mL of gas remained116. Thus, using multiple 400 mL bags of 129Xe maintains higher average delivered polarization. C) Locations where the primary field and active shielding field intersect (red box at position (N,N,N)) can cause rapid relaxation of HP 129Xe. Characterizing the magnet's fringe field helps identify safe zones where reservoirs of HP 129Xe can be placed without rapid relaxation (green box at position (0,0,n)). Please click here to view a larger version of this figure.
Supplementary File 1: Managing polarization during transportation. Please click here to download this File.
- Measure the position-dependent T1 of HP 129Xe within the fringe field (Figure 5C).
- Create reference points with known distances and positions relative to the magnet isocenter along the X, Y, and Z dimensions. Label isocenter and label the other positions n through N. The number of positions to investigate will depend on the space available.
- Hyperpolarize a small volume of 129Xe (~250 mL) and transport it to the MRI control room. Fill a large syringe (50-100 mL) with 129Xe and place it at the isocenter within the magnet (Position 1 in Figure 5C). Play out a ~1˚ flip angle pulse to measure the signal.
- Leave the syringe in position for ~10 min, then acquire another spectrum. Remeasure the signal every 10 min until the signal has decayed at least 1 T1 (i.e., the signal has decayed to ~1/3rd its initial value).
- Start a new T1 experiment with a new syringe of 129Xe by repeating Step 1.4.2.
- Move the syringe to a new position (e.g., Position n in Figure 5C) and leave it there for 10 min. Return the syringe to the isocenter to acquire an additional ~1˚ flip angle spectrum.
- Repeat this process: move the syringe to Position n, wait for 10 min, put it back to the isocenter, and remeasure the signal until it has decayed at least 1 T1.
- Repeat Steps 1.4.4 - 1.4.6 for the remaining reference positions covering the X, Y, and Z directions.
- The initial signal (S0) will decay monoexponentially over n RF pulses with a θ flip angle. Fit the signal (S) as a function of time (t) in the fringe field to calculate the T1 in each position:
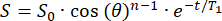 (2)
(2) - Identify a position with sufficient T1 (>20 min) for 129Xe reservoir placement.
- Create metal-free intubation cannulas (Figure 6).
- Obtain two veinous indwelling polytetrafluoroethylene (PTFE) catheters with Luer connectors. Discard needles into a sharps disposal container.
NOTE: For mice >25 g, use 18 G and 20 G catheters. For smaller animals, use 20 G and 22 G catheters. - Cut the Luer connector off the catheters. Feed the smaller catheter into the upper end of the larger catheter to create a more sharply tapered and longer cannula. Cut the composite cannula to ~4.6 cm with a beveled end, not including the Luer base (Figure 6A, B).
- Cut the wider end off of a 200 µL pipette tip to a length of ~2.6 cm (Figure 6C).
- Coat the inside of the pipette tip with general mold release lubricant. Use another pipette tip inserted inside to thinly distribute the lubricant. Fill the pipette tip with acetoxy silicone vulcanizing paste epoxy (Figure 6D, E).
- Plug the cannula with 22 G wire extending out of both ends. Feed the cannula tube through silicone in the pipette tip. Extend the tube ~7 mm past the end of the pipette tip (Figure 6F, G).
- Slide the cannula tube on the wider side of the pipette tip through a plastic male slip Luer connector, gluing the pieces together with the epoxy. Trim tubing that extends past the Luer connector (Figure 6H).
- Wait for the epoxy to dry (>24 h), then carefully remove the silicone intubation cannula from the pipette tip mold. Remove the wire from the cannula, ensuring the tube has not been occluded (Figure 6I).
- To make a handle for easy intubation, connect tubing (1/16" or 1/8") to a female Luer connector. When ready for intubation, connect this female Luer connector to the male Luer intubation cannula. This piece can be easily detached post-intubation (Figure 6J).
- Sanitize before each use on animals: wipe the outside of the cannula with 70% alcohol. Wipe 20 G wire with sanitizer then feed the wire through the cannula to sanitize the inside and ensure no blockages.
- Obtain two veinous indwelling polytetrafluoroethylene (PTFE) catheters with Luer connectors. Discard needles into a sharps disposal container.

Figure 6: Creating MRI and HP 129Xe compatible mouse intubation cannulas. These cannulas are constructed of venous catheters, pipette tips, and silicon sealant, as described in Step 1.5. Please click here to view a larger version of this figure.
2. Daily data collection
NOTE: See Supplementary File 2: Preclinical scan QA checklist.
Supplementary File 2: Preclinical scan QA checklist. Please click here to download this File.
- Complete daily scanner quality control and setup ventilator (Figure 4).
- With phantoms, run QA protocols on the scanner (see Table 1 for QA scan parameters and Step 1.2 for daily quality control steps).
- Calibrate Ventilator according to the method of J. Nouls et al.38. See Supplementary File 3: Ventilator calibration, Supplementary Figure 2, and Supplementary Figure 3.
- Set the ventilator settings for imaging at end-inspiration (Table 2). Place the animal bed on the scanner rack and the life support module (i.e., mechanical ventilator parts) on the table next to the scanner.
- Activate the site-specific animal heating system. Set a heater to 35.5 - 40 °C, turn on circulating air, and place the air hose within ~5" of where the animal's head will rest to pre-warm the bore of the scanner.
| Ventilation Setting | Recommendation for HP 129Xe MRI | Notes |
| Tidal volume (TV) | 8–10 mL/kg of ideal body weight | Moderate TV; low TV requires higher BR which may cause motion artifacts in images |
| Positive end-expiratory pressure (PEEP) | 2–6 cmH2O | |
| Breath rate (BR) | 80–120 br/min | |
| Recruitment maneuvers (RMs) | ~35 cmH2O for 6 s every 5 min | |
| Ventilation duration; Position | < 6 h; supine | Supine to better see chest motion |
| Fraction of inspired oxygen (FIO2) | 0.3–0.5 | Prevent hypoxia in anesthetized mice |
| Inspiratory to expiratory ratio (I:E) | 1:2–1:4 | |
| Inspiratory to total cycle duration | 0.2–0.4 | |
| Minute ventilation | ≥0.57 mL·g-1·min-1 | |
| Our standards: | ||
| BR = 80 br/min, inspiration duration = 200 ms, FIO2 = 0.3 | ||
| Imaging at end-inspiration: breath hold = 200 ms, trigger delay = 200 ms after start of inspiration | ||
| Imaging during breath hold: breath hold = 250 ms, trigger delay = 250 ms after start of inspiration | ||
| Imaging at end-expiration: breath hold = 200 ms, trigger delay = 650 ms after start of inspiration | ||
Table 2: Recommended ventilator settings for 129Xe imaging. Parameters can be fine-tuned for specific study objectives and experimental conditions117,118,119,120,121,122,123,124. Please click here to download this Table.
Supplementary File 3: Ventilator calibration. Please click here to download this File.
- Sedate and intubate animal.
- Turn on the incubator to 27.7 °C and/or the electric heating pad to 37.7 °C. Measure and record the body mass of the animal. Calculate sedative dosing based on mass. See Table 3 for a typical dosing regimen.
- Inject the sedative intraperitoneally. Note the time of injection and set the timer for the next dose of sedative.
NOTE: Complete the remaining steps in Section 2 (Daily data collection) as quickly as possible to minimize the time under sedation and the risk of overdose. - Apply eye lubricant to the animal's eyes and place the animal in a cage on the heating pad or within an incubator to prevent hypothermia.
- Confirm the animal is fully sedated by performing a toe pinch test 10-15 min after sedative injection68. Intubate following procedures outlined in Das et al.69.
NOTE: The article by Das et al.69 is accompanied by a comprehensive video demonstration of the technique. The steps are as follows: - Hang the animal supine by the teeth on a slanted board. Use a rodent tongue depressor to pull out the tongue.
- To ensure vocal cords are visible, supply white light via a fiber optic cable within the intubation cannula or a bright light placed on the outside of the throat. Insert the cannula less than 5 mm past the vocal cords.
- Ensure the cannula is in the trachea, not the esophagus, by connecting it to a piece of tubing with a small droplet of water inside. If the water droplet moves in time with the animal's breaths, the positioning is likely correct.
| Agent | Dose | Route | Duration | Comments | |||
| Inhaled Agents | |||||||
| Isoflurane | Induction: 4%–5% Maintenance: 1%– 3% or to effect | Inhaled | During continuous flow | • Requires use of calibrated vaporizer | |||
| Injectable Agents | |||||||
| Recommended: Ketamine + xylazine + acepromazine | 90 + 9 + 3 mg/kg | Intraperitoneal | 20–60 min | • Creates susceptibility to hypothermia | |||
| • For repeated dosing, it is recommended to switch to a ketamine + xylazine mixture to prevent overdose | |||||||
| • Causes shaking as it wears off. For imaging, strictly adhere to dosing schedule | |||||||
| • May cause bradycardia | |||||||
| Ketamine + xylazine | 90 + 9 mg/kg | Intraperitoneal | 20–40 min | • See above (Ketamine + xylazine + acepromazine) | |||
| Pentobarbital | 50 - 70 mg/kg | Intraperitoneal | 20–60 min | • Depresses respiratory rate and motion | |||
| • Expense may be cost prohibitive | |||||||
| • Pharmaceutical grade may not be available | |||||||
| Disclaimer: These are general guidelines. Consult a veterinarian for more information before implementation. | |||||||
Table 3: Common anesthetic formulary for mice. Please click here to download this Table.
- Ventilate animal (Table 2).
- Attach the animal to ventilator via Luer connector on the intubation cannula. Monitor diaphragmatic motion and peak inspiratory pressure (~10-12 cm H2O for a tidal volume of 10 mL/kg of body weight). If the pressure or breathing motion is abnormal, carefully adjust the neck angle and depth of the cannula as needed.
- Ensure the intubation cannula is airtight by performing a recruitment maneuver: prevent exhalation (e.g., with a finger, block the exhale port) such that the animal inhales multiple times without exhaling.
NOTE: If the airway pressure reaches 35 cmH2O peak inspiratory pressure over ~6 s, the airway seal is sufficiently tight. If not, see the discussion for troubleshooting. - Allow normal exhalation to resume. Perform recruitment maneuvers between scans and every ~5 min when not scanning to maintain lung compliance and prevent atelectasis. Connect the PEEP valve to the exhale line. Set PEEP to 4 cmH2O. Watch the peak inspiratory pressure increase by that amount.
- After successful intubation, plan and initialize the production of HP 129Xe around the sedative redose schedule to prevent the animal from waking during a scan. Monitor body temperature throughout the experiment.
- Data acquisition: Collect ventilation images.
NOTE: Data Acquisition Steps 2.4, 2.5, and 2.6 can be done in any order- Set the ventilator according to Table 2 for imaging at end-inspiration.
- Load the following setup protocols: proton animal localizer, single pulse to center on gas frequency in the mouse lungs, and 129Xe animal localizer. See Table 4 for scan parameters.
- Position the animal at the isocenter and confirm that the thoracic cavity is in the center of the field of view with proton localization. If using single-frequency coils, replace the proton coil with a 129Xe-tuned RF coil.
- Record 129Xe polarization and transport it to the MRI scanner. See Supplementary File 4: Xenon polarization QA checklist.
- Place a bag of 129Xe within the ventilator cannister and seal. Connect the canister in line with the ventilator and allow the cannister to pressurize (3 - 6 psig).
- Initiate 129Xe mechanical ventilation. Each time 129Xe ventilation is activated, allow the animal to complete ~5 breaths prior to starting a scan to turn over the functional residual capacity of the lungs.
NOTE: Switch to the N2/O2 mixture between 129Xe scans to conserve hyperpolarized gas. - Using a single pulse, adjust the working frequency to match the in vivo resonance frequency of gaseous 129Xe (~83.07 MHz at 7 T). Copy the frequency to all subsequent gas phase 129Xe scans. Perform 129Xe localization to confirm lungs are at isocenter.
- Load and run the 129Xe radial ventilation sequence. Monitor peak inspiratory pressure.
NOTE: If the 129Xe gas runs out before the protocol is finished, the peak inspiratory pressure will decline rapidly. A 400 mL bag of 129Xe can ventilate a 30 g mouse for ~24 min when ventilated with 70% 129Xe at 80 breaths per minute with a tidal volume of 10 mL/kg of ideal body weight. - When the scan is finished, switch to ventilating with the N2/O2 mixture and remove the empty bag of 129Xe.
- For imaging at end-expiration, change the breath hold duration and trigger delay according to Table 2 and repeat Steps 2.4.2 - 2.4.9.
- Export the raw data from the scanner.
| Protocol Short-Hand Name | Sequence Description | Trigger | TR (ms) | TE (ms) | Repetitions | Flip Angle (˚) | Matrix Size or Npts | FOV (mm2) | RF BW (kHz) | Slice/Slab Thickness (mm) | Scan Duration |
| Single pulse | Pulse acquire (gas phase) | Optional | 1000 | 1 | 60 | 2048 | 10 | 1 s | |||
| Animal localizer | 2D GRE | Yes | 50 | 1.7 | 1 | 60 | 642 | 322 | 3 | 25 | 60 s |
| Radial Ventilation | 3D Multi-echo radial | Yes | 20 | See caption | 1 | 30 | 613 | 223 | 32.05 | 30 | 16 min |
| Dissolved phase single pulse | Pulse acquire (dissolved phase) | No | 80 | 1 | 90 | 512 | 10.35 | 80 ms | |||
| Dissolved phase dynamic spec. | Pulse acquire (dissolved phase) | No | 50 | 1000 | 90 | 512 | 10.5 | 50 s | |||
| Diffusion weighted | 2D GRE | Yes | 12.2 | 8.1 | 4 | 45 | 642 | 322 | 3 | 1.5 | 18 min |
Table 4: In vivo sequence parameters. The 3D multi-echo radial ventilation sequence described previously39 acquires images at 6 echo times. Results are shown for the first echo image (TE = 1.12 ms, Figure 7). Please click here to download this Table.
Supplementary File 4: Xenon polarization QA checklist. Please click here to download this File.
- Data acquisition: Run dynamic dissolved phase spectroscopy.
- Set the ventilator according to Table 2 for imaging during breath hold. Set the BR to 100 breaths/min. Prepare for a new bag of 129Xe as in Steps 2.4.2 - 2.4.5.
- Initiate 129Xe mechanical ventilation. Load and run a single pulse to adjust the working frequency to match the dissolved frequency (~83.084 MHz at 7 T). Copy the working frequency to the dissolved phase dynamic spectroscopy.
NOTE: This will be a single peak in mice with wild-type hemoglobin54. - Load and run the dynamic spectroscopy sequence centered on the dissolved frequency in animal lungs. When the scan is finished, switch to ventilating with the N2/O2 mixture and remove the empty bag of 129Xe. Export the raw data from the scanner.
- Data acquisition: Collect diffusion-weighted images.
- Set the ventilator according to Table 2 for imaging during breath hold. Prepare for a new bag of 129Xe as in Steps 2.4.2 - 2.4.7.
- Load and run the diffusion-weighted sequence. When the scan is finished, switch to ventilating with the N2/O2 mixture and remove the empty bag of 129Xe. Export the raw data from the scanner.
3. Concluding the experiment
- Recover animal.
- Pull the intubation cannula straight out of the mouth of the animal. If the animal does not immediately begin breathing spontaneously, administer light chest compressions. If available, administer a light flow of medical-grade oxygen near the animal's face to support sedation recovery.
- Once the animal is steadily breathing on its own and if the animal was sedated for >2 h, administer 0.5 - 1 mL of normal saline subcutaneously to prevent dehydration.
- Return the animal to a cage by itself. Place the cage in an incubator or on a heating pad.
NOTE: Sedated animals are vulnerable to cannibalism and cannot be placed in a cage with cage mates until fully recovered (i.e., ambulating independently). Sedated animals cannot regulate their body temperature. Use the back of the hand to feel the temperature of the animal every few minutes. - Monitor the animal closely until their righting reflex has returned (i.e., it can independently flip from a supine to a prone position).
- Remove the animal from heat support once it can ambulate independently. Return the animal to a cage with its cage mates.
NOTE: if the animal has no cage mates, it is more susceptible to sedation-induced hypothermia. Provide the animal with extra bedding, and if available, leave the animal in an incubator overnight. - Record the animal's weight once per week for 2 weeks to monitor its health.
NOTE: If the animal sustained an injury to the mouth or esophagus from intubation, it may stop eating. If the animal loses >20% of its initial body weight, consult a veterinarian about euthanasia.
- Analyze ventilation images (Figure 7).
- Load raw data into a programming platform. Download the open-source reconstruction framework for non-Cartesian Images91.
- Reconstruct images according to open-source framework instructions. Normalize the first point on each radial projection39.
- Segment the lung parenchyma in the images, including voxels with low or no 129Xe signal. Do not include large airways. Segment the background noise in the image, excluding lungs, airways, and imaging artifacts.
NOTE: The proton localizer image can aid in determining parenchymal boundaries. - Calculate the SNR using the formula:
 (3)
(3) - Quantify defective ventilation.
NOTE: Various methods have been proposed to quantify impaired ventilation in small animal models. Analysis methods remain an open area of inquiry, but easily implemented approaches include: (i) Semi-quantitative manual segmentation92, (ii) Histogram approach using the signal from the trachea to normalize parenchymal signal47, and (iii) Segmenting the total lung volume (TLV) and defining a signal threshold (e.g., <60% of the whole lung mean) to divide the lungs into defective volume and ventilated lung volume (VV). Quantify VDP93,94 according to:
 (4)
(4)
- Analyze dissolved phase dynamic spectroscopy (Figure 8).
- Load raw data into a programming platform. Perform a fast Fourier transform and phase the spectra (simultaneous manual, zero-order phasing of spectra is sufficient for this application.)
- Calculate standard nuclear magnetic resonance (NMR) data95,96: SNR, full width half max, integrated area, chemical shift, and phase of both peaks.
- From the magnitude data, divide the signal amplitude of the dissolved spectrum by that of the gas spectrum for each repetition to find the dissolved-to-gas ratio over time.
- Analyze diffusion-weighted images (Figure 9).
- Load raw data into a programming platform. Segment the lung parenchyma of the b0 image as in Step 3.2.3. Calculate the SNR for each b-value image.
- Calculate the signal-value-to-noise ratio, SVNR0, by dividing the signal in each voxel of the b0 image by the standard deviation of the image noise. Exclude voxels with SVNR0 < 2.5 times the image noise97.
NOTE: The SVNR0 is an individual voxel metric, while the parenchymal SNR is a whole-lung metric. - Calculate ADC by fitting the signal decay over the b-values (bi) according to Equation 598,99:
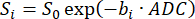 (5)
(5)
Results
Ventilation Images
If animal preparation and ventilation procedures are implemented properly, 3D radial imaging can successfully capture ventilation patterns when data acquisition is performed at either inspiration or expiration (Figure 7). While these images are collected over many breaths, the method described here is similar to the single-breath imaging method used in humans. This is because all lines of k-space are collected at a specific time during the breath cycle (e.g., all at end-inspiration), cumulatively creating an image representative of a single inhalation. In these images, the major airways (trachea and bronchi) are clearly visible, no obvious image artifacts are present, and the lung parenchyma appears fully inflated with high SNR (~15 or higher, depending on initial polarization). While counterintuitive, the images acquired at inspiration (Figure 7A, SNR = 15) display lower SNR than those acquired at expiration (Figure 7B, SNR = 27). This is related to the modest driving pressure and slow flow used when ventilating this mouse. For inspiration images, the HP gas had not yet fully washed into the parenchyma of the lungs, causing the 129Xe (and thus signal) to be concentrated in larger airways. This effect is visually exacerbated by k-space scaling during image reconstruction because the high airway signal dominates the dynamic range of the data. Nonetheless, the analysis should be straightforward if the parenchyma is segmented such that the large airways are omitted.
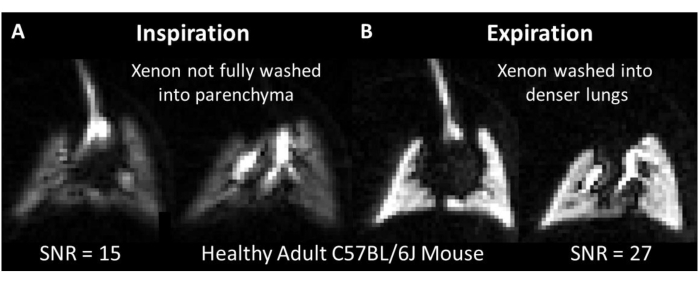
Figure 7: Representative 3D radial ventilation images in an adult C57BL/6J mouse acquired using previously published methods39. While both images have relatively high SNR, images at (A) inspiration have lower SNR than images at (B) expiration largely because the 129Xe gas had not yet had the time to wash into the lungs. Please click here to view a larger version of this figure.
It is important to distinguish between pathologically obstructed ventilation and other causes of low SNR. In Figure 7A, no ventilation obstruction is expected (i.e., low VDP) because the animal was a healthy, wild-type mouse. If ventilation were obstructed, defects would typically appear as regions with low to no 129Xe signal. Nonpathological causes of low signal commonly include:
(i) Low 129Xe polarization: This causes low SNR in lung parenchyma and airways without accompanying artifacts. Low polarization can typically be attributed to incorrectly following polarization procedures, depolarization during transport, or excessive O2 gas content in the HP 129Xe delivery tubing (e.g., from an incorrectly calibrated ventilator).
(ii) Intubation cannula inserted too deeply: If inserted too deeply into the trachea, the cannula may deflect down the left or right bronchus. If lodged tightly enough, the whole-lung tidal volume may be confined to only a few lobes and airways while the rest of the lung receives none. Should this occur, the hyperinflated lobes could be injured, and it may be necessary to euthanize the animal. Alternatively, the tip of the cannula may come in direct contact with tissue. In this case, 129Xe will be primarily confined to the interior of the cannula, resulting in a bright signal from a visibly straight tube and minimal signal within the parenchyma. In this case, the cannula position should be corrected promptly, and the animal should be monitored for signs of distress (e.g., abnormal, absent, or asynchronous chest motion compared to the ventilator).
(iii) Intubation cannula placed too shallowly: When a bolus of gas is delivered too shallowly in the oropharynx, the resistance to that bolus flowing into the lungs can be stronger than the resistance to that bolus flowing around the exterior of the cannula and out of the mouth. This can result in poor lung inflation. Tracheal SNR may be high, while parenchymal SNR will be low. Motion artifacts may be present due to gas flowing up the trachea during data acquisition. The character of the artifact will vary depending on sequence type (GRE, radial, etc.).
(iv) Incorrect acquisition timing: If RF pulsing and data acquisition occurred during periods of inspiratory or expiratory gas flow, severe motion artifacts will usually be present — particularly when using Cartesian sequences — and parenchymal SNR will be low.
(v) Spontaneous respiration: If the animal is insufficiently sedated during data acquisition, the spontaneous respiratory motion will be superimposed asynchronously on top of the mechanical ventilation pattern. This will result in variable breath-to-breath 129Xe delivery, moderate to severe motion artifacts, and poor parenchymal SNR. Careful tracking of sedative dose and timing is critical.
(vi) Animal death: In rare instances, mice may die (e.g., due to incorrect cannula position, insufficient O2 delivery, sedative overdose, or severe experimental pathology). If this occurs, lung tissue stiffens rapidly, and the lung parenchyma will inflate poorly. The upper trachea may remain bright, but lung parenchyma will be very low in signal.
Gas Exchange Spectroscopy
In human subjects, spectroscopy-derived parameters (e.g., signal intensity, spectral width, chemical shift, and phase) offer insights into cardiopulmonary dynamics and disease state52,53. Unlike in humans86, dogs100, and rats20,101, whole-lung spectra from mice obtained when 129Xe magnetization is confined to the lung parenchyma display a single, broad, dissolved phase resonance corresponding to 129Xe dissolved in the capillary membrane tissue, blood plasma, and RBCs54 (Figure 8A, 197 ppm). When transported by blood flow to more distal tissues, 129Xe produces distinct resonance frequencies from various tissues, including adipose tissue54, muscle, gray matter, and white matter102,103. However, due to relatively long transit times (several seconds) and modest solubility104 (Ostwald solubility = 0.1 - 0.3), these signals are typically weak and only observed during dedicated, careful experiments. The exception is 129Xe dissolved in fatty tissues (Figure 8A, 189 ppm). From these tissues, a 129Xe signal can appear within a few seconds (e.g., over the initial few breaths during animal setup). Moreover, signal intensity can be high, because the Ostwald solubility of 129Xe is nearly 20-fold greater in adipose tissues than in blood plasma (i.e., 1.7 vs 0.094, respectively, at 37 ˚C104). The longitudinal relaxation times are also expected to be relatively long (in vivo T1 is ~20 s in lipids105 vs. 3-8 s in blood106). As a result, the 129Xe signal from adipose tissue can be relatively high compared to other tissues.
Due to differing signal dynamics, 129Xe spectroscopy experiments can be tailored to interrogate adipose tissue (e.g., to target brown adipose tissue thermometry107) or gas uptake in the lungs by selecting the correct acquisition parameters. When 129Xe spectra from the lungs are acquired with the settings described in Table 4 (i.e., TR = 50 ms and a 90˚ flip angle centered on the dissolved phase peak), both gas phase and dissolved phase peaks are observed (Figure 8A,B). Notably, the gas volume in the mouse lung is 2-3x higher than the tissue volume, and the solubility of 129Xe in this tissue is only ~10%104,108,109,110,111. Despite this, the amplitude of the dissolved signal exceeds that of the gas phase by approximately 2-fold because the gas phase excitation is far off-resonance (~15 kHz at 7 T; Figure 8B, SNR = 102 vs. 43, respectively). Therefore, the 20-to-30-fold greater magnetization pool in the gas phase can be approximated as a stable (i.e., non-depleted) reservoir for the dissolve-phase signal during static portions of the breath cycle.
With a new breath of magnetization every ~600 ms and a TR of 50 ms, the signal intensity from both gas phase and dissolved phase 129Xe oscillates. For the gas signal, this is attributable to the change in total gas volume (i.e., high at inspiration and low at expiration). The situation is more complex for the dissolved signal because the source gas magnetization, T2*, and capillary blood volume may fluctuate with the inflation state. As a result, the dissolved-to-gas signal amplitude ratio also oscillates with respect to the breath cycle (Figure 8C, mean dissolved:gas ratio = 1.9 ± 0.39).
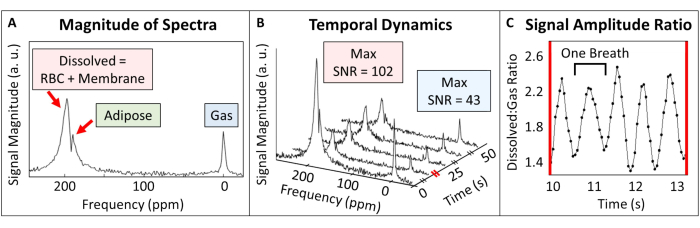
Figure 8: Representative dynamic spectroscopy in an adult C57BL/6J mouse. (A) A single pulse spectrum shows signal originating from 129Xe in the gas phase, dissolved in adipose tissue (189 ppm), and dissolved in both capillary membrane and blood tissues (197 ppm). (B) Dynamic spectroscopy showing the time-varying signal intensity of both dissolved and gaseous 129Xe (repetition time = 50 ms). To aid visualization, only five spectra are displayed. Diagonal lines on the time axis represent scale breaks. (C) The ratio of dissolved-to-gas signal amplitude over a 2 s interval (corresponding to the red scale break in panel B shows clear variation with the breathing cycle (0.6 s per breath). Please click here to view a larger version of this figure.
The following is a list of factors that could compromise 129Xe gas exchange spectroscopy results. In each case, the likely result is low SNR, which may make the spectral fitting and resulting metrics (e.g., dissolved-to-gas signal amplitude ratio, chemical shift, spectral width, and phase) inaccurate.
(i) Wrong working frequency: Because of the significantly higher gas volume and low tissue solubility, RF pulses centered on the gas phase frequency will produce no dissolved phase signal. RF pulses should be centered at or near the dissolved phase frequency.
(ii) Too broad of a bandwidth: The excitation bandwidth should be broad enough that some gas phase 129Xe is excited in order to measure the ratio of the dissolved-to-gas signal amplitude. But if the bandwidth is too broad, the excitation pulse will destroy magnetization in the gas phase before it dissolves into the tissue. This can be seen as a very high gas signal and low to no dissolved phase signal despite the pulse being centered on the dissolved phase frequency.
(iii) Unoptimized TR: To interrogate signal dynamics over a breath, Nyquist-Shannon sampling theorem requires the signal to be sampled at a minimum of twice its frequency (here, 100 breaths per minute were sampled every 50 ms, or ~12x per breath). Depending on experimental conditions, further interrogation of cardiopulmonary signal dynamics at a faster sampling rate is possible. The results of these experiments would depend on many factors, including excitation bandwidth, breathing rate, and heart rate. The xenon exchange time (i.e., the time for xenon to dissolve into tissue; ~56 ms54) and pulmonary transit time (i.e., the time for blood to flow from right to left ventricle; ~830 ms for a heart rate of ~436 beats/min112) must also be considered. Sampling too often will result in the early destruction of magnetization and low signal, but it will also reduce the influence of blood flow. Sampling less frequently will allow the magnetization to dissolve further and build up more signal, but it will become more dependent on blood flow and less localized to the gas exchange regions in the alveoli.
Diffusion-Weighted Images
The diffusion-weighted images, used to quantify alveolar-airspace size, have been rigorously validated in small animal models through traditional histomorphometry methods98,113,114. Here, we restricted discussion to 2D, slice-selective imaging to minimize scan time and gas usage while matching previously validated protocols98. A minimum of 2 b-value images is sufficient for calculating the ADC, and 4 or more b-value images are necessary for advanced morphometry calculations (such as mean linear intercept). The acquisition of multiple b-value images offers a significant advantage by enhancing the accuracy of the calculations. Unlike imaging humans during a single breath hold, preclinical imaging over many breaths provides continuously replenished signal. Because of this, it is possible to acquire many high SNR b-value images, limited only by the volume of HP gas available and animal sedation duration. Here, we acquired 7 b-value images, each with SNR >15, using 400 mL of 129Xe gas over 18 min of scan time (Figure 9). The signal in each image decays with increased diffusion gradient strength (b-value). This signal decay is proportional to the ADC of the 129Xe within the lung. Large airways can be distinguished from acinar tissue by comparing b0 and b6 images. In regions that are anatomically likely to contain airways, pixels will appear very bright in the b0 image but appear dark in the b6 image (red arrows, Figure 9). These pixels should be excluded from the analysis of lung parenchyma.

Figure 9: Representative diffusion-weighted images in an adult C57BL/6J mouse. Large airways (red arrows), through which 129Xe freely diffuses while diluted with oxygen, display increased signal attenuation relative to the lung parenchyma. This results in bright airways in the b0 image and low signal airways in the b6 image. Mice and rats have similarly small ADC values relative to humans, but the values will depend on biological and experimental factors like age, tidal volume, pressure, and diffusion parameters. Please click here to view a larger version of this figure.
The ADC of this healthy, adult, wild-type mouse follows the expected result based on the literature, its age, SNR, acquisition parameters, and more. Factors that may deleteriously impact the precision and accuracy of the ADC results include:
(i) Low SNR: Importantly, low SNR in the images bias the ADC calculation towards lower values99,115. For this reason, we exclude voxels with SVNR0 < 2.5 times the image noise97.
(ii) Improper segmentation: Poor segmentation (e.g., using signal thresholding without visual inspection for quality control) can skew results. If the images contain artifacts (like those caused by motion), they may exceed the threshold and be incorrectly categorized as parenchyma. Further, it is particularly important to exclude pixels near large airways, as partial volume effects may bias their ADC toward higher values.
(iii) Miscalibrated ventilator: In general, dilution of 129Xe with lighter gases (e.g., O2 or N2) can shift the ADC toward higher values, so ventilation with known gas mixtures is necessary for quantitative comparisons. If ventilating with a 129Xe/O2 mixture, an increase in oxygen concentration prior to delivery will increase T1 relaxation and reduce 129Xe magnetization in the gas delivery tubing, thus decreasing SNR.
(iv) Lung derecruitment: In anesthetized and mechanically ventilated animals, alveolar collapse (atelectasis) can occur over time. When this happens during constant volume ventilation, the same tidal volume is necessarily redistributed to the alveoli that remain recruited. As moderate to severe atelectasis develops, the rest of the lung can become hyperinflated, causing injury and increased ADC in those regions. Regular recruitment maneuvers and PEEP prevent alveolar collapse, avoid decreased lung compliance, and minimize the risk of lung injury that can skew ADC results.
(v) Poor data fitting: The method of ADC estimation must be taken into consideration. Log-linear fitting is the most computationally efficient but introduces bias in low SNR images. Therefore, it is best applied to high SNR images (>20). The weighted linear method reduces this bias and is appropriate in images with SNR > 15. Bayesian estimation can yield estimates of ADC with low uncertainty even with SNR <10 but is computationally expensive99.
(vi) Suboptimal diffusion time: The optimal diffusion time is influenced by the maximum gradient strength of the MRI scanner and the acinar airway radius in mice (radius ~100 µm49), which is approximately three times smaller than that in humans. To ensure optimal conditions, the diffusion time is set to achieve a diffusion length larger than the average alveolar radius but smaller than the mean length of the alveolar ducts. The optimal diffusion time for the site-specific maximum gradient strength can be determined using the methods outlined by Sukstanskii and Yablonskiy49. Using a suboptimal diffusion time may lead to nonlinear decreases in 129Xe ADC, complicating the interpretation of results.
(vii) Suboptimal b-values: There is a tradeoff between maximizing the SNR and maximizing the contrast-to-noise ratio (CNR) generated across b-value images. If CNR is sacrificed by the use of only low b-values (i.e., diffusive signal decay as if acquiring only the first 3 b-value images in Figure 9), then the ADC estimation will be inaccurate due to poor fitting. If the diffusion weighting is too strong, the resulting SNR will be insufficient, and the ADC will be biased toward lower values.
Discussion
Hyperpolarized 129Xe MRI is emerging as a sophisticated and powerful technique to study lung microstructure and function in small animal models. This protocol is intended to guide initial site preparation and describe experimental procedures needed to quantify ventilation, diffusion, and gas exchange in mouse lungs with HP 129Xe. Key prerequisites for experiments include establishing a 129Xe gas phantom and ensuring high gas polarization is delivered to the animal. The latter requires careful planning of polarization parameters, mitigating polarization losses during gas transport (e.g., building a magnetic carrying case), and characterizing (and ideally avoiding) T1 relaxation within the fringe field of the preclinical MRI magnet116,125. In parallel to developing the necessary HP 129Xe infrastructure, it is imperative to establish robust animal handling protocols and procedures. These include effective sedation to enable robust mechanical ventilation, non-injurious intubation to enable longitudinal studies, and ventilation strategies that balance the need to deliver high 129Xe magnetization with safe and physiologically relevant ventilation conditions.
To some degree, the methods, procedures, and results described here depend on the specific hardware used (e.g., ventilator, polarizer, and MRI scanner type). If alternative equipment is available, experimental techniques can be modified relatively easily. Further, the daily quality assurance procedures may be optimized (Step 1.2, Figure 4, and Table 1). Meticulous record keeping during the ~30 minutes of QA is critical during the establishment of a new site. It allows early detection of hardware issues and the ability to rule them out during troubleshooting. Once the day-to-day variations in QA metrics are well characterized, the flip angle calibration and ~21-min QA scan may be scaled back for efficiency. Additionally, the general approach can be extended to other common small animal species (e.g., rats and rabbits) by adjusting the anesthetic dosages, ventilator settings, and acquisition parameters. The basic protocols can be used to explore ventilator-dependent physiology like the impact of respiratory rate, volume, and PEEP levels on gas exchange or alveolar recruitment. If longitudinal data are not required or a longitudinal study is completed, the intubation cannula allows for bronchoalveolar lavage or rapid lung fixation and harvest for conventional analyses (e.g., histology or morphometric measurements). Moreover, the potential for modification of this protocol extends to MRI sequences and parameters, representing a dynamic aspect of 129Xe MRI research.
Cannula placement is critical to animal safety and image quality. Because the intubation cannula is not rigidly affixed — for example, by suturing around the trachea — relatively small displacements (e.g., movements when connecting physiological monitoring equipment) can break the airtight seal with the trachea. This airtight seal is necessary for recruitment maneuvers (~35 cm H2O peak inspiratory pressure), which are crucial for maintaining lung compliance and preventing atelectasis. Should the recruitment maneuver not reach the target pressure, the investigator must weigh the corrective strategies in the context of the study goals (e.g., diffusion-weighted imaging vs. ventilation imaging; single time-point vs. longitudinal). Mitigation strategies can include 1) removing the cannula and reintubating, 2) adjusting the cannula position to increase contact with the tracheal walls while still inserted, and 3) proceeding with the experiment without obtaining a gas-tight seal. From the standpoint of data fidelity, reintubation is the most robust option, but it can be time-consuming and lead to unwanted 129Xe magnetization decay. Adjusting the cannula position is more rapid, but it should be undertaken cautiously, as it carries a greater risk of injury. Continuing the experiment without RMs is best used when the mouse will be ventilated for <20 min to avoid atelectasis-induced changes in lung dynamics117.
Beyond the obvious need for safe animal handling, the key to developing a robust and reliable HP 129Xe mouse MRI program — and to troubleshooting its challenges — is meticulous record keeping. For HP 129Xe production, it is advisable to track laser power, accumulation time, flow rate, and polarization. During phantom imaging and spectroscopy, it is best to record image SNR, peak position, spectral linewidth, and the RF power needed to obtain a 90˚ pulse. For in vivo imaging, similar QA data should be recorded along with physiological monitoring parameters (peak inspiratory pressure, body temperature, etc.) and a detailed timeline of animal handling events. A few key pieces of equipment can make ventilator troubleshooting easier. These include an O2 sensor, a small animal spirometer, and a manometer to calibrate ventilation and PEEP pressure. An oscilloscope and voltmeter can assist in confirming that the ventilator timing is precise and matched to MRI scanner gating. Finally, a hand-held gaussmeter is indispensable for confirming the integrity of a magnetic carrying case if it is required for transport. Regarding MRI sequence troubleshooting, it is recommended to use both the gas phantom described in this protocol and a syringe filled with HP gas. The use of a syringe containing HP 129Xe gas is particularly beneficial for troubleshooting diffusion-weighted sequences, as the accuracy of the data can be assessed based on whether it reflects a known ground truth: the free diffusion coefficient of pure 129Xe within the syringe.
Several limitations must be acknowledged when applying this protocol. Firstly, it depends on specialized equipment and expertise, which reduces accessibility for new sites. However, with recent regulatory approval for clinical use in the U.S., the number of sites equipped with 129Xe MRI capabilities is expected to increase. The technique is also constrained by inherent limitations of hyperpolarized gas, including a relatively short polarization lifetime and the need for rapid data acquisition. The use of a mechanical ventilator typically improves image SNR and intrinsically avoids motion artifacts, but it masks natural breathing dynamics that could be captured by employing a free-breathing gas delivery device. Furthermore, findings from small animal models using this protocol should be interpreted cautiously when extrapolating to human physiology, given significant species variations. Another limitation is the challenging nature of oral intubation and mechanical ventilation, which are difficult in mice weighing less than 25 g. This may exclude males younger than ~8 weeks and females younger than ~20 weeks unless dedicated equipment and procedures are used. The most glaring limitation of this protocol is the absence of pulse programming and advanced image reconstruction techniques. The inclusion of such methods in a generalized protocol is impractical due to their intricate technical details and specialized knowledge. Finally, this protocol describes basic quantitative metrics like SNR, VDP, and ADC but not more advanced measures like regional fractional ventilation, diffusion morphometry, and gas exchange imaging. These advanced metrics will be more easily implemented once the basic infrastructure described here is set up.
Although HP 129Xe gas MRI of small animals is resource-intensive and experimentally challenging, it has the potential to significantly advance preclinical lung research. It offers a minimally invasive and sensitive means to quantify regional ventilation, alveolar-airspace size, and gas exchange dynamics. As such, it can provide a unique means to assess lung physiology, disease progression, and therapeutic interventions over time. It, therefore, represents an improvement over traditional preclinical techniques, including histology, biochemical analyses, ionizing imaging modalities, and invasive lung mechanics measurements. Moreover, small animal 129Xe MRI remains an actively developing subfield of research, and balancing the relevant imaging physics with preclinical requirements represents substantial research opportunities in its own right. Finally, as clinical 129Xe MRI becomes more widely accepted and used clinically, the opportunities for mouse-to-human and back translational studies will continue to grow with time.
Supplementary Figure 1: A circuit diagram for the magnetic carrying case. A solenoid made of enameled copper wire, L1, is connected in parallel with V1, a 12 Volt rechargeable sealed lead acid battery. The resistance of resistor R1 can be selected to control the current through V1 and L1. A panel indicator LED lamp, R2 and D1, is also wired in parallel with V1. A toggle on-off switch, S1, is connected in series with V1. Please click here to download this File.
Supplementary Figure 2: Ventilator calibration results for nitrogen. The volume output from 30 breaths was measured in triplicate. A linear regression was performed to determine the driving pressure necessary to produce relevant gas volumes. Please click here to download this File.
Supplementary Figure 3: Ventilator pressure settings cheat sheet. The slope and intercept results from the linear regression of each gas are used in Equation S1. This equation was used to determine the driving pressure necessary to produce a tidal volume of 10 ml/kg of ideal body weight for gas mixtures of 70% N2 + 30% O2 and 70% 129Xe + 30% O2 for pertinent animal weights. Please click here to download this File.
Disclosures
Peter Niedbalski is a consultant for Polarean Imaging, Plc.
Acknowledgements
The authors extend their heartfelt gratitude to Jerry Dalke for being a guiding light in ventilator construction. We'd like to thank Carter McMaster for brewing HP 129Xe gas. We'd also like to thank Dr. Matthew Willmering and Dr. Juan Parra-Robles for their thought-provoking scientific discussions. Figures created with BioRender.com. This work was funded by the National Institutes of Health (Grant Nos: NHLBI R01HL143011, R01HL151588)
Materials
| Name | Company | Catalog Number | Comments |
| 1 mL syringe | fisher scientific | Catalog No.14-955-464 | https://www.fishersci.com/shop/products/sterile-syringes-single-use-12/14955464 |
| 10 mL graduated cylinder | Cole-Parmer | UX-34502-69 | https://www.coleparmer.com/i/cole-parmer-essentials-graduated-cylinder-glass-hexagonal-base-10-ml-2-pk/3450269?PubID=UX&persist=true&ip=no& gad_source=1&gclid=CjwKCAi A6KWvBhAREiwAFPZM7h3do -ssjascARuVviKd7V7kC5ztdIB6 _70DnMr-K3qk9RKeJ7-IrhoCeT 0QAvD_BwE |
| 18 G - veinous PFTE catheters (nonsterile) | Terumo Surflo | SROX1832CA | https://www.shopmedvet.com/product/iv-catheter-18-x-1-25inch?r=GSS17&p=GSS17&utm_source= google&utm_medium=google_ shopping&gad_source=1&gclid= CjwKCAiA0bWvBhBjEiwAtEsoW 4oTvZkAgWQCda6ocVtQlulVrG 2536FNbu5soMVSFN8xK_g1Uh pXIRoCGwoQAvD_BwE |
| 20 G - veinous PFTE catheters (nonsterile) | Terumo Surflo | SROX2051CA | https://www.shopmedvet.com/product/iv-catheter-20-x-2inch?r=GSS17&p=GSS17&utm_source =google&utm_medium=google_ shopping&gad_source=1&gclid= CjwKCAiA0bWvBhBjEiwAtEsoW 87ggCkgToD_XF_UgpQBTpmN dgSNfCml6TkDKlW8k27Dq_daR itPuhoCnBQQAvD_BwE |
| 22 G - veinous PFTE catheters (nonsterile) | Terumo Surflo | SROX2225CA | https://www.shopmedvet.com/product/iv-catheter-22-x-1inch?r=GSS17&p=GSS17&utm_source= google&utm_medium=google_ shopping&gad_source=1&gclid =CjwKCAiA0bWvBhBjEiwAtEso W9IM6mpee6m7e-lBfR8dZhSN KYbMUs7qgEU4gYCRTW_rJAs W_lGkthoCm30QAvD_BwE |
| 400 mL tedlar bags | Jensen Inert Products | GST-001S-3507TJC | NA |
| 60 mL syringe | fisher scientific | Catalog No.14-955-461 | https://www.fishersci.com/shop/products/sterile-syringes-single-use-12/14955461 |
| 70% alcohol | Cole-Parmer | UX-80024-34 | https://www.coleparmer.com/i/labchem-isopropyl-alcohol-70-v-v-500-ml/8002434?PubID=UX&persist=true&ip= no&gad_source=1&gclid=CjwKC AiA6KWvBhAREiwAFPZM7gGh p8g7MBHBBKadaRCAwfEMgV gna5fhYRsuXIuqoqOiToCC4fem nhoCGMEQAvD_BwE |
| Dewar for liquid nitrogen | Terra Universal | 4LDB | https://www.laboratory-equipment.com/tw-4ldb-liquid-nitrogen-dewar-ic-biomedical.html?srsltid=AfmBOooxwMtOA1Z2TweR P8V5Iy5EvYT3alZuzoiY 3UF3Ib9RgFnDxVTfWP0 |
| Eye lubricant | Refresh | REFRESH P.M. | https://www.refreshbrand.com/Products/refresh-pm |
| Fiber optic light | AmScope | HL250-AY | https://amscope.com/products/hl250-ay?tw_source=google &tw_adid=&tw_campaign= 16705014684&gad_source= 1&gclid=CjwKCAiA6KWvBhA REiwAFPZM7p-DpyvHJaGxR pAD1385hzGf1oPdKHHLFDR Sp8yrtxry11SNJeJnKxoCtAoQ AvD_BwE |
| Gaussmeter | Apex Magnets | GMHT201 | https://www.apexmagnets.com/magnets/accessories/ht-digital-gaussmeter-with-peak-hold-can-display-gauss-or-tesla |
| Glass vessel (phantom) | Ace Glass | 8648-24 | https://aceglass.com/results.php?t=8648-24&t=8648-24 |
| Heating pad | Office Depot | 9206211 | Pure Enrichment PureRelief Express Designer Series Heating Pad 12 x 15 Palm Aqua - Office Depot |
| Hyperpolarizer | Polarean | 9820 | https://polarean.com/xenon-mri-platform/ |
| Intubation board | Hallowell EMC | 000A3467 | https://hallowell.com/product/rodent-tilting-workstand/ |
| Intubation supplies | Parts list published elsewhere | NA | https://app-jove-com.remotexs.ntu.edu.sg/t/50318/a-simple-method-of-mouse-lung-intubation |
| Isotopically enriched xenon cylinder | Linde Isotopes | XE-129(1%)N2(10%)HE CGMP 302SZ | NA |
| Liquid nitrogen | Linde | NI LC160-22 | https://www.lindedirect.com/store/product-detail/nitrogen_n2_nitrogen_liquid _lc160_22_psi_ni_lc160_22 /ni-lc160-22?cat_id=shop&node=b89 |
| Male slip luer | Cole-Parmer | UX-21943-27 | https://www.coleparmer.com/i/diba-omnifit-t-series-solvent-waste-cap-adapter-polypropylene-male-luer-slip-x-1-16-id-hose-barb-5-pk/2194327 |
| Manometer | Grainger | 3T294 | https://www.grainger.com/product/3T294?gucid=N:N:PS: Paid:GGL:CSM-2295:4P7A1P: 20501231&gad_source=1&gclid =CjwKCAiAi6uvBhADEiwAWiyR dltxrPJmmcm0bFiYLuPrB25HV QFdEfKMBqvgJBNdQUs3DZ7b TLr8CRoCanAQAvD_BwE& gclsrc=aw.ds |
| Minivent ventilator | harvard apparatus | 73-0044 | https://www.harvardapparatus.com/minivent-ventilator-for-mice-single-animal-volume-controlled-ventilators.html |
| Mouse ear puncher | fisher scientific | 13-812-201 | https://www.fishersci.com/shop/products/fisherbrand-animal-ear-tag-punch/13812201 |
| Mouse tongue depressor | Medical Tools | VRI-617 | https://medical-tools.com/shop/rodent-tongue-depressor.html |
| Mouse weight scale | Cole-Parmer | UX-11712-12 | https://www.coleparmer.com/i/adam-equipment-cqt2000-core-portable-balance-2000g-x-1g-220-v/1171212?PubID=UX&persist=true&ip=no&gad _source=1&gclid=CjwKCAiA6K WvBhAREiwAFPZM7iYnAG5Ilc Z5DZWrdJ6wcLDZSCSfNJHOH m2PQOpyyWe0TjFa75R3tBoCjB sQAvD_BwE |
| MRI scanner | Bruker | 7T Biospec horizontal system | https://www.bruker.com/de/products-and-solutions/preclinical-imaging/mri/biospec.html |
| Multimeter | Home Depot | 1007898529 | https://www.homedepot.com/p/Klein-Tools-600-Volt-Digital-Multi-Meter-Manual-Ranging-MM325/320822947 |
| Natural abundance xenon | Linde Isotopes | UN 2036 | NA |
| Needle | fisher scientific | 305194 | https://www.fishersci.com/shop/products/bd-general-use-precisionglide-hypodermic-needles-20/148266C?keyword=true |
| Needle safe syringe holder | fisher scientific | NC2703873 | https://www.fishersci.com/shop/products/ndlsafe-ii-syr-uncap-deca/NC2703873#?keyword=needlesafe |
| Nitrogen cylinder | Linde | NI M-K | https://www.lindedirect.com/store/product-detail/nitrogen_n2_nitrogen_nf_k/ni-m-k?cat_id=shop&node=b89 |
| Oxygen cylinder | Linde | OX M-K | https://www.lindedirect.com/store/product-detail/oxygen_o2_oxygen_usp_k/ox-m-k?cat_id=shop&node=b90 |
| Oxygen sensor | Apogee instruments | MO-200 | https://www.apogeeinstruments.com/mo-200-oxygen-sensor-with-handheld-meter/ |
| Oxygen sensor inline flowhead | Apogee instruments | AO-002 | https://www.apogeeinstruments.com/ao-002-oxygen-meter-sensor-flow-through-head/ |
| PEEP valve | Hallowell EMC | 000A6556A | https://hallowell.com/product/adjustable-peep-valve-with-exhaust-port-range-5-20cm-disposable/ |
| Pipette tips | fisher scientific | Catalog No.02-707-108 | Fisherbrand Stack-Rack Space-Saver Tips: 101-1000 L Standard; Blue; Volume: | Fisher Scientific |
| Plunger valve | Ace glass | 8648-20 | https://www.aceglass.com/results.php?t=8648 |
| Preclinical coil | Doty scientific | custom built | https://dotynmr.com/products/bmax-xy-low-e/ |
| Pressure regulators | Cole-Parmer | UX-98202-11 | https://www.coleparmer.com/i/cole-parmer-single-stage-regulator-1500-scfh-capacity-346-cga-fitting/9820211?PubID=UX&persist=true&ip=no& gad_source=1&gclid=CjwKCAi A6KWvBhAREiwAFPZM7pruR xCAiaj52nA_8Y1nveQZRsD6B f0QO65o2DKFYqRoz0PopSkX QxoCxqcQAvD_BwE |
| Pressure-drop ventilator | Parts list published elsewhere | NA | https://sites.duke.edu/driehuyslab/resources/ |
| PVC pipe for phantom | Home Depot | 193682 | https://www.homedepot.com/p/IPEX-1-2-in-x-10-ft-White-PVC-SCH-40-Potable-Pressure-Water-Pipe-30-05010HD/319692959 |
| SAI animal heating system | SAII | Model 1030 | https://i4sa.com/product/model-1030-monitoring-gating-system/ |
| Saline | Farris Laboratories Inc. | 0409488820-1 | https://www.farrislabs.com/products/bacteriostatic-sodium-chloride-0-9-30ml-bottle?variant=42807174824167¤cy =USD&utm_medium=product_ sync&utm_source=google&utm_ content=sag_organic&utm_ campaign=sag_organic&utm_ campaign=gs-2021-09-24&utm _source=google&utm_medium =smart_campaign&gad_source =1&gclid=CjwKCAiA6KWvBh AREiwAFPZM7oS3-hFDETO_2f6OWOoKyBMb WuDuWqYxdWRYUWEkY M2Py73VfGzVtRoC2FQQAvD_BwE |
| Sharps container | fisher scientific | 22-730-455 | https://www.fishersci.com/shop/products/sharps-container-47/p-7250579#?keyword=needle%20safe |
| Silicone epoxy | Grainger | 3KMY7 | https://www.grainger.com/product/3KMY7?gucid=N:N:PS:Paid:GGL:CSM- 2295:4P7A1P:20501231&gad_ source=1&gclid=CjwKCAiA6KW vBhAREiwAFPZM7voahkm8tda t1Euql1A8DFhC6AZVJ0wXzCE PfE6iUzrIJXV-Hl8o4xoCQLYQA vD_BwE&gclsrc=aw.ds |
| Silicone mold release lubricant | Grainger | 19MW95 | https://www.grainger.com/product/CRC-Mold-Release-Agent-16-oz-19MW95 |
| Spirometer | ADInstruments | FE141 | https://www.adinstruments.com/products/spirometer |
| Spirometer - mouse flowhead | ADInstruments | MLT1L | https://www.adinstruments.com/products/respiratory-flow-heads |
| Tubing - 1/4 OD | Clippard | URH1-0402-CLT-050 | https://www.clippard.com/part/URH1-0402-CLT-050 |
| Tubing - 1/8 OD | Clippard | URH1-0804-CLT-050 | https://www.clippard.com/part/URH1-0402-CLT-050 |
| Vacuum pump | Cole-Parmer | UX-60062-11 | https://www.coleparmer.com/i/environmental-express-diaphragm-pump-high-volume-120v/6006211?PubID=UX&persist=true&ip=no&gad _source=1&gclid=CjwKCAiA6K WvBhAREiwAFPZM7uFGwmW pRelHNFgZVvJJV09vDUVyfyG HoKeZTiFNIiVTe-05IpJJPxoCO PoQAvD_BwE |
| Wire - 18 gauge | Digikey | 2328-18H240-ND | https://www.digikey.com/en/products/detail/remington-industries/18H240/15202027?s=N4 IgjCBcoOwBxVAYygMwIYBsDOB TANCAPZQDa4YATPAGwgC6h ADgC5QgDKLATgJYB2AcxAB fQmAAMAFkqIQKSBhwFiZEA GZNATi0SGzNpE48BwsSErqw 6uQqV5CJSOQCsMF%2Bq11 GIVuy58QqLmss4gALbogvy4L AAEAO683LgMIkA |
| Xenon polarization measurement station | Polarean | NA | https://polarean.com/xenon-mri-platform/ |
References
- World Health Organization. Global health estimates 2020: Deaths by cause, age, sex, by country and by region, 2000-2019. World Health Organization. , (2020).
- Middleton, P. G., et al. Elexacaftor-Tezacaftor-Ivacaftor for cystic fibrosis with a single Phe508del allele. N Engl J Med. 381 (19), 1809-1819 (2019).
- Taylor-Cousar, J. L., et al. Tezacaftor-Ivacaftor in patients with cystic fibrosis homozygous for Phe508del. N Engl J Med. 377 (21), 2013-2023 (2017).
- Richeldi, L., et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 370 (22), 2071-2082 (2014).
- King, T. E., et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 370 (22), 2083-2092 (2014).
- Yong, K. S. M., Her, Z., Chen, Q. Humanized mice as unique tools for human-specific studies. Arch Immunol Ther Exp (Warsz. 66 (4), 245-266 (2018).
- Li, H., Auwerx, J. Mouse systems genetics as a prelude to precision medicine. Trends Genet. 36 (4), 259-272 (2020).
- Eppig, J. T., Motenko, H., Richardson, J. E., Richards-Smith, B., Smith, C. L. The International Mouse Strain Resource (IMSR): cataloging worldwide mouse and ES cell line resources. Mamm Genome. 26 (9-10), 448-455 (2015).
- Vedi, M., et al. 2022 updates to the rat genome database: A Findable, Accessible, Interoperable, and Reusable (FAIR) resource. Genetics. 224 (1), 042 (2023).
- Ghorani, V., Boskabady, M. H., Khazdair, M. R., Kianmeher, M. Experimental animal models for COPD: a methodological review. Tob Induc Dis. 15, 25 (2017).
- Semaniakou, A., Croll, R. P., Chappe, V. Animal models in the pathophysiology of cystic fibrosis. Front Pharmacol. 9, 1475 (2018).
- Moore, B. B., et al. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol. 49 (2), 167-179 (2013).
- Jenkins, R. G., et al. An official American thoracic society workshop report: Use of animal models for the preclinical assessment of potential therapies for pulmonary fibrosis. Am J Respir Cell Mol Biol. 56 (5), 667-679 (2017).
- Gomez-Arroyo, J., et al. A brief overview of mouse models of pulmonary arterial hypertension: problems and prospects. Am J Physiol Lung Cell Mol Physiol. 302 (10), L977-L991 (2012).
- Dignam, J. P., Scott, T. E., Kemp-Harper, B. K., Hobbs, A. J. Animal models of pulmonary hypertension: Getting to the heart of the problem. Br J Pharmacol. 179 (5), 811-837 (2022).
- Woodrow, J. S., Sheats, M. K., Cooper, B., Bayless, R. Asthma: The use of animal models and their translational utility. Cells. 12 (7), 1091 (2023).
- Van de Velde, G., et al. Longitudinal in vivo microcomputed tomography of mouse lungs: No evidence for radiotoxicity. Am J Physiol Lung Cell Mol Physiol. 309 (3), L271-L279 (2015).
- Mata, J. F., et al. Evaluation of emphysema severity and progression in a rabbit model: comparison of hyperpolarized 3He and 129Xe diffusion MRI with lung morphometry. J Appl Physiol (1985). 102 (1985), 1273-1280 (2007).
- Boudreau, M., Xu, X., Santyr, G. E. Measurement of 129Xe gas apparent diffusion coefficient anisotropy in an elastase-instilled rat model of emphysema. Magn Reson Med. 69 (1), 211-220 (2013).
- Cleveland, Z. I., et al. 3D MRI of impaired hyperpolarized 129Xe uptake in a rat model of pulmonary fibrosis. NMR Biomed. 27 (12), 1502-1514 (2014).
- Kimura, A., et al. Inflammation during lung cancer progression and ethyl pyruvate treatment observed by pulmonary functional hyperpolarized 129Xe MRI in mice. Contrast Media Mol Imaging. 2021, 9918702 (2021).
- Kimura, A., et al. Treatment response of ethyl pyruvate in a mouse model of chronic obstructive pulmonary disease studied by hyperpolarized 129Xe MRI. Magn Reson Med. 78 (2), 721-729 (2017).
- Ouriadov, A., et al. Early stage radiation-induced lung injury detected using hyperpolarized 129Xe Morphometry: Proof-of-concept demonstration in a rat model. Magn Reson Med. 75 (6), 2421-2431 (2016).
- Willmering, M. M., et al. Improved pulmonary 129Xe ventilation imaging via 3D-spiral UTE MRI. Magn Reson Med. 84 (1), 312-320 (2020).
- Kaushik, S. S., et al. Single-breath clinical imaging of hyperpolarized 129Xe in the airspaces, barrier, and red blood cells using an interleaved 3D radial 1-point Dixon acquisition. Magn Reson Med. 75 (4), 1434-1443 (2016).
- Zanette, B., Munidasa, S., Friedlander, Y., Ratjen, F., Santyr, G. A 3D stack-of-spirals approach for rapid hyperpolarized 129Xe ventilation mapping in pediatric cystic fibrosis lung disease. Magn Reson Med. 89 (3), 1083-1091 (2023).
- Zanette, B., Stirrat, E., Jelveh, S., Hope, A., Santyr, G. Physiological gas exchange mapping of hyperpolarized 129Xe using spiral-IDEAL and MOXE in a model of regional radiation-induced lung injury. Med Phys. 45 (2), 803-816 (2018).
- Collier, G. J., et al. Dissolved 129Xe lung MRI with four-echo 3D radial spectroscopic imaging: Quantification of regional gas transfer in idiopathic pulmonary fibrosis. Magn Reson Med. 85 (5), 2622-2633 (2021).
- Qing, K., et al. Assessment of lung function in asthma and COPD using hyperpolarized 129Xe chemical shift saturation recovery spectroscopy and dissolved-phase MRI. NMR Biomed. 27 (12), 1490-1501 (2014).
- Ruppert, K., Brookeman, J. R., Hagspiel, K. D., Mugler, J. P. Probing lung physiology with xenon polarization transfer contrast (XTC). Magn Reson Med. 44 (3), 349-357 (2000).
- Kern, A. L., et al. Investigating short-time diffusion of hyperpolarized 129Xe in lung air spaces and tissue: A feasibility study in chronic obstructive pulmonary disease patients. Magn Reson Med. 84 (4), 2133-2146 (2020).
- Amzajerdian, F., et al. Simultaneous quantification of hyperpolarized xenon-129 ventilation and gas exchange with multi-breath xenon-polarization transfer contrast (XTC) MRI. Magn Reson Med. 90 (6), 2334-2347 (2023).
- Perron, S., et al. Application of a 2D frequency encoding sectoral approach to hyperpolarized 129Xe MRI at low field. J Magn Reson. 336, 107159 (2022).
- Loza, L. A., et al. Quantification of ventilation and gas uptake in free-breathing mice with hyperpolarized 129Xe MRI. IEEE Trans Med Imaging. 38 (9), 2081-2091 (2019).
- Imai, H., et al. Regional fractional ventilation mapping in spontaneously breathing mice using hyperpolarized 129Xe MRI. NMR Biomed. 28 (1), 24-29 (2015).
- Friedlander, Y., et al. Effect of inhaled oxygen concentration on 129Xe chemical shift of red blood cells in rat lungs. Magn Reson Med. 86 (3), 1187-1193 (2021).
- Virgincar, R. S., et al. A portable ventilator with integrated physiologic monitoring for hyperpolarized 129Xe MRI in rodents. J Magn Reson. 295, 63-71 (2018).
- Nouls, J., Fanarjian, M., Hedlund, L., Driehuys, B. A constant-volume ventilator and gas recapture system for hyperpolarized gas MRI of mouse and rat lungs. Concepts Magn Reson Part B Magn Reson Eng. 39B (2), 78-88 (2011).
- Niedbalski, P. J., et al. Preclinical hyperpolarized 129Xe MRI: ventilation and T2* mapping in mouse lungs at 7 T using multi-echo flyback UTE. NMR Biomed. 33 (7), e4302 (2020).
- Akinyi, T. G. . An Affordable Open-Source Small Animal MR and Hyperpolarized Gas Compatible Ventilator: Feasibility in Preclinical Imaging. , (2017).
- Smith, L. J., et al. The assessment of short and long term changes in lung function in CF using 129Xe MRI. Eur Respir J. 6, 2000441 (2020).
- Svenningsen, S., et al. Reproducibility of hyperpolarized 129Xe MRI ventilation defect percent in severe asthma to evaluate clinical trial feasibility. Acad Radiol. 28 (6), 817-826 (2021).
- Kirby, M., et al. Hyperpolarized 3He and 129Xe MR imaging in healthy volunteers and patients with chronic obstructive pulmonary disease. Radiology. 265 (2), 600-610 (2012).
- Virgincar, R. S., et al. Quantitative analysis of hyperpolarized 129Xe ventilation imaging in healthy volunteers and subjects with chronic obstructive pulmonary disease. NMR Biomed. 26 (4), 424-435 (2013).
- Ebner, L., et al. Multireader determination of clinically significant obstruction using hyperpolarized 129Xe-ventilation MRI. AJR Am J Roentgenol. 212 (4), 758-765 (2019).
- Driehuys, B., et al. 3He MRI in mouse models of asthma. Magn Reson Med. 58 (5), 893-900 (2007).
- Mistry, N. N., Thomas, A., Kaushik, S. S., Johnson, G. A., Driehuys, B. Quantitative analysis of hyperpolarized 3He ventilation changes in mice challenged with methacholine. Magn Reson Med. 63 (3), 658-666 (2010).
- Costa, M., et al. Noninvasive assessment of in vivo mouse lung microstructural changes due to aging and PEEP. Am J Respir Crit Care Med. 207, A4713 (2023).
- Sukstanskii, A. L., Yablonskiy, D. A. Lung morphometry with hyperpolarized 129Xe: theoretical background. Magn Reson Med. 67 (3), 856-866 (2012).
- Chan, H. F., Stewart, N. J., Norquay, G., Collier, G. J., Wild, J. M. 3D diffusion-weighted 129Xe MRI for whole lung morphometry. Magn Reson Med. 79 (6), 2986-2995 (2018).
- Bier, E. A., et al. Noninvasive diagnosis of pulmonary hypertension with hyperpolarised 129Xe magnetic resonance imaging and spectroscopy. ERJ Open Res. 8 (2), 00035 (2022).
- Bier, E. A., et al. A protocol for quantifying cardiogenic oscillations in dynamic 129Xe gas exchange spectroscopy: The effects of idiopathic pulmonary fibrosis. NMR Biomed. 32 (1), e4029 (2019).
- Wang, Z., et al. Diverse cardiopulmonary diseases are associated with distinct xenon magnetic resonance imaging signatures. Eur Respir J. 54 (6), 1900831 (2019).
- Freeman, M. S., Cleveland, Z. I., Qi, Y., Driehuys, B. Enabling hyperpolarized 129Xe MR spectroscopy and imaging of pulmonary gas transfer to the red blood cells in transgenic mice expressing human hemoglobin. Magn Reson Med. 70 (5), 1192-1199 (2013).
- Iguchi, S., et al. Direct imaging of hyperpolarized 129Xe alveolar gas uptake in a mouse model of emphysema. Magn Reson Med. 70 (1), 207-215 (2013).
- Imai, H., et al. Noninvasive detection of pulmonary tissue destruction in a mouse model of emphysema using hyperpolarized 129Xe MRS under spontaneous respiration. Magn Reson Med. 64 (4), 929-938 (2010).
- Silk, S. B., Hampton, L. L., Brown, P. A. What investigators need to know about the use of animals. ILAR J. 54 (3), 324-328 (2014).
- Cereda, M., et al. Mild loss of lung aeration augments stretch in healthy lung regions. J Appl Physiol. 120 (1985), 444-454 (2016).
- Constantinides, C., Murphy, K. Molecular and integrative physiological effects of isoflurane anesthesia: The paradigm of cardiovascular studies in rodents using magnetic resonance imaging. Front Cardiovasc Med. 3, 23 (2016).
- Irwin, M. R., Curay, C. M., Choi, S., Kiyatkin, E. A. Basic physiological effects of ketamine-xylazine mixture as a general anesthetic preparation for rodent surgeries. Brain Res. 1804, 148251 (2023).
- Roth, D. M., Swaney, J. S., Dalton, N. D., Gilpin, E. A., John Ross, J. Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol. 282 (6), H2134-H2140 (2002).
- Massey, C. A., Richerson, G. B. Isoflurane, ketamine-xylazine, and urethane markedly alter breathing even at subtherapeutic doses. J Neurophysiol. 118 (4), 2389-2401 (2017).
- Janssen, B. J. A., et al. Effects of anesthetics on systemic hemodynamics in mice. Am J Physiol Heart Circ Physiol. 287 (4), H1618-H1624 (2004).
- Lenzarini, F., Di Lascio, N., Stea, F., Kusmic, C., Faita, F. Time course of isoflurane-induced vasodilation: A Doppler ultrasound study of the left coronary artery in mice. Ultrasound Med Biol. 42 (4), 999-1009 (2016).
- Mondonedo, J. R., et al. Volatile anesthetics and the treatment of severe bronchospasm: A concept of targeted delivery. Drug Discov Today Dis Models. 15, 43-50 (2015).
- Nolan, J. P. Anaesthesia and Neuromuscular Block. Clinical Pharmacology. , 295-310 (2012).
- Arras, M., Autenried, P., Rettich, A., Spaeni, D., Rülicke, T. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med. 51 (5), 443-456 (2001).
- Navarro, K. L., et al. Mouse anesthesia: The art and science. ILAR J. 62 (1-2), 238-273 (2021).
- Das, S., MacDonald, K., Chang, H. Y., Mitzner, W. A simple method of mouse lung intubation. J Vis Exp. (73), e50318 (2013).
- Thomas, J. L., et al. Endotracheal intubation in mice via direct laryngoscopy using an otoscope. J Vis Exp. (86), e50269 (2014).
- Slutsky, A. S., Ranieri, V. M. Ventilator-induced lung injury. N Engl J Med. 369 (22), 2126-2136 (2013).
- Matute-Bello, G., et al. An official American thoracic society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 44 (5), 725-738 (2011).
- Kulkarni, H. S., et al. Update on the features and measurements of experimental acute lung injury in animals: An official American thoracic society workshop report. Am J Respir Cell Mol Biol. 66 (2), e1-e14 (2022).
- Tsuchida, S., et al. Atelectasis causes alveolar injury in nonatelectatic lung regions. Am J Respir Crit Care Med. 174 (3), 279-289 (2006).
- Fan, E., et al. An official American thoracic society/European society of intensive care medicine/society of critical care medicine clinical practice guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 195 (9), 1253-1263 (2017).
- Santos, R. S., Silva, P. L., Pelosi, P., Rocco, P. R. Recruitment maneuvers in acute respiratory distress syndrome: The safe way is the best way. World J Crit Care Med. 4 (4), 278-286 (2015).
- Mekontso Dessap, A., et al. Conflicting physiological and genomic cardiopulmonary effects of recruitment maneuvers in murine acute lung injury. Am J Respir Cell Mol Biol. 46 (4), 541-550 (2012).
- García-Fernández, J., et al. Recruitment manoeuvres in anaesthesia: How many more excuses are there not to use them. Rev Esp Anestesiol Reanim (Engl Ed). 65 (4), 209-217 (2018).
- Allen, G. B., Suratt, B. T., Rinaldi, L., Petty, J. M., Bates, J. H. Choosing the frequency of deep inflation in mice: balancing recruitment against ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 291 (4), L710-L717 (2006).
- da Silva, A. C. L., et al. Sigh maneuver protects healthy lungs during mechanical ventilation in adult Wistar rats. Exp Biol Med. 245 (15), 1404-1413 (2020).
- Riva, D. R., et al. Recruitment maneuver: RAMP versus CPAP pressure profile in a model of acute lung injury. Respir Physiol Neurobiol. 169 (1), 62-68 (2009).
- Schwarte, L. A., Zuurbier, C. J., Ince, C. Mechanical ventilation of mice. Basic Res Cardiol. 95 (6), 510-520 (2000).
- Joelsson, J. P., Ingthorsson, S., Kricker, J., Gudjonsson, T., Karason, S. Ventilator-induced lung-injury in mouse models: Is there a trap. Lab Anim Res. 37 (1), 30 (2021).
- Jameson, C. J., Jameson, A. K., Hwang, J. K. Nuclear spin relaxation by intermolecular magnetic dipole coupling in the gas phase. 129Xe in oxygen. J Chem Phys. 89 (7), 4074-4081 (1988).
- Kelley, M., Branca, R. T. Theoretical models of spin-exchange optical pumping: Revisited and reconciled. J Appl Phys. 129 (15), 1-16 (2021).
- Norquay, G., Leung, G., Stewart, N. J., Wolber, J., Wild, J. M. 129Xe chemical shift in human blood and pulmonary blood oxygenation measurement in humans using hyperpolarized 129Xe NMR. Magn Reson Med. 77 (4), 1399-1408 (2017).
- Norquay, G., Collier, G. J., Rao, M., Stewart, N. J., Wild, J. M. 129Xe-Rb spin-exchange optical pumping with high photon efficiency. Phys Rev Lett. 121 (15), 153201 (2018).
- Ball, J. E., Wild, J. M., Norquay, G. Investigating Rubidium density and temperature distributions in a high-throughput 129Xe-Rb spin-exchange optical pumping polarizer. Molecules. 28 (1), 11 (2022).
- Plummer, J. W., et al. A semi-empirical model to optimize continuous-flow hyperpolarized 129Xe production under practical cryogenic-accumulation conditions. J Magn Reson. 320, 106845 (2020).
- Nikolaou, P., et al. Near-unity nuclear polarization with an open-source 129Xe hyperpolarizer for NMR and MRI. Proc Natl Acad Sci U S A. 110 (35), 14150-14155 (2013).
- Robertson, S. H., et al. Optimizing 3D noncartesian gridding reconstruction for hyperpolarized 129Xe MRI-focus on preclinical applications. Concepts Magn Reson Part A Bridg Educ Res. 44 (4), 190-202 (2015).
- Thomas, A. C., et al. A robust protocol for regional evaluation of methacholine challenge in mouse models of allergic asthma using hyperpolarized 3He MRI. NMR Biomed. 22 (5), 502-515 (2009).
- Niedbalski, P. J., et al. Protocols for multi-site trials using hyperpolarized 129Xe MRI for imaging of ventilation, alveolar-airspace size, and gas exchange: A position paper from the 129Xe MRI clinical trials consortium. Magn Reson Med. 86 (6), 2966-2986 (2021).
- Woodhouse, N., et al. Combined helium-3/proton magnetic resonance imaging measurement of ventilated lung volumes in smokers compared to never-smokers. J Magn Reson Imaging. 21 (4), 365-369 (2005).
- Brown, R. W., Cheng, Y. C. N., Haacke, E. M., Thompson, M. R., Venkatesan, R. . Magnetic Resonance Imaging: Physical Principles and Sequence Design. , (2014).
- Levitt, M. H. . Spin Dynamics: Basics of Nuclear Magnetic Resonance. , (2013).
- Salerno, M., et al. Emphysema: hyperpolarized helium 3 diffusion MR imaging of the lungs compared with spirometric indexes—initial experience. Radiology. 222 (1), 252-260 (2002).
- Niedbalski, P. J., et al. Validating in vivo hyperpolarized 129Xe diffusion MRI and diffusion morphometry in the mouse lung. Magn Reson Med. 85 (4), 2160-2173 (2021).
- Bdaiwi, A. S., et al. Improving hyperpolarized 129Xe ADC mapping in pediatric and adult lungs with uncertainty propagation. NMR Biomed. 35 (3), e4639 (2022).
- Ruppert, K., Brookeman, J. R., Hagspiel, K. D., Driehuys, B., Mugler, J. P. NMR of hyperpolarized 129Xe in the canine chest: spectral dynamics during a breath-hold. NMR Biomed. 13 (4), 220-228 (2000).
- Driehuys, B., et al. Imaging alveolar-capillary gas transfer using hyperpolarized 129Xe MRI. Proc Natl Acad Sci U S A. 103 (48), 18278-18283 (2006).
- Nakamura, K., et al. 129Xe spectra from the heads of rats with and without ligation of the external carotid and pterygopalatine arteries. Magn Reson Med. 53 (3), 528-534 (2005).
- Kershaw, J., et al. Confirming the existence of five peaks in 129Xe rat head spectra. Magn Reson Med. 57 (4), 791-797 (2007).
- Chen, R. Y., et al. Tissue-blood partition coefficient for xenon: temperature and hematocrit dependence. J Appl Physiol. 49 (2), 178-183 (1980).
- Möller, H. E., et al. Magnetic resonance angiography with hyperpolarized 129Xe dissolved in a lipid emulsion. Magn Reson Med. 41 (5), 1058-1064 (1999).
- Norquay, G., et al. Relaxation and exchange dynamics of hyperpolarized 129Xe in human blood. Magn Reson Med. 74 (2), 303-311 (2015).
- Zhang, L., et al. Absolute thermometry of human brown adipose tissue by magnetic resonance with laser polarized 129Xe. Commun Med. 3 (1), 147 (2023).
- Barshishat-Kupper, M., et al. Protein oxidation in the lungs of C57BL/6J mice following X-irradiation. Proteomes. 3 (3), 249-265 (2015).
- Olsson, L. E., et al. Measurement of MR signal and T2* in lung to characterize a tight skin mouse model of emphysema using single-point imaging. J Magn Reson Imaging. 25 (3), 488-494 (2007).
- Crowle, A. J. Delayed hypersensitivity in mice. J Allergy. 30 (2), 151-164 (1959).
- Guo, J., Cao, X., Cleveland, Z. I., Woods, J. C. Murine pulmonary imaging at 7T: T2* and T1 anisotropic UTE. Magn Reson Med. 79 (4), 2254-2264 (2018).
- Sonobe, T., et al. Imaging of the closed-chest mouse pulmonary circulation using synchrotron radiation microangiography. J Appl Physiol. 111 (1), 75-80 (2011).
- Wang, W., et al. Imaging lung microstructure in mice with hyperpolarized 3He diffusion MRI. Magn Reson Med. 65 (3), 620-626 (2011).
- Ouriadov, A. V., et al. Application of a stretched-exponential model for morphometric analysis of accelerated diffusion-weighted 129Xe MRI of the rat lung. Magn Reson Mater Phy. 34 (1), 73-84 (2021).
- O'Halloran, R. L., Holmes, J. H., Altes, T. A., Salerno, M., Fain, S. B. The effects of SNR on ADC measurements in diffusion-weighted hyperpolarized He-3 MRI. J Magn Reson. 185 (1), 42-49 (2007).
- Moller, H. E., Cleveland, Z. I., Driehuys, B. Relaxation of hyperpolarized 129Xe in a deflating polymer bag. J Magn Reson. 212 (1), 109-115 (2011).
- Reiss, L. K., Kowallik, A., Uhlig, S. Recurrent recruitment manoeuvres improve lung mechanics and minimize lung injury during mechanical ventilation of healthy mice. PLoS One. 6 (9), e24527 (2011).
- Cagle, L. A., et al. Effects of positive end-expiratory pressure and recruitment maneuvers in a ventilator-induced injury mouse model. PLoS One. 12 (11), e0187419 (2017).
- Cannizzaro, V., et al. Lung volume recruitment maneuvers and respiratory system mechanics in mechanically ventilated mice. Respir Physiol Neurobiol. 169 (3), 243-251 (2009).
- Hohlbaum, K., et al. Impact of repeated anesthesia with ketamine and xylazine on the well-being of C57BL/6JRj mice. PLoS One. 13 (9), e0203559 (2018).
- Blevins, C. E., Celeste, N. A., Marx, J. O. Effects of oxygen supplementation on injectable and inhalant anesthesia in C57BL/6 mice. J Am Assoc Lab Anim Sci. 60 (3), 289-297 (2021).
- Cannizzaro, V., et al. Impact of supplemental oxygen in mechanically ventilated adult and infant mice. Respir Physiol Neurobiol. 165 (1), 61-66 (2009).
- Sembroski, E., Sanghavi, D. K., Bhardwaj, A. Inverse Ratio Ventilation. StatPearls Publishing. , (2023).
- Boros, S. J. Variations in inspiratory:expiratory ratio and airway pressure wave form during mechanical ventilation: the significance of mean airway pressure. J Pediatr. 94 (1), 114-117 (1979).
- Zheng, W., Cleveland, Z. I., Moller, H. E., Driehuys, B. Gradient-induced longitudinal relaxation of hyperpolarized noble gases in the fringe fields of superconducting magnets used for magnetic resonance. J Magn Reson. 208 (2), 284-290 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved