Method Article
Direct Liquid-Culture Screening for Evaluating the Production of Heterologous Proteins Using an Auxotrophic Mutant of Aspergillus Oryzae
In This Article
Summary
A direct liquid-culture (DLC) screening method has been developed which significantly reduces the time required for polyethylene glycol (PEG)-mediated protoplast transformation of the filamentous fungus, Aspergillus oryzae, when employed for evaluation of the secretory production of heterologous proteins. This method dramatically increases the throughput of the evaluation protocol.
Abstract
Aspergillus oryzae, a filamentous fungus, is one of the most widely used hosts for industrial applications including large-scale production of proteins. A polyethylene glycol (PEG)-mediated protoplast transformation method is generally used for the introduction of heterologous genes into A. oryzae. The conventional method typically requires three weeks for the screening of favorable transformants. Here, a new technique, the direct liquid-culture (DLC) screening method, is introduced which reduces the screening time to six days in a 200 mL flask format or to 10 days in a 24 well microplate format. The DLC screening method ensures the acquisition of positive transformants and evaluation of the secretory production of heterologous proteins in a single step, unlike the conventional screening method where two separate steps are required for the same. The protocol for PEG-mediated protoplast transformation of A. oryzae is described, which consists of five steps: preparation of fresh spore suspension, preculture, preparation of protoplasts, introduction of DNA, and DLC screening. For successful results in DLC screening, it is critical to use a nutrient-rich medium with optimized osmotic pressure. The protocol should further popularize the use of A. oryzae as a host of choice in the industrial production of proteins.
Introduction
Aspergillus oryzae is an important microorganism in the Japanese food industry that has been used for over 1,000 years in the production of fermented foods, such as sake (rice wine), shoyu (soy sauce), and miso (soybean paste)1,2. It has the ability to secrete a large amount of proteins, such as proteases and amylases3. Genome sequence information for A. oryzae is also available4. Moreover, powerful and practically useful genetic engineering techniques have been established for this fungus5,6,7,8,9,10. Favorable transformants have been used as hosts for secretory production of heterologous proteins11,12,13,14.
Electroporation, Agrobacterium-mediated transformation, and polyethylene glycol (PEG)-mediated protoplast transformation are the techniques used for introducing heterologous genes into A. oryzae15,16,17. The PEG-mediated protoplast transformation method has been widely used since it was first reported for Neurospora crassa in 197918. In this method, protoplasts are prepared and mixed with PEG and the heterologous gene that is to be introduced into the cells. The walls of protoplasts are enzymatically compromised, which makes the cells vulnerable to physical stress and changes in osmotic pressure19,20. The conventional screening method employed in the secretory production of heterologous proteins includes three steps: acquisition of positive transformants (on soft agar plate), selection of true and false transformants (on agar plate), and evaluation of the secretory production of heterologous proteins (in liquid culture); each of these steps takes seven days (Figure 1). Thus, the conventional method typically requires about three weeks.
The time required for screening of transformed A. oryzae cells is much longer than that required for other microorganisms commonly used in biotechnology research, making the process more cumbersome. For example, when using Escherichia coli as a host, it takes approximately two days from the introduction of DNA to confirmation of its effect21.
To circumvent the limitation associated with the use of A. oryzae as mentioned above, herein, a new direct liquid-culture (DLC) screening method is introduced, which enables a more rapid and simple screening for evaluation of the secretory production of heterologous proteins (Figure 1). In the DLC method, a uridine auxotrophic mutant and a nutrient-rich liquid medium are used. Using this method, the screening step can be completed within six days after the introduction of DNA into the protoplasts in a 200 mL flask format or within 10 days in a 24 well microplate format. Furthermore, the time-consuming and laborious preparation of agar plate media is not needed in this method. There is a huge advantage in using the newly described method, especially considering the fact that the conventional method requires two different media: the soft agar plate for acquisition of positive transformants, which requires careful handling and temperature control, and solid agar plate for selection of true transformants.

Figure 1: Schematic of polyethylene glycol (PEG)-mediated protoplast transformation of the filamentous fungus, Aspergillus oryzae.
(Top panel) Conventional screening method. (Bottom panel) Direct liquid-culture (DLC) screening method. Please click here to view a larger version of this figure.
Protocol
1. Preparation of fresh spore suspension
- Inoculate 20 μL of a stock spore suspension (1 x 107 spores/mL) in the center of a culture plate containing Czapek-Dox (CD) medium with 20 mM uridine (autoclaved at 121 °C for 20 min, Table 1).
- Incubate at 30 °C for 7 days to promote spore formation.
- Add 1.5 mL of 0.01 % Tween 20 solution (autoclaved at 121 °C for 20 min) to the culture plate containing spores and suspend the spores by scraping with a cell spreader.
- Collect the spore suspension in a sterile 1.5 mL microcentrifuge tube using a pipette.
- Store the suspension at 4 °C until use.
NOTE: Use the spore suspension in the following procedure within 2 weeks. To make a stock for long-term storage, add glycerol to the spore suspension to a final concentration of 15% and store at -80 °C.
2. Preculture
- Add 100 mL of polypeptone-dextrin (PD) medium (Table 2) in a 500 mL Erlenmeyer flask, and autoclave it at 121 °C for 20 min.
- After confirming that the temperature of the medium is reduced, add the uridine solution, sterilized by passing through a filter with a pore size of 0.22 μm, to a final concentration of 20 mM.
- Add 200 μL of the spore suspension (1 x 107 spores/mL) prepared in step 1.4, and incubate at 30 °C with shaking at 120 rpm for 36 h.
3. Preparation of protoplasts
- Harvest the fungal biomass prepared in step 2.3 on a glass filter with pore size of 30 μm (autoclaved at 121 °C for 20 min).
- Pour 100 mL of distilled water (DW) (autoclaved at 121 °C for 20 min) on the glass filter and stir several times with a spatula (autoclaved at 121 °C for 20 min).
- After washing with DW, pour 100 mL of sodium chloride (NaCl) buffer (autoclaved at 121 °C for 20 min, Table 3) on the glass filter and stir several times with a spatula.
- Transfer about 1−2 mL (wet volume) of the cells to a 50 mL conical tube using a spatula (autoclaved at 121 °C for 20 min).
- After adding 15 mL of the enzyme solution (sterilized by passing through a 0.22 μm pore sized filter, Table 4) to a 50 mL conical tube, add NaCl buffer until the total volume reaches 30 mL.
- Close the lid and seal with paraffin film. Incubate at 30 °C with shaking at 60 rpm for 2 h.
- Flow the resulting solution through a 70 μm cell strainer attached to the 50 mL conical tube to remove the unreacted mycelia.
- Centrifuge the protoplast solution in a 50 mL conical tube at 2,150 x g and 4 °C for 20 min.
- Gently discard the supernatant to prevent pellet from dislodging. Add 1 mL of ice-cold sterilized solution B (autoclaved at 121 °C for 20 min, Table 5) and gently suspend the precipitated protoplasts by pipetting.
- Transfer the suspended protoplasts to a sterile 1.5 mL tube.
- Centrifuge at 2,220 x g and 4 °C for 5 min and remove the supernatant. Then, add 1 mL of ice-cold solution B and gently suspend the pellet by pipetting.
- Repeat step 3.9.
- Using a hemocytometer, measure the number of protoplasts under a microscope.
- Prepare a protoplast suspension (1−3 x 107 protoplasts/mL) in solution B and store it on ice until use.
NOTE: Use the protoplast suspension immediately in the following procedure.
4. Introduction of DNA for secretory production of protein
- Using a plasmid containing a DNA cassette for secretory production of protein as a template, amplify the DNA fragment required for secretory production by PCR.
- Prepare the DNA sample to be used for transformation by processing the PCR product using a PCR purification kit.
- Add 1 mL of the protoplast solution, 200 μL of solution C (autoclaved at 121 °C for 20 min, Table 6), and 50 μL of the DNA sample (0.5−1 μg/μL) in a sterile, precooled 15 mL conical tube, and mix the solution gently by pipetting.
NOTE: Solution C is highly viscous and can be difficult to handle; ensure thorough mixing by pipetting. - Incubate the tube on ice for 30 min.
- Add 1.5 mL of solution C, mix gently by pipetting, and then, leave the solution at room temperature for 20−30 min.
5. Direct liquid culture screening
NOTE: Select the culture system using Erlenmeyer flasks (section 5.1) or microplates (section 5.2).
- Culture system using a 200 mL Erlenmeyer flask
- Add 50 mL of PD medium containing 0.8 M sorbitol to a 200 mL Erlenmeyer flask, and sterilize by autoclaving at 121 °C for 20 min.
- After confirming that the temperature of the medium is reduced, add the protoplast suspension prepared in step 4.5 to a final concentration of 1 x 105 protoplasts/mL.
- Incubate the culture for 6 days at 30 °C with shaking at 120 rpm.
- Collect 1 mL of the medium and store in a 1.5 mL tube at 4 °C. Store the culture sample at -20 ° C, if not analyzed immediately.
- Mix 20 μL of culture sample and 20 μL of sodium dodecyl sulfate (SDS) sample buffer (Table 7) in a 1.5 mL tube and boil at 95 ° C for 5 min.
- After cooling on ice, load 20 μL of the boiled sample and 5 μL of prestained protein standard on a precast gel and analyze the secretory production of the target heterologous protein by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE)10.
- Culture system using a 24 well microplate
- Add the protoplast suspension prepared in step 4.5 to sterilized 0.8 M sorbitol-containing PD medium to a final concentration of 1 x 105 protoplasts/mL, and gently mix by pipetting.
- Add 1 mL of the solution prepared in step 5.2.1 to three wells in a 24 well microplate.
- Affix the lid and incubate at 30 °C with shaking at 175 rpm for 10 days.
- Collect 1 mL of medium and store in a 1.5 mL tube at 4 ° C. Store the culture sample at -20 ° C, if not analyzed immediately.
- Mix 20 μL of the culture sample and 20 μL of SDS sample buffer in a 1.5 mL tube and boil at 95 ° C for 5 min.
- After cooling on ice, load 20 μL of the boiled sample and 5 μL of prestained protein standard on a precast gel and analyze the secretory production of the target heterologous protein by SDS-PAGE10.
Results
The results for the introduction of the DNA expression cassette coding for Talaromyces cellulolyticus cellobiohydrolase (CBH: GenBank Accession Number E39854) into a uridine auxotroph of A. oryzae strain HO422 and screening for the secretory production of the heterologous protein are described below.
Preparation of fresh spore suspension
The final yield of spore suspension from one agar plate was 1 mL (1 x 107 spores/mL).
Preculture
After incubation for 36 h, ~5 mL (wet volume) of the fungal biomass was obtained from one flask when harvested at step 3.1. The yield sufficed for at least two trials of step 3.2.
Preparation of protoplasts
As the enzymatic reaction proceeded, the aggregated mycelia dissolved and the solution became cloudy (Figure 2), indicating the formation of protoplasts.
Introduction of DNA for secretory production of protein
The DNA cassette for secretory production of CBH was amplified by PCR using the plasmid pPPAe8-CBH1 as a template22. The molecular weight of CBH calculated from the amino acid sequence excluding the putative signal peptide is 52 kDa.
Culture system using a 200 mL Erlenmeyer flask
Protoplasts mixed with the DNA were added to PD medium containing 0.8 M sorbitol as well as to medium containing 0 and 1.6 M sorbitol (suboptimal levels). After incubation for 6 days, prolific cell growth was observed in the medium with 0.8 M sorbitol, whereas the growth was less in the presence of 1.6 M sorbitol; no growth was observed in the absence of sorbitol (Figure 3A). The secretion of CBH in the supernatant of the culture with 0.8 M sorbitol was confirmed by SDS-PAGE10 (Figure 3B). As a negative control, 200 μL of a spore suspension (1 x 107 spores/mL) of the HO4 strain was added to 100 mL of PD medium, and the supernatant from a sample cultured for 6 days was analyzed by SDS-PAGE10. As shown in Figure 3B, a band of the same size as reported previously using the same cbh gene was observed11.
Culture system using a 24 well microplate
Protoplasts that were mixed with DNA and those that were not mixed were added to PD medium containing 0.8 M sorbitol and cultured for 10 days (Figure 4A). The wells 1−3 contained samples in which DNA was mixed. The well 4 contained a sample in which DNA was not mixed (negative control). On day 6, growth was observed in all the 4 wells. On day 10, an increase in the amount of mycelium was observed in wells 1 and 2 relative to the growth on day 6, whereas growth in wells 3 and 4 appeared to have ceased after day 6. From these results, it is predicted that wells 1 and 2 had the true transformants and well 3 was a false positive case. The secretion of CBH in the supernatant collected from wells 1, 2, and 4 was confirmed by SDS-PAGE10 (Figure 4B). As shown in Figure 4B, a band of the same size as reported previously using the same cbh gene was confirmed11.
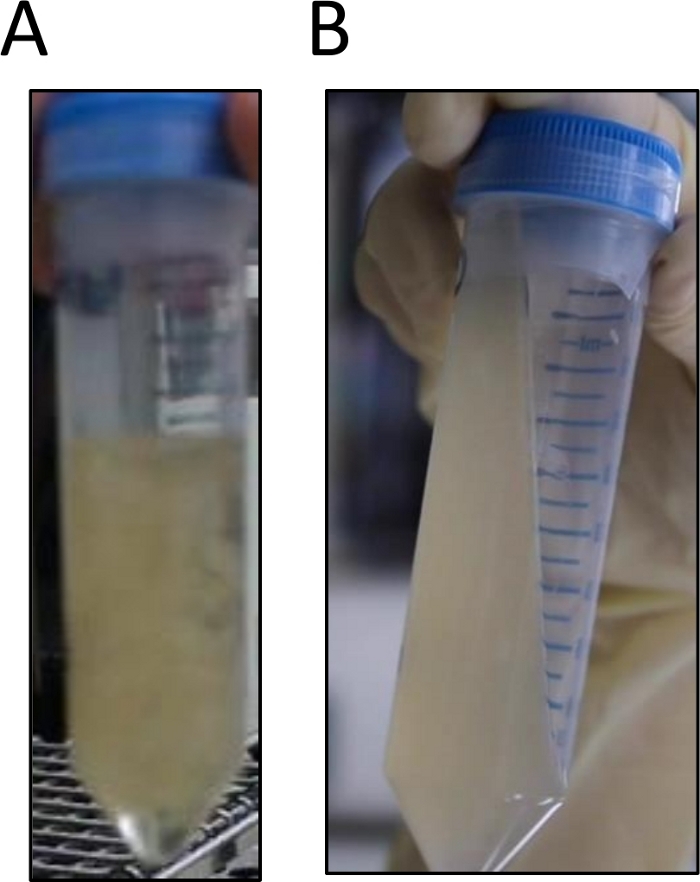
Figure 2: Picture showing protoplast suspension prepared in protocol section 3.
(A) Before and (B) after the enzymatic treatment. Please click here to view a larger version of this figure.
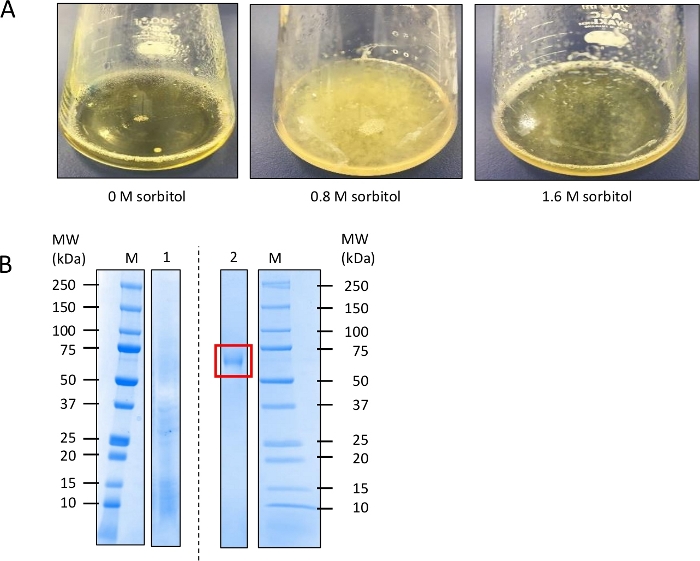
Figure 3: Direct liquid-culture (DLC) screening of Aspergillus oryzae strain HO4 transformed with an expression cassette of Talaromyces cellulolyticus cellobiohydrolase (CBH) in a 200 mL Erlenmeyer flask.
(A) Comparison of growth in culture medium containing 0, 0.8, and 1.6 M sorbitol after incubation for 6 days. (B) Confirmation of CBH secretion in the supernatant of the culture with 0.8 M sorbitol by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. M: Marker; 1: Culture supernatant of non-transformed A. oryzae strain HO4; 2: Culture supernatant (0.8 M sorbitol) of transformed A. oryzae strain HO4. Red rectangle shows the target heterologous protein. Supernatant on day 6 of culture was used for the assay. Please click here to view a larger version of this figure.

Figure 4: Direct liquid-culture (DLC) screening of Aspergillus oryzae strain HO4 transformed with an expression cassette of Talaromyces cellulolyticus cellobiohydrolase (CBH) in a 24 well microplate.
(A) Comparison of growth in culture medium containing 0.8 M sorbitol after incubation for 3, 6, and 10 days. Wells 1−3 contain samples mixed with DNA. Well 4 contains a sample to which DNA was not added (negative control). (B) Confirmation of CBH secretion in the supernatant of the culture with 0.8 M sorbitol by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. M: Marker; 1: Culture supernatant from well 1; 2: Culture supernatant from well 2; 4: Culture supernatant from well 4. Red rectangle shows the target heterologous protein. Supernatant on day 10 of culture was used for the assay. Please click here to view a larger version of this figure.
| Component | Amount (g) per liter medium |
| Dextrin hydrate | 30 |
| KCl | 2 |
| KH2PO4 | 1 |
| NaNO3 | 3 |
| MgSO4·7H2O | 0.5 |
| FeSO4·7H2O | 0.01 |
| Agar | 15 |
Table 1: Composition of Czapek-Dox (CD) medium. Adjust the pH of the medium to 6 using 1 M NaOH and make the volume to 1 L with distilled water. After autoclaving, add uridine solution, sterilized by passing through a filter with a pore size of 0.22 μm, to a final concentration of 20 mM.
| Component | Amount (g) per liter medium |
| Dextrin hydrate | 20 |
| Polypeptone peptone | 10 |
| Casamino acids | 1 |
| KH2PO4 | 5 |
| NaNO3 | 1 |
| MgSO4·7H2O | 0.5 |
Table 2: Composition of polypeptone-dextrin (PD) medium. Adjust the pH of the medium to 6 using 1 M NaOH and make the volume to 1 L with distilled water. After autoclaving, add uridine solution, sterilized by passing through a filter with a pore size of 0.22 μm to a final concentration of 20 mM.
| Component | Amount |
| 5 M NaCl | 16 mL |
| 1 M NaH2PO4 | 10 mL |
| Distilled water | Volume to 100 mL |
Table 3: Composition of NaCl buffer.
| Component | Amount |
| Lytic enzyme | 0.1 g |
| Yatalase | 0.1 g |
| Cellulase R-10 | 0.05 g |
| NaCl buffer | Volume to 30 mL |
Table 4: Composition of the enzyme solution.
| Component | Amount |
| D-Sorbitol | 21.86 g |
| CaCl2 | 0.555 g |
| 1M Tris-HCl (pH 7.5) | 1 mL |
| Distilled water | Volume to 100 mL |
Table 5: Composition of solution B.
| Component | Amount |
| Polyethylene glycol | 50 g |
| 1 M Tris-HCl (pH 7.5) | 1 mL |
| Distilled water | Volume to 100 mL |
Table 6: Composition of solution C.
| Component | Amount |
| 2-Mercaptoethanol | 2.5 mL |
| Sodium dodecyl sulfate | 1 g |
| Sucrose | 2.5 g |
| Bromophenol blue | 1 mg |
| 1 M Tris-HCl (pH 6.8) | 6.25 mL |
| Distilled water | Volume to 25 mL |
Table 7: Composition of SDS sample buffer.
Discussion
We have developed a system that allows the screening of A. oryzae transformants more rapidly than the conventional method, by conducting liquid culture of the protoplasts. The most critical aspect of this method is the osmotic pressure of the liquid medium. The osmotic pressure suitable for liquid culture was optimized using sorbitol. The growth of A. oryzae strain HO4 was most active in the presence of 0.8 M sorbitol (Figure 3A). In the conventional method using soft agar medium, an osmotic pressure of 0.5−1.2 M was reported to be suitable for N. crassa, Aspergillus sp., and Trichoderma sp.21. Osmotic pressure regulators include sorbitol, NaCl, potassium chloride (KCl), and magnesium sulfate (MgSO4)21. Depending on the type of strain used, the osmotic pressure regulator and the target osmotic pressure may need to be adjusted.
It will be necessary to consider the composition of the enzyme solution for producing protoplasts when using other filamentous fungi as a host for secretory production of heterologous proteins. The composition used in this protocol was according to Tamano et al.23. Endo et al.24 used a different enzyme solution without cellulase for Aspergillus nidulans.
In this protocol, PD medium is used in the screening method, which is suitable for the uridine-requiring strain. To establish an alternative protocol using an antibiotic or an auxotroph requiring a compound other than uridine for selection, the nutrient composition of the liquid medium must be optimized. For example, when using pyrithiamine for A. oryzae25, it is necessary to use a medium that does not contain thiamine. Similarly, when using the niaD gene as an auxotrophic marker for A. oryzae26, nitrate must be used as a single nitrogen source.
The volume and format of liquid culture affect the screening time. In the representative results, the 24 well microplate format required a longer time compared to the 200 mL Erlenmeyer flask format. This may be because of the differences (i) in the initial number of viable cells and (ii) in the dissolved oxygen content, which depends on the degree of aeration. In the 24 well microplate format, the selection between true and false transformants is easily achieved by visual inspection (Figure 4A).
In recent years, genetic modification using genome-editing technology has been reported in filamentous fungi27,28. The simultaneous introduction of two genes in a single transformation procedure using genome-editing technology has also been reported29. It may be possible to rapidly and simultaneously introduce multiple heterologous protein genes by combining genome-editing techniques and the DLC screening method using microplates. In this perspective, the merits ascribed to DLC should be much appreciated.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge Rinkei Okano for help with the experiments. The authors thank Professor Katsuya Gomi of Tohoku University and Professor Masayuki Machida of Kanazawa Institute of Technology for valuable discussions. This work was funded by Honda Research Institute Japan Co., Ltd.
Materials
| Name | Company | Catalog Number | Comments |
| 1 M NaOH | NACALAI TESQUE, INC. | 37421-05 | |
| 1 M Tris-HCl (pH 7.5) | FUJIFILM Wako Pure Chemical Corporation | 318-90225 | |
| 1 M Tris-HCl (pH 6.8) | FUJIFILM Wako Pure Chemical Corporation | 2106-100 | |
| 1.5-mL Microcentrifuge tube | AS ONE Corporation | 1-1600-03 | |
| 15-mL Conical centrifuge tube | Becton, Dickinson and Company | 352196 | |
| 2-mercaptoethanol | Bio-Rad Laboratories, Inc. | 1610710 | |
| 24-well Microplate | AGC TECHNO GLASS CO., LTD. | 3820-024 | |
| 50-mL Conical centrifuge tube | Becton, Dickinson and Company | 352070 | |
| 70-µm Cell strainer | Becton, Dickinson and Company | 352350 | |
| Agar | FUJIFILM Wako Pure Chemical Corporation | 010-15815 | |
| Autoclave | TOMY SEIKO CO.,LTD. | LSX-700 | |
| Bromophenol blue | FUJIFILM Wako Pure Chemical Corporation | 021-02911 | |
| CaCl2 | FUJIFILM Wako Pure Chemical Corporation | 038-24985 | |
| Casamino acid | FUJIFILM Wako Pure Chemical Corporation | 393-02145 | |
| Cellulase R-10 | Cosmo Bio Co., Ltd. | 16419 | |
| Dextrin hydrate | FUJIFILM Wako Pure Chemical Corporation | 044-00585 | |
| D-Sorbitol | FUJIFILM Wako Pure Chemical Corporation | 191-14735 | |
| e-PAGEL | ATTO CORPORATION | E-T/R1020L | Used for precast gel in SDS-PAGE |
| Electrophoresis device | ATTO CORPORATION | WSE-1150 | Used for SDS-PAGE |
| FeSO4·7H2O | FUJIFILM Wako Pure Chemical Corporation | 098-01085 | |
| Glass filter 17G3 | Tokyo Garasu Kikai Co., Ltd. | 0000094147 | |
| Glycerol | FUJIFILM Wako Pure Chemical Corporation | 070-04941 | |
| Hemocytometer | Funakoshi Co., Ltd. | 521-10 | |
| High speed refrigerated centrifuge | KUBOTA CORPORATION | 7780 | Used for centrifugation of samples in 50-mL conical centrifuge tubes |
| Incubator | TAITEC CORPORATION. | G·BR-200 | Used for flask liquid culture and preparation of protoplasts |
| Incubator | TAITEC CORPORATION. | BR-43FL | Used for microplate liquid culture and plate culture |
| KCl | FUJIFILM Wako Pure Chemical Corporation | 163-03545 | |
| KH2PO4 | NACALAI TESQUE, INC. | 28721-55 | |
| Lysing enzyme | Sigma-Aldrich | L1412-10G | |
| MgSO4·7H2O | FUJIFILM Wako Pure Chemical Corporation | 131-00405 | |
| Micro refrigerated centrifuge | KUBOTA CORPORATION | 3740 | Used for centrifugation of samples in 1.5-mL microcentrifuge tubes |
| Microscope | Leica Microsystems | DMI6000 B | |
| NaCl | FUJIFILM Wako Pure Chemical Corporation | 190-13921 | |
| NaH2PO4·2H2O | NACALAI TESQUE, INC. | 31718-15 | |
| NaNO3 | FUJIFILM Wako Pure Chemical Corporation | 195-02545 | |
| Parafilm M | Bemis Company, Inc | PM-996 | |
| PCR Purification Kit | QIAGEN K.K | 28104 | |
| Petri dish | Sumitomo Bakelite Co., Ltd. | MS-11900 | Used as culture plate |
| Polyethylene glycol | Sigma-Aldrich | P3640-500G | |
| Polypeptone peptone | Becton, Dickinson and Company | 211910 | |
| Protein ladders | Bio-Rad Laboratories, Inc. | 161-0377 | Used as molecular weight marker in SDS-PAGE |
| Sodium dodecyl sulfate | Bio-Rad Laboratories, Inc. | 1610301 | |
| Sterile filter | Merck KGaA | SLGP033RB | |
| Sucrose | NACALAI TESQUE, INC. | 30404-45 | |
| Tween 20 | Tokyo Chemical Industry Co., Ltd. | T0543 | |
| Uridine | Sigma-Aldrich | U3750-25G | |
| Yatalase | Takara Bio Inc. | T017 |
References
- Tsuboi, H., et al. Improvement of the Aspergillus oryzae enolase promoter (P-enoA) by the introduction of cis-element repeats. Bioscience, Biotechnology, and Biochemistry. 69, 206-208 (2005).
- Nemoto, T., Maruyama, J. -. I., Kitamoto, K. Contribution ratios of amyA, amyB, amyC genes to high-level α-amylase expression in Aspergillus oryzae. Bioscience, Biotechnology, and Biochemistry. 76, 1477-1483 (2012).
- Shinkawa, S., Mitsuzawa, S. Feasibility study of on-site solid-state enzyme production by Aspergillus oryzae. Biotechnology for Biofuels. 13, 31 (2020).
- Machida, M., et al. Genome sequencing and analysis of Aspergillus oryzae. Nature. 438 (7071), 1157-1161 (2005).
- Veenstra, A. E., van Solingen, P., Bovenberg, R. A. L., vander Voort, L. H. M. Strain improvement of Penicillium chrysogenum by recombinant DNA techniques. Journal of Biotechnology. 17 (1), 81-90 (1991).
- Takahashi, T., Masuda, T., Koyama, Y. Enhanced gene targeting frequency in ku70 and ku80 disruption mutants of Aspergillus sojae and Aspergillus oryzae. Molecular Genetics and Genomics. 275 (5), 460-470 (2006).
- Mizutani, O., et al. A defect of LigD (human Lig4 homolog) for nonhomologous end joining significantly improves efficiency of gene-targeting in Aspergillus oryzae. Fungal Genetics and Biology. 45 (6), 878-889 (2008).
- Bergès, T., Barreau, C. Isolation of uridine auxotrophs from Trichoderma reesei and efficient transformation with the cloned ura3 and ura5 genes. Current Genetics. 19 (5), 359-365 (1991).
- Takeno, S., et al. Cloning and sequencing of the ura3 and ura5 genes, and isolation and characterization of uracil auxotrophs of the fungus Mortierella alpina 1S-4. Bioscience, Biotechnology, and Biochemistry. 68 (2), 277-285 (2004).
- Ji, Y. W., et al. Application of membrane filtration method to isolate uninuclei conidium in Aspergillus oryzae transformation system based on the pyrG marker. Food Science and Biotechnology. 22 (1), 93-97 (2013).
- Mitsuzawa, S., Fukuura, M., Shinkawa, S., Kimura, K., Furuta, T. Alanine substitution in cellobiohydrolase provides new insights into substrate threading. Scientific Reports. 7 (1), 16320 (2017).
- Fleissner, A., Dersch, P. Expression and export: recombinant protein production systems for Aspergillus. Applied Microbiology and Biotechnology. 87, 1255-1270 (2010).
- Yoon, J., Maruyama, J. -. I., Kitamoto, K. Disruption of ten protease genes in the filamentous fungus Aspergillus oryzae highly improves production of heterologous proteins. Applied Microbiology and Biotechnology. 89, 747-759 (2011).
- Lin, H., et al. Engineering Aspergillus oryzae A-4 through the chromosomal insertion of foreign cellulase expression cassette to improve conversion of cellulosic biomass into lipids. PLoS One. 9, 108442 (2014).
- Chakraborty, B. N., Patterson, N. A., Kapoor, M. An electroporation-based system for high-efficiency transformation of germinated conidia of filamentous fungi. Canadian Journal of Microbiology. 37 (11), 858-863 (1991).
- Sun, Y., et al. A dual selection marker transformation system using Agrobacterium tumefaciens for the industrial Aspergillus oryzae 3.042. Journal of Microbiology and Biotechnology. 29 (2), 230-234 (2019).
- Gomi, K., Iimura, Y., Hara, S. Integrative transformation of Aspergillus oryzae with a plasmid containing the Aspergillus nidulans argB Gene. Agricultural and Biological Chemistry. 51 (9), 2549-2555 (1987).
- Case, M. E., Schweizer, M., Kushner, S. R., Giles, N. H. Efficient transformation of Neurospora crassa by utilizing hybrid plasmid DNA. Proceedings of the National Academy of Sciences of the United States of America. 76 (10), 5259-5263 (1979).
- Lim, F. Y., Sanchez, J. F., Wang, C. C., Keller, N. P. Toward awakening cryptic secondary metabolite gene clusters in filamentous fungi. Methods in Enzymology. 517, 303-324 (2012).
- Li, D., Tang, Y., Lin, J., Cai, W. Methods for genetic transformation of filamentous fungi. Microbial Cell Factories. 16 (1), 168 (2017).
- Chung, C. T., Niemela, S. L., Miller, R. H. One-step preparation of competent Escherichia coli: transformation and storage of bacterial cells in the same solution. Proceedings of the National Academy of Sciences of the United States of America. 86 (7), 2172-2175 (1989).
- Shinkawa, S., et al. Base sequence for protein expression and method for producing protein using same. U.S. Patent. , (2018).
- Tamano, K., et al. The β-1, 3-exoglucanase gene exgA (exg1) of Aspergillus oryzae is required to catabolize extracellular glucan, and is induced in growth on a solid surface. Bioscience, Biotechnology, and Biochemistry. 71 (4), 926-934 (2007).
- Endo, Y., et al. Novel promoter sequence required for inductive expression of the Aspergillus nidulans endoglucanase gene eglA. Bioscience, Biotechnology, and Biochemistry. 72 (2), 312-320 (2008).
- Kubodera, T., Yamashita, N., Nishimura, A. Pyrithiamine resistance gene (ptrA) of Aspergillus oryzae: cloning, characterization and application as a dominant selectable marker for transformation. Bioscience, Biotechnology, and Biochemistry. 64 (7), 1416-1421 (2000).
- Yamada, O., Lee, B. R., Gomi, K. Transformation system for Aspergillus oryzae with double auxotrophic mutations, niaD and sC. Bioscience, Biotechnology, and Biochemistry. 61 (8), 1367-1369 (1997).
- Nødvig, C. S., Nielsen, J. B., Kogle, M. E., Mortensen, U. H. A CRISPR-Cas9 system for genetic engineering of filamentous fungi. PLoS One. 10 (7), 0133085 (2015).
- Shi, T. Q., et al. CRISPR/Cas9-based genome editing of the filamentous fungi: the state of the art. Applied Microbiology and Biotechnology. 101 (20), 7435-7443 (2017).
- Katayama, T., et al. Forced recycling of an AMA1-based genome-editing plasmid allows for efficient multiple gene deletion/integration in the industrial filamentous fungus Aspergillus oryzae. Applied Environmental Microbiology. 85 (3), 01896-01918 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved