Method Article
Evaluation of the Impact of a New Cooling Cell Processor System on Islet Cell Isolation Facility
In This Article
Summary
The protocol here describes a custom cooling cell processor system that is compatible with a good manufacturing practices (GMP) cleanroom to improve cell viability post-processing.
Abstract
Standard cell therapy equipment, including the gold standard cell processor to purify human islets for clinical transplantation, is rarely refrigerated, potentially exposing cells to elevated temperatures during the centrifugation step. Custom cooling systems have a direct benefit on human islet viability and function. The current study was designed to test the effectiveness of a newly developed, readily available cooled cell processor system requiring minimal modifications and to evaluate its impact on human cell viability and the GMP cleanroom environment. The cooler system, a mechanically refrigerated heat exchanger set at -30 °C was used to deliver cooled medical grade dry air to the cell processor bowl through a hole drilled in the centrifuge cover. With the limited availability of pancreas donors in Qatar, system validation was done with continuous density gradient purification of pooled human bone marrow buffy coat. Sterility, turbulence, and particle count were measured in class C and class B clean room environments. No turbulence developed around the cooled cell processor, and no excess 0.5 µm and 5 µm airborne particulates were generated as per cleanroom GMP standards. At the beginning and end of the collection steps, the temperature rose respectively to 21.50 °C ± 0.34 °C and 21.93 °C ± 0.20 °C in the non-cooled cell processor and to only 10.9 °C ± 0.17 °C and 11.16 °C ± 0.35 °C in the cooled- cell processor (p <0.05). The cooled cell processor led to both improved recovery (98%) of the mononuclear cell fraction and viability (100% ± 2%) post-processing. The new cooling system effectively reduces the heat produced by the cell processor while having no particulate impact on the GMP clean room environment. The cooled cell processor described here is an inexpensive ($16,000 without including taxes, customs clearance, and transportation) and minimally invasive method to provide robust cooling. Currently, this technology in the GMP cell therapy facility is being applied to human islet cell isolation and transplantation for the clinical program.
Introduction
Islet transplantation for severe Type 1 diabetes can restore endogenous insulin secretion and has become the standard of care in a number of countries1. Islet isolation is a multi-step procedure that includes warm enzymatic and mechanical digestion of the pancreas, followed by cooled phases for recovery of digested tissue and islet purification with continuous or discontinuous density gradients on a cell processor. Rapid cooling slows the enzymatic digestion phase and protects islets from digestive enzymes liberated by contaminating acinar cells. The cell processor was originally designed to separate cellular blood components at ambient temperature2. It was repurposed to purify human pancreatic islets of Langerhans in 19893. For three decades, human islets have been purified for clinical trials and, more recently, standard-of-care, either in a cell processor with discontinuous gradients4 or continuous density gradients1 made with a gradient maker1 or equivalent computer-operated mixer5,6,7. Other groups have reported islet purification from large mammals and humans using large cylindrical plastic bottles8,7,9, which substantially improved the efficacy of purification and islet quality.
Indeed, considering the gold standard to purify human islets for clinical transplantation10,11, the manufactured cell processor was without refrigeration. As a direct consequence of the lack of refrigeration, islets were exposed to increasing temperatures during the purification step. During centrifugation, an uncontrolled increase in temperature occurs due to the mechanical friction of the rotor12,13. Cell processors were adapted with a cooling system in islet isolation centers that statistically increased glucose-induced insulin secretion and cell viability, confirming the advantage of keeping pancreatic islets at low temperatures during the purification procedure14,15.
It should be noted that in the late 1980s, 10 refrigerated prototypes were built to reduce islet damage during density gradient purification but received no further attention. The cell processor prototypes were based on spindle and bowl cooling with chilled polyethylene glycol. In particular, islet purification solutions, including organ preservation solutions and polysaccharide density gradients, may be deleterious for islet viability when used at or above room temperature16,17,18. A comparison of different cooling strategies of the cell processor for islet cell purification versus the original non-refrigerated cell processor was evaluated to combat the uncontrolled increase of temperature that occurs due to the friction of the rotor. Several attempts have been made to cool down the cell processor during human islet purification. The cell processor can be temporarily or permanently housed in a specially designed cold room to maintain a temperature of 0-8 °C19 during the gradient centrifugation which is currently used at the islet isolation centers at the University of Illinois Chicago19, University of Miami14, University of Oxford facilities15, and the University of San Francisco (the custom cooling unit encases the cell processor). Another modification includes core cooling of the cell processor spindle with a cooling system using electronically controlled liquid nitrogen cooling in Geneva, Sweden, and the Czech Republic15,19. Another alternative in the mid of 2000 was the core cooling of the cell processor with an air-conditioning unit12 or by liquid cooling of the shaft at the University of Lille.
Cooling modifications of the cell processor are not readily available and come with risks (humidity, condensation build-up) inside GMP cleanroom environments, including loss of warranty and refusal of the constructor to service the machine. Consequently, some islet centers, including Milan, Italy, Oslo, Norway, and Edinburgh, Scotland, do not use a cooling system for cell processors during the islet purification process15.
The current study was designed to test the effectiveness of a newly developed readily available cooler in conjunction with a minimally modified cell processor system. Briefly, the air-based cooler described here is a mechanically refrigerated heat exchanger controlling the temperature of 1 standard cubic foot per minute (SCFM) of dry gas between -40 °C to -100 °C. This cooling system requires a dry medical air supply. The air will flow through the line and heat exchanger and out through the (7.9 mm) nozzle. A heater located within the nozzle supplies heat to control the temperature of the air stream. A spare solid plastic centrifuge cover was purchased, and minimal modifications were made. A hole of 13.3 mm diameter was drilled in the cover, and cooled air was delivered from the cooler nozzle to the cell processor via the hole in the centrifuge cover. The cooled medical air was injected inside the centrifugation bowl of the cell processor with constant control of the temperature of the liquids or density gradients.
We evaluated the technical performance of the cooled cell processor and the impact of the cooling element on the GMP cleanroom environment. The main focus was to achieve a robustly low (<10 °C) temperature during human islet purification to preserve viability. Using a buffy coat density separation as a proxy for islet isolation, we determined the efficacy of this cooling protocol on cell viability.
Protocol
This protocol used discarded whole blood not fit for use as per institution discard guidelines. IRB approval and informed consent are not applicable.
1. Cooled cell processor setup
NOTE: All procedures must be done in a clean room environment, and staff must respect clean-room garment procedures.
- Purchasing of equipment
- Purchase a spare plexiglass lid. Purchase the cooling equipment system, cooler with mechanically refrigerated gas stream temperature controller, air dryer, pressure and flow regulator, and the warranty (optional). See Table of Materials for references.
NOTE: The total cost is $16,133 without shipping or customs fees. - Order medical dry air, a compressed medical gas that complies with standard grade (European pharmacopoeia reference 1238) from the local medical gas supplier. Purchase 2 cylinders of 6.8 m3 (47 L: 23 cm x 153 cm; diameter x length).
- Purchase a spare plexiglass lid. Purchase the cooling equipment system, cooler with mechanically refrigerated gas stream temperature controller, air dryer, pressure and flow regulator, and the warranty (optional). See Table of Materials for references.
- Set-up of the equipment (Figure 1 and Figure 2)
- Enter the equipment in the GMP cleanroom (disinfect before entering the cleanroom according to GMP procedures.
- For the medical grade compressed air, install a gas line instead of placing the gas cylinder directly in the cleanroom.
- Modification of the cell processor for cooling
- First, remove the plexiglass cover. Keep the original plexiglass cover in case there is a problem with the warranty. Drill a hole in the spare lid of the cell processor with a diameter to match and fit the outer diameter of the cooling device nozzle tip to prevent cracking the centrifuge cover (13.3 mm recommended).
- Fix brackets together with a circular piece of foam. This is done to prevent breakage and/or scratching the lid, to hold the cooler nozzle in place above the cell processor, to protect the lid from excessive bending due to the high pressure of injected air, to support the weight of the nozzle, and lastly to ensure the maximum safety to the operator to have free and safe access to all required cell processor functions during the experimental procedure.
- Connect the cooling system to the cell processor by attaching the cooling nozzle to the hole in the plexiglass. Secure it with brackets and foam cushions.
- Place the refrigeration housing up to 8 feet (2.44 m) away from the nozzle. House the temperature controller in a separate cabinet and place it within 6 feet (1.83 m) of the refrigeration housing.
- Set the cooler set point at -40 °C for cooling the air stream on the cooler. The exit air temperature is continuously indicated on the digital indicator to a resolution of 0.1 °C. Set the outlet temperature digitally to 0.1 °C with the temperature controller.
- Control the pressure and the flow rate of the air supply on the pressure and flow regulator module (Figure 2) before feeding to the cooler. Set them to 55 psi and 4 SCFM flow rates, respectively, to reach the optimum conditions on an air stream cooled at -30 °C ± 2 °C.
- Verify that the medical gas supply is regulated at an out pressure of 5 bar and can last at least 90 min, as this is required for the compressed air to reach -30 °C.
NOTE: Medical dry air is compressed medical gas, and fully pressurized dry air complies with standard grade. - Deliver 5 bar from a cooler nozzle connected to the cell processor lid through a hole drilled in the centrifuge cover. Inject the cooled medical air inside the centrifugation bowl of the cell processor (Figure 2). Select the position of the air injection in a way that the cooled air is directed perpendicularly to the aluminum rim of the centrifuge rotor.
- When preparing the cell processor, ensure the rotor is at room temperature (18 °C). Set the automatic thermo-regulator of the cooling system to -30 °C and direct cool air injections to the rotor.
NOTE: Different trial conditions were tested to reach an optimum temperature gradient of +4 °C, which is necessary to overcome the thermo-resistance of the cell processor cell separator system and heating of the rotor during centrifugation. The programmed thermo-regulator will maintain the optimum target temperature inside the cell processing machine during the procedure, particularly during the centrifugation, at a clean room temperature of +18 °C.- Set up the cell processor with the first pre-cooling phase to cool down the processor (without the cell processor kit) at +3 °C for 30 to 45 min, and the second pre-cooling phase of running the cell processor should be with the kit precooled at +4 °C at 134 x g, for 5 min.
2. Airborne particulate count
- Assess the compliance of the cooling device with the GMP cleanroom environment as per ISO 14644-1 standard to evaluate the impact of heat devices during the operation phase.
- Use particle count monitoring to provide evidence that the required level of cleanliness is achieved at critical control points at rest and in operation.
- The minimum number of sample locations depends on the area measured. Calculate this using the equation

where A is the area of the cleanroom or clean air-controlled space in m2, and NL is the number of sample locations rounded up to a whole number. - Airborne sampling volume is the minimum volume at each location. Ensure that the air volume is large enough to count 20 particles of the largest particle size specified. Calculate the airborne sampling volume through the equation
V= 20/C x 1000
where V is the minimum single sample volume per location, expressed in L, and C is the class limit (number of particles/m3). - For each location, depending on the area, use one or more samples. Ensure the least volume sampled at each location is 2 L and the minimum sample time is at least 1 min.
- The minimum number of sample locations depends on the area measured. Calculate this using the equation
- Before sampling, purge the particle counter device with a purging filter. Remove the filter and place it with the isokinetic probe for sampling.
- Place the particle counter instrument in the identified sampling location. Initiate sample collection from the main screen of the particle counter device.
- Once the sampling is over, then take the particle counter device to the next location. When all locations for sampling are finished, review the data, and print a report.
- When the average particle concentration for all sampling points in the area falls below the class limit stated in Table 1, consider the room passed for having the allowable particle concentration.
3. Gradient preparation and cell separation processing
- Prior to the experimentation, precool the light and heavy density gradients and the cell processor kits overnight at +4 °C in a refrigerator.
- Precool the cell processor for 45 min at rest (i.e., switch on the cell processor prior to use). Maintain the temperature between +2 °C and +5 °C. Insert the cell processor kit pre-cooled at +4 °C.
- For the standard ethanol cooled double jacketed gradient maker (optional; Figure 3) connect the 2 glass chambers and place them on a magnetic stirrer. Clamp the tubing between the 2 chambers. Cool the gradient maker with an ethanol-circulating cooling chiller at 0 °C.
- First, prepare to load the heavy layer at the bottom of the bag by filling the chamber with 130 mL of heavy density gradient (HD;1.087 g/mL). Load at a pump speed of 150 mL/ min.
- Once the heavy gradient is in the bag, add 130 mL of high density (HD) to the front beaker and 140 mL of low density (LD; 1.063 g/mL) gradients to the back (clamp closed).
- De-clamp the hemostat/clamp between the 2 chambers and create a continuous gradient at 50 mL/min pump speed.
- Prepare in advance human mononuclear buffy coat cells for purification from multiple pooled anonymous buffy coats collected from whole blood waste products (40 mL to 60 mL of whole blood was added to 10 buffy coat bags) depending on the availability from the blood bank.
- MNC Separation
- Use density gradient centrifugation for low-density human mononuclear cell isolation. To do this, follow the steps described below.
- For cell preparation, calculate the volume of blood (anticoagulated whole blood or suspended buffy coat). Dilute the blood with 2x-4x buffer solution by mixing inversion. The more diluted the blood, the better the purity of the mononuclear cells.
- Prepare buffer by mixing phosphate buffer with an anticoagulant. Calculate the number of tubes needed by dividing the volume of blood by 35 mL and pour 15 mL of 1.073 density gradient solution in each tube.
- Gradually layer 35 mL of the diluted blood suspension over the density gradient by gently running on the surface of the density gradient by using a 60 mL syringe connected to a needle; for small volumes, use a pipette. Try not to disrupt the interface, as this will result in a poor mononuclear layer.
- Centrifuge at 400 x g for 20 min at 20 °C without any breaks (acceleration 0, deceleration 0). After centrifugation, gently harvest the interface layer of mononuclear cells using a sterile transfer pipette. Transfer the cells into another new 50 mL conical tube.
- Wash the cells 2x with buffer (PBS with added anticoagulant). For the first wash, fill the tube containing the cells with buffer till 50 mL. Centrifuge at 300 x g for 10 min at 20 °C to pellet cells (acceleration 9, deceleration 0). Gently remove all supernatant carefully.
- Perform the second wash for the removal of platelets. Resuspend the pellet to a volume of 50 mL with buffer. Centrifuge at 200-300 x g for 10 min at 20 °C (acceleration 9, decceleration 0).
- Reconstitute the cells to a final volume of 5 mL, these cells should have specific gravity resembling low-density MNC <1.073. Measure the specific density of the gradient with a densiometer.
- Resuspend the pellet to a final volume of 100 mL with 95 mL of high-density gradient medium (1.083 g/mL) in a transfer bag. Use this product for purification using a cell separator. The final volume of 100 mL contains cells and 1.083 high-density gradient medium.
NOTE: The buffy coat may be stored in the refrigerator overnight in PBS containing 0.5% BSA or autologous plasma. The buffy coat can also be washed.
- MNC Separation
- Top load the preformed gradients with a maximum of 30 mL of MNC isolated from the buffy coat suspended in 100 mL of concentrated conservation medium using a peristaltic pump at 25 mL/min. Keep the gradient at +4 °C.
- Continuously measure the temperature inside the cell processor with a pre-calibrated thermoprobe. Using a digital thermocouple connected to a sterile thermoprobe, monitor the temperature of gradients and cells during the process.
4. Optimization of different steps of gradient loading
- Insert the cell processor kit pre-cooled at +4 °C. Load the heavy gradient (130 mL) at + 4.5 °C. At the end of gradient loading, set the centrifuge at a relatively low speed (537 x g) in order to minimize the heat generated by the rotor.
- Turn off the cell processor to allow the hydraulic system to go back to pre-run levels and release air.
- Restart the cell processor at 537 x g and add the heavy (130 mL) and low (140 mL) density gradients to create a continuous density gradient at +4.3 °C and a flow rate of 50 mL/min.
- Load the buffy coat (30 mL) with 100 mL of cold storage medium at +6 °C and a flow rate of 25 mL/min. Add 50 mL (25 mL x 2) of washing solution.
- After 5 min at +5 °C, collect spun gradient in 12 bottles at a flow rate of 100 mL/min.
- Fill tubes with wash media (M199 + 20% human albumin + 10% Penicillin streptomycin). Fill tube 1 with 100 mL of wash media and 150 mL of cells and tubes 2-12 with 225 mL of wash media and 25 mL of cells.
Results
The impact of the cooled cell processor on the GMP cleanroom was first quantified. Standardized methods used a particle counter measuring the airborne particle contamination in a GMP grade class C and class B clean room environment at critical points in proximity to the cell processor and pressurized air cooler system. Results demonstrated that no turbulence developed around the equipment. Recorded data showed that there was no excess of 0.5 µm and 5 µm airborne particulate as per cleanroom GMP grade C, grade B, and BSL III standards (Figure 4).
All experiments were performed with continuous polysaccharide density gradients. First, temperature measurements of gradient solutions were tested without cells at different time points in 6 runs in the cooled and non-cooled cell processor. In subsequent studies, human cells were purified on density gradients using the cooled cell processor.
All data were expressed as a mean of 3 independent trials ± standard deviation (SD) and analyzed with commercial analysis software. Statistical significance was set as p<0.05. As shown in Figure 5, there was no significant difference in the gradient temperatures measured in the cooled and non-cooled cell processor during the first four steps; the recorded temperature was approximately +4.5 °C. However, after the 5 min centrifugation period and during the collection steps, the temperature rose at the beginning and the end of the collection steps to 21.50 °C ± 0.34 °C and 21.93 °C ± 0.20 °C in the non-cooled cell processor, respectively, and to only 10.9 °C ± 0.17 °C and 11.6 °C ± 0.35 °C in the cooled- cell processor (p <0.05), respectively (Figure 5).
Subsequent studies strove to determine the impact of the cooled cell processor on the efficiency of human cell purification and viability. Three concentrated human-pooled buffy coats were used in this study. The rationale for using a buffy coat rather than an islet preparation was due to the limited availability of pancreas donors in Qatar.
The temperature in the clean room was at +16.5 °C ± 1.5 °C, whereas the temperature inside the biosafety cabinet was +18.8 °C ± 0.47 °C. At the end of the gradient loading, the cooled cell processor temperature dropped to +3.3 °C ± 2.3 °C. Cells and cell processor temperature during loading, centrifugation, and collection was +4 °C. After centrifugation, the temperature of the collected cells (from tubes number 1 to 12) rose slightly to +8.5 °C ± 3.5 °C (Figure 6).
Active air sampling was performed in grade A BSLIII using calibrated air sampler to count the number of viable and living organisms per cubic meter of air (blood agar/Sabouraud dextrose agar plate). Sterility screening was tested on the 3 sample trials at the reception and post-processing in the cooled cell processor system, using pediatric blood culture bottles for aerobic and anaerobic organisms and fungi; 3 mL of drawn samples from each buffy coat showed no growth (Figure 7).
Finally, we evaluated the viability of the human cells after density gradient purification in the new cooled cell processor compared to the non-cooled processor. CD34+ viability was investigated by 7-AAD staining and trypan blue exclusion assay23 as well as total nucleated cells were assessed before and after the purification procedure on the concentrated buffy coat cells (Table 1). Mononuclear cell MNC was the fraction of interest being purified from starting material of TNC, MNC% increased after purification. The CD34 cells isolated from the TNC maintained their viability during the process of purification in the cooled cell processor to a greater extent than in the non-cooled processor (viability with 7-AADc: cooled 100% + 0.58% versus non-cooled 76% + 7.8% p=0.1, with trypan blue cooled 98% + 0.58% vs non-cooled 75% ± 7.2% (p<0.05)).
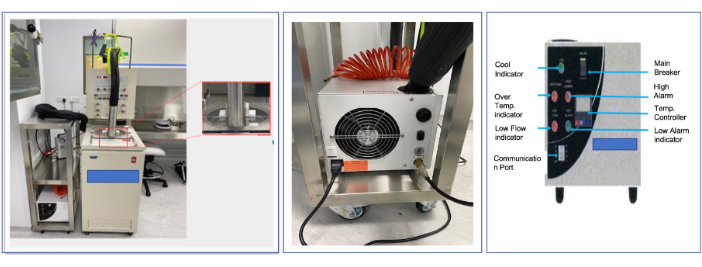
Figure 1. General presentation of the cooling system and the cooler. The figure shows the various equipment used in this study. Please click here to view a larger version of this figure.
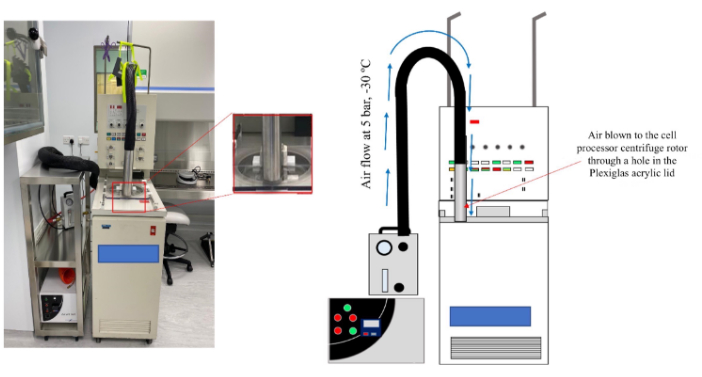
Figure 2. Pre-cooling setup of the cell processor to cool down the centrifuge bowl. The figure shows how the setup was done. Please click here to view a larger version of this figure.
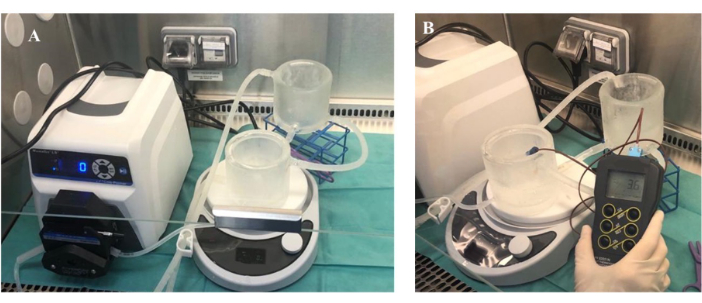
Figure 3. Temperature monitoring of jacketed gradient to cool polysaccharide gradient. The figure shows the temperature measured in the polysaccharide gradient. Please click here to view a larger version of this figure.
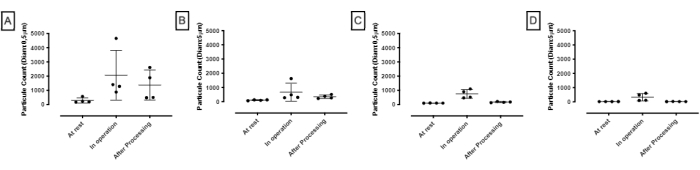
Figure 4: Airborne particle assessment in clean room environment grade C and grade B. (A,B) The measurements of the airborne particle contamination were performed before, during, and after processing. The data was from 2 different sampling sites in a 33 m2 room at 2 different time intervals (n=4). The GMP standards for 0.5 µm particulate in the clean room class C at rest and in operation are 352,000 and 3,520,000, respectively. Those for 5 µm particulate are 2,900 and 29,000, respectively. (C,D) The data was from 2 different sampling sites in a 30 m2 room at 2 different time intervals (n=4). The GMP standards for 0.5 µm particulate in the class B clean room at rest and in operation are 3,520 and 352,000, respectively, and for 5 µm particulate, are 29 and 2,900, respectively. The data shows mean + SD. Please click here to view a larger version of this figure.

Figure 5: Temperature of density gradient media with and without cooled cell processor. Temperatures of polysaccharide gradients were measured during the processing in the absence of human cells. Data is expressed as the mean of 6 independent experiments ± S.D. *p < 0.05; ***p<0,001 cooling versus non-cooling (2 Way ANOVA + Sidak post-test). Please click here to view a larger version of this figure.

Figure 6: Temperature of the cooled versus uncooled cell processor and further optimization. (A) Data are shown as mean ± SD of 3 independent trials: cleanroom environment: clean room; 1st phase precooling: 1st phase of cell processor pre-cooling, 2nd phase precooling: 2nd phase of cell processor precooling; Abbreviations = HD-G: temperature of heavy density gradient; Cell Processor-HD: temperature in the cell processor at the end of heavy density gradient loading; HD+LD loading: heavy and light density gradient loading; End G-loading: Temperature of the gradient at the end of loading; Cell loading: temperature of cells at loading; Cells-Cell processor: temperature of cell processor during cell loading; centrifugation: temperature of cell processor during centrifugation; Tube number 1 to 12: temperature of cells.(B) Optimization of the cooling system. Data are shown as mean ± SD of 3 independent trials. Abbreviations = cleanroom environment BSLIII: biosafety cabinet level 3; 1st precooling: 1st phase of cell processor pre-cooling, 2nd precooling: 2nd phase of cell processor precooling; HD-G: temperature of heavy density gradient; cell processor -HD: temperature in the cell processor at the end of heavy density gradient loading; HD+LD loading: heavy and light density gradient loading; End G-loading: Temperature of the gradient at the end of loading; Cell loading: temperature of cells at loading; Cells-cell processor: temperature of cell processor during cell loading; Centrifugation: temperature of cell processor during centrifugation; Collection: Temperature of cell processor during collection; Tube number 1 to 12: temperature of cells. Custom conical cooling tray for collection, kept at -20 °C overnight. Cell processor kit, kept at + 4 °C. Measured temperature of the cooled cell processor during the post-processing phase remained low at +4 °C. (C) Effect of the non-cooled cell processor on cell temperature. Please click here to view a larger version of this figure.
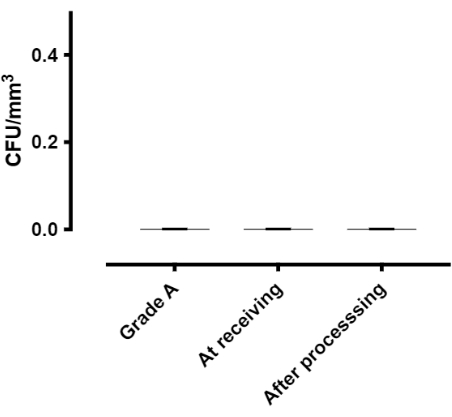
Figure 7. Air sampling/sterility screening benchmark. Sterility screening was tested (n=3) during the process in grade A room, at the time of the receiving sample and at the end of the procedure. The benchmark value is 0 CFU/m3. Please click here to view a larger version of this figure.
Table 1: Cell viability before and after purification in the cooled cell processor. Abbreviations = aTNC: Total nucleated cells count; bMNC: mononuclear cells; c 7-ADD: 7-aminoactinomycin D. Please click here to download this Table.
Table 2: Average particle concentration. Please click here to download this Table.
Discussion
Islet isolation facilities around the world have adopted for decades human islet purification in a repurposed cell processor. Although some islet production facilities continue to process islets in an unrefrigerated cell processor, most centers use a refrigerated version of the cell processor10,11,15. Centers either place the cell processor in a cold room which has the major disadvantage of exposure to humidity and condensation, or technically make major modifications to the cell processor itself, including the shaft, which has immediate repercussions on the warranty of the machine and its preventive maintenance. The presented new GMP cell therapy compatible cell processor cooling system can be beneficial to perform cell processing at a defined low temperature (+ <10 °C), which cannot be achieved during centrifugation with the original cell processor cell-separator at room temperature.
The air-cooling technology was first used by the group in Brussels20 and implemented in Leiden21. The new version of the cooling system that requires compressed medical-grade air, available in most hospital settings, was tested for cooling the cell processor in a GMP cleanroom setting Doha-Qatar. Indeed, compressed air is less of a risk in the confined space of a clean room than nitrogen19. Based on the literature and our own experience, the critical step, which was the aim of this study, was to reduce the temperature to < +10 °C. This can be achieved with the cooled cell processor but also requires the use of pre-cooled solutions and maintaining the temperature of the gradients during the continuous or discontinuous gradients by using a cooled ethanol jacketed gradient maker or an automated gradient maker5,6,7 with cooling packs around the gradient and on the tubing toward the cell processor. Note that the near-zero temperatures observed during the precooling were in the absence of cells: zero or subzero-degree temperatures must be avoided once cells are introduced into the system. One must also ensure before starting that there is a sufficient supply of medical gas for the entire procedure.
Temperature has been considered an important part of cell processing and purification of the MNC fraction. Indeed, intracellular organ preservation solutions have to be used at hypothermic temperatures. The rather recent routine use in islet isolation labs of gradient medium diluted to the appropriate density with cold storage organ preservation medium in human islet purification22 suggests that increases in temperature above hypothermic temperatures may have detrimental effects on cells.
Continuous measurement of the temperature identified that increases in temperatures occurred at the end of centrifugation and the start of collection (Figure 6A). These changes appeared to be due to the intermittent cut-out of the compressor. Temperature increased from +4 °C to +7.2 °C during the last gradient density-phase HD-G (Figure 6B). To avoid the increase in temperature during this critical period, we further optimized cooling (Figure 6B) by using several measures: extending the first pre-cooling phase from 30 to 45 min, pre-cooling the cell processor kit overnight in the +4 °C refrigerator, as well as using a conical cooling tray taken from a -20 °C freezer. We document further improvement in the collection temperature as well as the media. Caution must be taken with the -20 °C racks used to pre-cool the system prior to the addition of cells since the temperature drops to 0 °C and HDG from 0- 5.8 °C. Hence, we are currently using -5 °C racks to avoid the potential risk of freezing cells.
The data shown (Figure 4) demonstrated that no turbulence developed around the equipment, and the newly developed cooling system presented no contamination risk in a GMP environment since we did not detect any excess of 0.5 µm and 5 µm airborne particulate as per cleanroom GMP grade C and BSL III standards. Furthermore, the concentrated buffy coat cells showed no contamination or growth during the purification step (Figure 7) with the cooled cell processor system.
Finally, we tested the impact of the cooled and non-cooled cell processor on human cell viability and purification efficiency after density gradient purification with a human cell concentrated buffy coat. Quality controls for the MNC purified fraction on pre- and post-processing confirmed excellent cell viability measured with 7-aminoactinomycin D on flow cytometer was (100% ± 2%) or using trypan Blue (98%) and 98% purity of isolated MNC fraction (Table 1) which was superior to the viability observed on a non-cooled cell processor.
The described technology here requires drilling a small 13.3 mm hole in the plexiglass lid of the cell processor. To avoid the risk of the constructor refusing to service the machine, a discussion with the constructor must be engaged prior to the modification. A spare plexiglass lid should be ordered, and if necessary, the original lid (with no hole) can always be placed back on the processor prior to maintenance. The authors have not encountered such a problem. Note that the majority of the cell separator processing machines used throughout the world no longer have a warranty since they have been in service for several decades. Further optimization may include injecting cooled air elsewhere on the shaft. However, one must avoid condensation around electrical circuits. Other optimizations include encasing the cell therapy apparatus in a cooled environment without modifying the structure (i.e., no hole) and blowing air in the encasement (this was not tested here).
Alternative methods, including bottle purification8,9, may indeed be superior to purification in the cell processor-separator. However, in a GMP environment, one strives to eliminate open systems; this is the ultimate goal23.
In conclusion, the new pressurized cooling system efficiently maintains a cooled temperature of the cell processor during density gradient purification without any particle impact on GMP clean room environment. The cooled cell processor described here is a readily available, simple, inexpensive ($16,000 without taxes, customs clearance, and transportation), minimally invasive method, to provide robust cooling and maintenance of temperature during cell processing. Currently, the cell therapy laboratory at HMC/Qatar and CHU Lille will apply this cooling technology for human islet cell isolation and purification. We foresee that this technology of cooling may be extended to other non-refrigerated devices in GMP cleanrooms.
Disclosures
The authors declare no conflict of interest.
Acknowledgements
The authors would like to acknowledge the European genomic institute for diabetes (ANR-10- LABEX-0046 to FP) and Qatar metabolic institute - Hamad medical corporation, Qatar. The authors thank as well the Medical Research Center at Hamad Medical Corporation for article processing fees support.
Materials
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Air particles counter | Lasair III | ||
| AirJet XR40 | SP Scientific | FTS system XR40 | |
| Biosafety Cabinet | Thermo Scientific | EQ-1301 | |
| Circulating Cooling Chiller | Julabo | CF30 | |
| COBE 2991 | Terumo BCT | ||
| Conical Cooling Tray | Biorep | CCT-01 | |
| Double jacketed gradient maker | customized in house | ||
| KJT-Thermocouple Thermometer | Hanna Instruments | HI93551N | For measuring liquids temperature |
| Magnetic stirrer | Thermo Scientific | 88880014 | |
| Peristaltic pump | MasterFlex | MK-77921-79 | |
| Thermometer | Extech Instruments | RMS 430 | For COBE temperature |
| Waterless Bead Bath | Cole-Parmer | 10122-00 | |
| Materials and reagents | |||
| BD stem cell enumeration kit | BD Biosciences | 344563 | |
| COBE 2911 tubing kit | Terumo BCT | 90819 | |
| Conical Tubes 250ml | Corning | 430776 | |
| Gradient density 1.1 | Biochrom | L6155 | |
| Graduated disposable bottles | Thermo fisher | 382019-1000 | |
| Human albumin 20% | Kedrion Biopharma | 8091600 | |
| M199 washing solution | Corning | 99-784-CM | |
| Masterflex tubes | Masterflex | 96400-6 | |
| Medical Dry Air | Linde Healthcare | Medi On 22 | |
| Pencillin-streptomycin | Gibco | 15140122 | |
| Thermoprobe | Biorep | TC-02 | Thermosensor |
| Trypan blue | Sigma Aldrich | 15250061 | |
| University of Wisconsin Belzer (UW) | Bridge of Life | RM/N 4055 | Conservation Medium |
References
- Shapiro, A. M., et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. The New England Journal of Medicine. 343 (4), 230-238 (2000).
- Vesilind, G. W., Simpson, M. B., Colman, R. E., Kao, K. J. Evaluation of a centrifugal blood cell processor for washing platelet concentrates. Transfusion. 28 (1), 46-51 (1988).
- Lake, S. P., et al. Large-scale purification of human islets utilizing discontinuous albumin gradient on IBM 2991 cell separator. Diabetes. 38, 143-145 (1989).
- Ricordi, C., et al. Human islet isolation and allotransplantation in 22 consecutive cases. Transplantation. 53 (2), 407-414 (1992).
- Friberg, A. S., Stahle, M., Brandhorst, H., Korsgren, O., Brandhorst, D. Human islet separation utilizing a closed automated purification system. Cell transplantation. 17 (12), 1305-1313 (2008).
- Bampton, T. J., et al. Australian experience with total pancreatectomy with islet autotransplantation to treat chronic pancreatitis. ANZ Journal of Surgery. 91 (12), 2663-2668 (2021).
- Doppenberg, J. B., Engelse, M., de Koning, E. J. P. PRISM: A Novel Human Islet Isolation Technique. Transplantation. 106 (6), 1271-1278 (2021).
- Shimoda, M., et al. Islet purification method using large bottles effectively achieves high islet yield from pig pancreas. Cell Transplantation. 21 (2-3), 501-508 (2012).
- Noguchi, H. Pancreatic Islet Purification from Large Mammals and Humans Using a COBE 2991 Cell Processor versus Large Plastic Bottles. Journal of Clinical Medicine. 10 (1), 10 (2021).
- Shapiro, A. M., et al. International trial of the Edmonton protocol for islet transplantation. The New England Journal of Medicine. 355 (13), 1318-1330 (2006).
- Ricordi, C. National Institutes of Health-Sponsored Clinical Islet Transplantation Consortium Phase 3 Trial: Manufacture of a Complex Cellular Product at Eight Processing Facilities. Diabetes. 65 (11), 3418-3428 (2016).
- Swift, S., Kin, T., Mirbolooki, M., Wilson, R., Lakey, J. R. T. Comparison of cooling systems during islet purification. Cell Transplantation. 15 (2), 175-180 (2006).
- Rilo, H. L. R., et al. Comparison of islet number and viability obtained during purification on Euro-Collins-Ficoll gradients processed on a refrigerated or nonrefrigerated COBE 2991 cell separator. Transplantation Proceedings. 26 (6), 3430 (1994).
- Chadwick, D. R., et al. Pancreatic islet purification using bovine serum albumin: The importance of density gradient temperature and osmolality. Cell Transplantation. 2 (4), 355 (1993).
- Nano, R., et al. Heterogeneity of human pancreatic islet isolation around Europe: results of a survey study. Transplantation. 104 (1), 190-196 (2020).
- Chadwick, D. R., et al. Storage of pancreatic digest before islet purification. Transplantation. 58 (1), 99-104 (1994).
- Amrani, M., et al. Detrimental effects of temperature on the efficacy of the University of Wisconsin solution when used for cardioplegia at moderate hypothermia. Comparison with the St. Thomas Hospital solution at 4 degrees C and 20 degrees C. Circulation. 86, 280-288 (1992).
- Fremes, S. E., Li, R. K., Weisel, R. D., Mickle, D. A., Tumiati, L. C. Prolonged hypothermic cardiac storage with University of Wisconsin solution. An assessment with human cell cultures. The Journal of Thoracic and Cardiovascular Surgery. 102 (5), 666-672 (1991).
- Adewola, A., et al. Comparing cooling systems for the COBE 2991 cell separator used in the purification of human pancreatic islets of Langerhans. Cryo Letters. 31 (4), 310-317 (2010).
- Keymeulen, B., et al. Implantation of standardized beta-cell grafts in a liver segment of IDDM patients: graft and recipients characteristics in two cases of insulin-independence under maintenance immunosuppression for prior kidney graft. Diabetologia. 41 (4), 452-459 (1998).
- de Kort, H., de Koning, E. J., Rabelink, T. J., Bruijn, J. A., Bajema, I. M. Islet transplantation in type 1 diabetes. BMJ. 342, (2011).
- Barbaro, B., et al. Improved human pancreatic islet purification with the refined UIC-UB density gradient. Transplantation. 84 (9), 1200-1203 (2007).
- Lemarie, C., et al. A new single-platform method for the enumeration of CD34+ cells. Cytotherapy. 11 (6), 804-806 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved