Method Article
Immunocytochemical Visualization of Proteins from Cyanobacterial Cells with High Autofluorescence of Phycoerythrin and Phycourobilin
In This Article
Summary
This protocol outlines the visualization and quantification of a particular protein within cells at the cellular level for the phycoerythrin-containing cyanobacterium, Crocosphaera watsonii.
Abstract
Presented is a protocol for visualizing and quantifying a specific protein in cells at the cellular level for the marine cyanobacterium Crocosphaera watsonii, a crucial primary producer and nitrogen fixer in oligotrophic oceans. One of the challenges for marine autotrophic N2 fixers (diazotrophs) is distinguishing probe-derived fluorescence signals from autofluorescence. C. watsonii was selected to represent chlorophyll-, phycoerythrin- and phycourobilin-containing cyanobacteria. The protocol allows for simple and semi-quantitative visualization of proteins in C. watsonii at a single-cell level, enabling investigation of protein production under different environmental conditions to evaluate the metabolic activities of the target cyanobacteria. Furthermore, the fixation and permeabilization methods are optimized to enhance the fluorescence signals from target proteins to distinguish them from autofluorescence, especially from phycoerythrin and phycourobilin. The enhanced signal can be visualized using confocal or widefield fluorescence microscopy. Additionally, fluorescence intensity was semi-quantified using Fiji software. This single-cell analysis workflow allows the evaluation of cell-to-cell variations of specific protein content. The protocol can be performed in any life science laboratory as it requires only standard equipment and can also be easily adapted to other phycoerythrin-containing cyanobacterial cells.
Introduction
The physiological variation from cell to cell (commonly referred to as "heterogeneity") in metabolic activities within microorganisms, including cyanobacteria, has been documented through studies on clone cultures1,2,3,4. This heterogeneity encompasses diverse metabolic activities such as cell division5, carbon assimilation6,7,8, and nitrogen assimilation9,10. For instance, recent investigations have indicated that N2 fixation activity in colonial cyanobacteria C. watsonii and C. subtropica (Cyanothece) exhibits single-cell level variability, being present in subpopulations of cells while absent in others within the community. Notably, the nitrogen uptake or N2 fixation activities also exhibit variability among cells in situ11,12,13. These findings have been substantiated by stable 15N isotope analyses conducted with isotope ratio mass spectrometers (NanoSIMS)14,15. However, despite NanoSIMS offering a novel avenue for analyzing isotopic composition at the individual cell level, its use remains constrained due to its technical complexity and cost.
An alternative approach to observe intracellular heterogeneity in metabolic activities is through immunodetection. Earlier reports have demonstrated the immunodetection of nitrogenase in individual cells, but this poses challenges due to the autofluorescence emitted by their photosynthetic pigments16,17,18. Marine cyanobacteria, particularly those adapted to oceanic waters such as the major oceanic diazotrophs C. watsonii and Trichodesmium, contain substantial amounts of phycobilins that emit autofluorescence in shorter wavelengths: phycoerythrin and phycourobilin19. To circumvent this autofluorescence, blue-emitting fluorochromes with UV excitation have been favored for cyanobacteria studies16,20,21. However, this strategy hasn't consistently yielded success, as cells treated solely with primary antibodies emitted strong blue to bluish-yellow autofluorescence under UV excitation20,21. Efforts have been made to mitigate this issue by subjecting cells to blue or UV light exposure prior to observation and by employing semiconducting nanocrystals22. The present study employs a different strategy that enhances protein fluorescence signals using the tyramide signal amplification system (TSA) to visualize proteins with low cellular content.
TSA, also known as catalyzed reporter deposition (CARD), is a highly sensitive enzymatic method enabling the detection of low-abundance targets in immunocytochemistry. This technique leverages peroxidase's catalytic activity to covalently deposit labeled tyramide in proximity to target proteins in situ23,24. In the presence of hydrogen peroxide, peroxidase catalyzes the oxidative condensation of tyramide into reactive tyramide radicals, which then bind to electron-rich moieties such as tyrosine, phenylalanine, and tryptophan25. This enhances signals by up to 10 to 200-fold compared to standard methods, making the signal detectable via standard chromogenic or fluorescent techniques. Consequently, this technique facilitates the rapid and simultaneous assessment of multiple proteins alongside phenotypic markers in heterogeneous populations and rare cell subsets. Notably, as of now, the amalgamation of immunolabeling and TSA systems for cyanobacteria has been limited to a single study that visualized saxitoxin in Cylindrospermopsis raciborskii26.
The method outlined herein permits the investigation of protein production under varying environmental conditions at the single-cell level, enabling the assessment of metabolic activities in target cyanobacteria. The availability of whole-cell immunofluorescence protein detection allows for swift and semi-quantitative visualization of proteins in C. watsonii at the single-cell level. Moreover, this method can be easily adapted for use with other cyanobacterial cells containing phycourobilin and phycoerythrin.
Protocol
1. Cyanobacteria cultivation
- Cultivate Crocosphaera watsonii PS0609 cells in Erlenmeyer flasks or photobioreactors in YBCII medium27. To determine the culture density, measure the cell density using a method of choice (e.g., microscopy, flow cytometry, cell counter, etc.).
- Maintain the cell densities between ~1 × 104 to ~5 × 106 cells mL-1.
2. Preparation of reagents
- Keep 100% methanol at -20 °C.
- Phosphate-buffered saline (PBS): Dissolve 1.36 g of KH2PO4, 1.42 g of Na2HPO4, 8.0 g of NaCl, and 0.224 g of KCl in 0.8 L of distilled water, fill to 1 L, adjust pH at 7.4 and autoclave it (see Table of Materials).
- 1% paraformaldehyde (PFA) in PBS
- Add 50 mL of PBS to a glass beaker on a stir plate in a ventilated hood, heat it to 60 °C, and mix by stirrer.
- Introduce 0.5 g of paraformaldehyde powder into the warmed PBS solution. Gradually elevate the pH by drip-feeding 1 N NaOH from a pipette until the solution becomes transparent. After the paraformaldehyde has fully dissolved, lower the temperature and proceed to filter the solution.
- Adjust the volume of the solution to 40 mL with PBS. Adjust the pH with small amounts of dilute HCl to approximately 6.9. Preserve the solution at -20 °C.
- PBG blocking solution (0.2% gelatin + 0.5% bovine serum albumin; BSA, see Table of Materials)
- Dissolve 0.2 g of gelatin and 0.5 g of BSA in 100 mL of PBS in a 200 mL glass beaker. Heat the mixture to 60 °C and stir. Then, aliquot it into a 15 mL tube and store it at -20 °C.
- 0.2% Triton X-100 in PBS: Add 20 µL of Triton X-100 into 10 mL of PBS. Prepare it in a 15 mL tube and preserve it at -20 °C.
- 0.5 M EDTA: Dissolve 1.46 g of EDTA into 10 mL of distilled water (DW) and adjust pH to 8.0 (EDTA will not dissolve in pH < 8.0). Prepare it in a 15 mL tube and preserve it at -20 °C.
- 1 M Tris HCl: Dissolve 1.58 g of Tris HCl into 10 mL of DW and adjust pH to 8.0. Prepare it in a 15 mL tube and preserve it at -20 °C.
- 50 mM Tris HCl: Dissolve 0.079 g of Tris HCl into 10 mL of DW and adjust the pH to 7.0. Prepare it in a 15 mL tube and preserve it at -20 °C.
- Add 10 mg/mL lysozyme: Add 100 mg of lysozyme, 1 mL of 0.5 M EDTA (pH 8), and 1 mL of 1 M Tris HCl (pH 8). Add DW up to 10 mL into a 15 mL tube. Prepare it freshly on the day of use.
- 10,000 units/mL achromopeptidase: Dissolve 0.011 g of 900 units/mg achromopeptidase (see Table of Materials) to 1 mL of DW, mix well, and preserve at -20 °C.
- 60 units/mL achromopeptidase working solution: Add 3 µL of 10,000 units/mL achromopeptidase in 0.5 mL of 50 mM Tris HCl. Prepare it freshly on the day of use.
- Prepare 100x tyramide stock solution: Dissolve the tyramide reagent in 150 µL of dimethyl sulfoxide (DMSO). Store at 2-8 °C for up to 6 months.
- 1x Reaction buffer: Add 1 drop of 20x Reaction buffer to 1 mL of DW. This can be replaced with Tris buffer, pH 7.4.
- Reaction stop reagent solutions
- Reaction stop reagent stock solution: Add 1.45 mL of 95% ethanol to one vial of Reaction stop reagent contained in the tyramide signal amplification kit (see Table of Materials). Store at -20 °C for 6 months
- Reaction stop reagent working solution: dilute the reaction stop reagent stock solution (1:11) in PBS. Prepare it freshly on the day of use.
- 100x H2O2 solution: Add 1 drop (about 50 µL) of H2O2 to 1 mL of DW. Prepare it freshly on the day of use.
- Tyramide working solution (for a final volume of 510 µL): Add 5 µL of 100x tyramide stock solution, 5 µL of 100x H2O2 solution, and 500 µL of 1x Reaction buffer into 1.5 mL tube in the mentioned order, and gently mix well by pipetting. Prepare it freshly on the day of use.
3. Harvesting cells
- Harvest 10 mL of C. watsonii culture into a 15 mL tube, collect cells by centrifugation at 5,500 × g at 4 °C for 8 min, and discard the supernatant. Keep the samples on ice between the procedure.
- Resuspend the pellet with the remaining supernatant, transfer the resuspended subsample to a 1.5 mL tube, and add 1 mL of PBS to the wash. Collect cells by centrifugation (5,500 × g, 4 °C, 5 min), and discard the supernatant.
4. Fixation and preservation of cells
- Add 1 mL of 1% PFA solution and incubate for 15 min at room temperature.
- Centrifuge the cells at 5,500 × g, 4 °C, 5 min, remove the supernatant by pipet and discard it into the assigned chemical waste bin, add 1 mL of PBS, and resuspend it.
- Centrifuge the cells (5,500 × g, 4 °C, 5 min), remove the supernatant by pipet and discard it. Add 1 mL of 100% methanol (kept at -20 °C) and resuspend using a vortex mixer. Keep the subsamples at -20 °C for 15 min to overnight.
5. Permeabilization and blocking
- Centrifuge the cells (5,500 × g, 4 °C, 5 min), remove the supernatant (methanol) by pipette and discard it into the assigned chemical waste bin, add 1 mL of PBS, and resuspend it.
- Centrifuge the cells (5,500 × g, 4 °C, 5 min), remove the supernatant by pipette and discard it, add 1 mL of 0.2% Triton X-100 and incubate for 15 min at room temperature.
- Centrifuge the cells (5,500 × g, 4 °C, 5 min), remove the Triton X-100-supernatant by pipette and discard it, add 1 mL of PBS and resuspend it.
- Centrifuge the cells (5,500 × g, 4 °C, 5 min), remove the supernatant using a pipette, add 0.5 mL of lysozyme (see Table of Materials) and 0.5 mL of PBG (step 2.4), and incubate for 3 h at 37 °C.
- Centrifuge the cells (5,500 × g, 4 °C, 5 min), carefully remove the supernatant using a pipette and discard it into an assigned waste bin, add 0.5 mL of 60 units achromopeptidase (step 2.10) and 0.5 mL of PBG and incubate for 30 min at 37 °C.
- Centrifuge the cells (5,500 × g, 4 °C, 5 min), remove achromopeptidase supernatant using a pipette, discard it into the assigned waste bin, and add the necessary amount of PBS.
NOTE: The necessary amount should be decided by the number of subsamples to be observed (one subsample is 90 µL for ~2 cm diameter circle). Ensure to prepare negative control(s); without 1st nor 2nd antibody, and without 1st antibody.
6. Preparation of samples for imaging
- Place samples on a glass slide and add the 1st antibody28.
- Draw two about ~2 cm diameter circles on a poly-lysin-coated slide glass with a liquid-repellent slide marker pen (see Table of Materials). Apply 90 µL of subsamples into the circles. Dry the applied subsample in a chemical hood. It takes about 30-60 min.
- Rehydrate the subsample and wash it with 90 µL of PBS, incubate for 2 min, and discard the PBS. Repeat the steps 2 times.
- Dilute the 1st antibody28 (1 mg mL-1) 200 times in PBG blocking solution. Adjust the amount of the solution depending on the number of samples.
- Apply 90 µL of primary (1st) antibody28 on each sub-sample except negative control. Put distilled water-wet tissue paper in a chamber, close it by a cover, and wrap it with grafting tape. Incubate overnight at 4 °C.
- Apply 2nd antibody.
- Remove the excess of 1st antibody by allowing the glass slide to stand on its long side on tissue paper, turn it to bring the cells up, and add 0.2% Triton X-100 into each circle using a pipette and incubate 2 min to rinse the cells. Repeat 5 times.
- Gently apply poly-horse radish peroxidase (HRP) conjugated secondary (2nd) antibody (goat, anti-rabbit IgG) (see Table of Materials), and incubate for 60 min at room temperature. Gently rinse the cells with PBS for 10 min; repeat 3 times.
- Tyramide signal amplification
- Add 90 µL of tyramide working solution onto samples, and incubate for 10 min at room temperature. Discard the solution by allowing the glass slide to stand on the long side on a tissue paper. Add 90 µL of reaction stop reagent and incubate for 3 min at room temp. Discard the solution.
NOTE: The incubation time should be tested for each target protein or for each microscope. The incubation time can be shortened, or the step can be omitted to observe the protein allocation using a confocal microscope to avoid oversaturation of the signal.
- Add 90 µL of tyramide working solution onto samples, and incubate for 10 min at room temperature. Discard the solution by allowing the glass slide to stand on the long side on a tissue paper. Add 90 µL of reaction stop reagent and incubate for 3 min at room temp. Discard the solution.
- Rinse the cells with PBS (5 times) every 30 min. Rinse the cells with DW. Remove the water briskly. Add one drop of mounting medium (see Table of Materials) and place the cover glass.
- Gently press the cover glass to remove the excess mounting medium and let it dry for about 30 min. Seal it with transparent nail polish and store it in the dark.
7. Detection of fluorescence signal using a fluorescence microscope
- Power on the fluorescence microscope, computer, and UV light source. Open the software for the camera connected to the microscope (see Table of Materials). Set to monitor live images. Switch off the light in the room.
- Insert the glass slide containing the cells on the specimen stage. Use the lens with the smallest magnification and adjust the objective and condenser lens to fine-tune the focus. Change the lens to larger magnifications one by one.
- Put immersion oil on top of the cover glass and change the lens to the oil immersion 100x lens. Cover the light source from the light source, select the DAPI filter set for Tyramide-350 analysis, and open the excitation UV light shutter.
- Test 3-4 exposure times and decide the best exposure time without oversaturation. Select the FITC filter set for phycoerythrin observation, repeat the procedure, and decide the best exposure time. Contemplate utilizing an increased gain or binning mode on the camera to expedite the procedure and mitigate signal degradation due to phototoxicity. Set the gain and binning mode back for image acquisition.
- Select the field of view, take a snapshot for a bright field image, tyramide image, and phycoerythrin image with the selected exposure time for each image.
8. Detection under a confocal microscope
- Power on the confocal microscope and computer and start microscopy software. Heat up the laser for 60 min. Switch off the light in the room. Place the glass slide containing the cells on the specimen stage using immersion oil on an objective lens.
- Select the 405 nm laser and set the main beam splitters (MBS) 405. Set the laser power to 0.5%. Set the measuring parameters to obtain a pixel size of 0.03 µm, zoom 10 (image size 512 x 512 pixels). Set pixel dwell time as 0.02 ms, which gives total scan time as 5.06 s.
- Set the detector range from 410 nm to 695 nm on GaAsP spectral detector enabling a spectral resolution of 9 nm. Set the optimum gain (800-900). Start the lambda scan.
NOTE: Optimum gain should be optimized for each microscope.
9. Quantify the intensity of the signal using Fiji
- Open the image captured with the DAPI filter under the fluorescent microscope. Select cells in the bright field image and define the region of interest (ROI). After defining the ROI for all cells, save the ROI.
- Close the bright field image and open the fluorescence image. Separate the RGB image into red, blue, and green to extract the Tyramide-350 signal.
- Apply ROI prepared by bright field image to blue image and measure the intensity of the signal29. Save the results.
Results
The fluorescence signal was observed from extracellular substances in the negative control, where the 1st antibody was not used (Figure 1A-C). The fluorescence signal of the tyramide-boosted reagent, conjugated to the large subunit of the Rubisco protein (RbcL), was successfully detected in C. watsonii under a fluorescence microscope using a DAPI filter with UV excitation (Figure 1D-F). Additionally, fluorescent signals were visible from the extracellular polymeric substance (EPS) (Figure 1C,F).
Moving forward, the protocol was applied to the enzyme responsible for nitrogenase Fe protein (NifH). To confirm the specificity of the fluorescence signal resulting from the immunochemical reaction, the fluorescence intensity was quantified using the Fiji software. The intensity of signals from the cells treated with the complete protocol was significantly higher than from cells lacking the 1st and/or 2nd antibodies (Figure 2) (Mann-Whitney test30, p < 0.001 and Steel-Dwass post-hoc test31, p < 0.001). The signals from the cells lacking the first and/or second antibodies were slightly higher than those from the control with no antibody or tyramide reagent, using the maximum excitation wavelength at 350 nm (Tyramide 350) (Mann-Whitney test, p < 0.001 and Steel-Dwass post-hoc test, p < 0.001). However, a small fraction of cells from the control and cells lacking the first and/or second antibodies exhibited high-intensity signals (~200). Nonetheless, these cells constituted less than 1% of all cells within a given treatment. In contrast, over 83% of cells treated with the full procedure displayed a signal intensity of 200 or higher. Furthermore, less than 3% of cells in the control group and less than 5% of cells lacking the first and/or second antibodies exhibited higher-intensity signals (>133) than the lowest signal measured for cells treated with the complete procedure (Figure 2).
The positive impact of the tyramide signal amplification system was verified by the relationship between the incubation time of the tyramide working solution (step 6.3) (Figure 3). The proportion of cells with positive signals increased with longer incubation times, up to 7.5 min. The positivity threshold for the signal was defined as being greater than 25% of the maximum value within the same image. The error bars represent the standard deviation32.
The fluorescence spectra and images obtained via a confocal microscope are presented as the final step. Due to the confocal microscope's minimum excitation wavelength of 405 nm, the Tyramide 405 reagent was employed instead of Tyramide 350. When the second antibody was conjugated with the Tyramide 405 reagent, fluorescence emission spectra peaking around 460 nm could be distinguished from fluorescence peaks emitted by phycourobilin at 503 nm and phycoerythrin at 566 nm (Figure 4). The error bars denote the standard deviation32.
The procedure is summarized as a flowchart (Figure 5).

Figure 1: Bright-field and fluorescence image of C. watsonii PS0609 culture under the fluorescence microscope. (A-C) Negative control, treated without primary antibody. (D-F) Positive control treated as described in the protocol (Figure 5). (A,D) Bright-field image. (B,E) Phycoerythrin autofluorescence detection with FITC filter set (Excitation 460-495 nm/Emission 510 nm). (C, F) Immunofluorescence detection of RbcL with DAPI filter set (Excitation 360-370 nm/Emission 420-460 nm). The blue signal shows the fluorescence from the Tyramide 350. The white arrow shows the Tyramide 350 attached to the extracellular polymeric substance. Scale bars: 10 µm. Please click here to view a larger version of this figure.

Figure 2: The confirmation of fluorescence signals being from the target protein. (A) Each dot represents fluorescence from a single cell. The parenthesis shows the number of cells analyzed. (B-E) Bright-field and fluorescence image of C. watsonii PS0609 culture under the fluorescence microscope. (F-I) Immunofluorescence detection of nitrogenase Fe protein (NifH)28 with DAPI filter set (Excitation 360-370 nm/Emission 420-460 nm). Cont.; no amendment, Tyramide; tyramide reagent. Please click here to view a larger version of this figure.

Figure 3: Optimization of booster incubation time. The average percentage of cells with the positive signal for nitrogenase Fe protein (NifH). Each incubation time of the tyramide reagent with standard deviation. The threshold of the positivity of the signal was defined as >25% of the maximum value in the same image. Parentheses show the number of cells observed under a given condition. Error bar shows standard deviation. Please click here to view a larger version of this figure.

Figure 4: The fluorescence emission spectra. Immunostaining against RbcL (A) and NifH (B). The average single-cell fluorescence emission spectra of C. watsonii PS0609 with (RbcL or NifH: black line) or without 1st nor 2nd antibodies (Cont.: grey line) incubation with RbcL (A) or NifH (B) as 1st antibody followed by Tyramide 405. Data are normalized at 503 nm. The parentheses show the number of cells analyzed. A typical image of C. watsonii PS0609 cells without 1st antibody (C) and with NifH antibody (D) obtained by Tyramide 405 channel (detected at 410-436 nm) confocal imaging. r. u.; relative unit. PUB: phycourobilin, PE: phycoerythrin, Tyramide: tyramide reagent. Error bar shows standard deviation. Please click here to view a larger version of this figure.
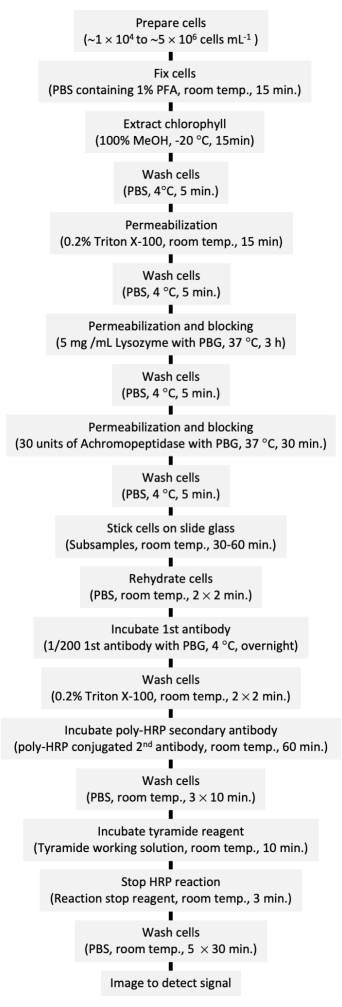
Figure 5: Flowchart of the present protocol. MtOH: Methanol, PFA: paraformaldehyde, PBS: phosphate-buffered saline, PBG: 0.2% gelatin + 0.5% BSA, HRP: horseradish peroxidase. Please click here to view a larger version of this figure.
Discussion
For cyanobacteria, the TSA system has found widespread use in TSA-fluorescence in situ hybridization (TSA-FISH, CARD-FISH), targeting specific rRNA. However, its application for proteins remains limited26. In this study, a TSA procedure was applied to enable whole-cell immunodetection of the N2-fixing cyanobacterium C. watsonii, incorporating modifications based on a previous reference20. Notable revisions encompassed permeabilization, accomplished through a combination of lysozyme and achromopeptidase22, and signal enhancement using TSA systems23. Furthermore, leveraging the TSA technique and RGB image separation through Fiji software enabled the visualization and quantification of antibody-conjugated fluorescence signals amidst the inherent auto-fluorescence of phycourobilin and phycoerythrin.
The visualization of proteins in unicellular cyanobacteria offers insight into cell-to-cell variations in protein content. Previously, analyses of cell-to-cell metabolic heterogeneity in cyanobacteria relied on isotope ratio mass spectrometers (NanoSIMS)11,12,13,14,15. Nonetheless, utility of NanoSIMS has been curtailed by instrument scarcity and high costs. In contrast, immunocytochemistry necessitates standard equipment present in most labs. The provided protocol can be readily adapted for other cyanobacterial cells. For instance, it has successfully been employed for nitrogenase (NifH) in C. watsonii WH8501 and Rubisco (RbcL) in Synechocystis sp. PCC 6803, yielding anticipated outcomes.
Critical protocol stages include cell envelope permeabilization. In the case of C. watsonii, achromopeptidase use was pivotal, a step introduced following the methodology of reference22. The same protocol yielded positive results with Synechocystis sp. PCC 6803. Nevertheless, since cell envelope structure varies among cyanobacteria species, optimization of incubation times and permeabilization agents might be required for other species.
While the signals obtained through the full protocol significantly exceeded those from control cells or cells lacking 1st and/or 2nd antibodies, it's noteworthy that high signals were recorded in certain cells missing antibodies or control cells. This phenomenon could relate to challenges in segregating EPS from cells, possibly due to fluorescence dye binding to EPS (Figure 1C,F). Hence, the rinsing step assumes significance in mitigating such issues. This method holds potential for quantifying protein content33,34, akin to quantification achieved through signal detection via flow cytometry35. The current protocol effectively enables the assessment of target protein presence in C. watsonii. This technique promises swifter and more cost-effective monitoring of intracellular metabolic heterogeneity compared to NanoSIMS11,14,15. This approach could be employed in forthcoming studies to identify organisms exhibiting target metabolic functions, such as in situ N2 fixation.
Disclosures
We confirm there are no conflicts of interest related to this study.
Acknowledgements
We appreciate Dr. Radek Kana and Barbora Šedivá for assistance with confocal microscopic analysis and Dr. Roman Sobotka and Dr. Kateřina Bišová for advice in immunodetection and fluorescence microscopy analysis. This research was financially supported by Czech Research Foundation GAČR (project 20-17627S to OP and TM), the Mobility plus project between JSPS and Czech Academy of Sciences (JPJSBP 120222502), and JSPS KAKENHI (project 23H02301).
Materials
| Name | Company | Catalog Number | Comments |
| Achromopeptidase | FUJIFILM | 014-09661 | |
| Alexa Fluor350 | Thermo Scientific | B40952 | Tyramide-350 |
| Alexa Fluor405 | Thermo Scientific | B48254 | Tyramide-405 |
| Alexa Fluor488 Tyramide SuperBoost Kit | Thermo Scientific | B40922 | Goat anti-rabbit IgG |
| Bovine serum albumin | Sigma-Aldrich | A2153 | |
| Centrifuge | Eppendorf | 5804 R | |
| Centrifuge tubes (15 mL) | VWR | 525-1085 | For harvesting cells |
| Confocal microscope | Zeiss | LSM880 | Equipped with Airyscan |
| Fluorescence microscope | Olympus | BX51 | DAPI filter: Ex.360-370 nm, Em. 420-460 nm |
| Gelatine | Merk | 4070 | |
| High precision microscope cover glasses for confocal microscope | Deckgläser | No. 1.5H | |
| Liquid Blocker Regular/Mini | Daido Sangyo Co., Ltd. | Part 6505 | For keeping the cells on the slide glass |
| Lysozyme | ITW Reagents | A4972 | |
| Methanol | Carl Roth | 67-56-1 | |
| Monopotassium Phosphate | Penta | 12290 | |
| Monunting medium | Sigma-Aldrich | 345789-20ML | FluorSave Reagent |
| Mounting medium | Vectashild | H-1300 | |
| Objective lens used in the confocal microscope | Zeiss | Plan-Apochromat 63x/1.4 Oil DIC M27 | |
| Paraformaldehyde | Sigma-Aldrich | 158127 | |
| Poly-lysine coated slide glass | Sigma-Aldrich | P0425-72EA | |
| Potassium chloride | Lach-Ner | ||
| Safe lock tube (1.5 mL) | Eppendorf | 0030 120.086 | For treating cells and storing chemicals |
| Sodium chloride | Penta | 16610 | |
| Sodium hydrogen phosphate | Penta | 15130 | |
| Triton X-100 | Sigma-Aldrich | X100 |
References
- Ackermann, M. A functional perspective on phenotypic heterogeneity in microorganisms. Nature Reviews Microbiology. 13 (8), 497-508 (2015).
- Schreiber, F., Ackermann, M. Environmental drivers of metabolic heterogeneity in clonal microbial populations. Current Opinion in Biotechnology. 62, 202-211 (2020).
- Takhaveev, V., Heinemann, M. Metabolic heterogeneity in clonal microbial populations. Current Opinion in Microbiology. 45, 30-38 (2018).
- van Heerden, J. H., et al. Lost in transition: start-up of glycolysis yields subpopulations of nongrowing cells. Science. 343 (6174), 1245114 (2014).
- Lindemann, D., Westerwalbesloh, C., Kohlheyer, D., Grunberger, A., von Lieres, E. Microbial single-cell growth response at defined carbon limiting conditions. RSC advances. 9 (25), 14040-14050 (2019).
- Kotte, O., Volkmer, B., Radzikowski, J. L., Heinemann, M. Phenotypic bistability in Escherichia coli's central carbon metabolism. Molecular Systems Biology. 10 (7), 736 (2014).
- Nikolic, N., et al. Cell-to-cell variation and specialization in sugar metabolism in clonal bacterial populations. PLoS Genetics. 13 (12), 1007122 (2017).
- Solopova, A., et al. Bet-hedging during bacterial diauxic shift. The Proceedings of the National Academy of Sciences. 111 (20), 7427-7432 (2014).
- Schreiber, F., et al. Phenotypic heterogeneity driven by nutrient limitation promotes growth in fluctuating environments. Nature Microbiology. 1, 16055 (2016).
- Zimmermann, M., et al. Substrate and electron donor limitation induce phenotypic heterogeneity in different metabolic activities in a green sulphur bacterium. Environmental Microbiology Reports. 10 (2), 179-183 (2018).
- Masuda, T., et al. Heterogeneous N2 fixation rates confer energetic advantage and expanded ecological niche of unicellular diazotroph populations. Communications Biology. 3, 172 (2020).
- Mohr, W., Vagner, T., Kuypers, M. M., Ackermann, M., Laroche, J. Resolution of conflicting signals at the single-cell level in the regulation of cyanobacterial photosynthesis and nitrogen fixation. PLoS One. 8 (6), 66060 (2013).
- Polerecky, L., et al. Temporal patterns and intra- and inter-cellular variability in carbon and nitrogen assimilation by the unicellular cyanobacterium Cyanothece sp. ATCC 51142. Frontiers in Microbiology. 12, 620915 (2021).
- Berthelot, H., et al. NanoSIMS single cell analyses reveal the contrasting nitrogen sources for small phytoplankton. ISME Journal. 13, 651-662 (2019).
- Foster, R. A., Sztejrenszus, S., Kuypers, M. M. Measuring carbon and N2 fixation in field populations of colonial and free-living unicellular cyanobacteria using nanometer-scale secondary ion mass spectrometry. Journal of Phycology. 49 (3), 502-516 (2013).
- Lin, S., Henze, S., Lundgren, P., Bergman, B., Carpenter, E. J. Whole-cell immunolocalization of nitrogenase in marine diazotrophic cyanobacteria, Trichodesmium spp. Applied and Environmental Microbiology. 64, 3052-3058 (1998).
- Berman-Frank, I., et al. Segregation of nitrogen fixation and oxygenic photosynthesis in the marine cyanobacterium Trichodesmium. Science. 294, 1534-1537 (2001).
- El-Shehawy, R., Lugomela, C., Ernst, A., Bergman, B. Diurnal expression of hetR and diazocyte development in the filamentous non-heterocystous cyanobacterium Trichodesmium erythraeum. Microbiology. 149, 1139-1146 (2003).
- Webb, E. A., Ehrenreich, I. M., Brown, S. L., Valois, F. W., Waterbury, J. B. Phenotypic and genotypic characterization of multiple strains of the diazotrophic cyanobacterium, Crocosphaera watsonii, isolated from the open ocean. Environmental Microbiology. 11 (2), 338-348 (2009).
- Taniuchi, Y., Murakami, A., Ohki, K. Whole-cell immunocytochemical detection of nitrogenase in cyanobacteria: improved protocol for highly fluorescent cells. Aquatic Microbial Ecology. 51, 237-247 (2008).
- Ohki, K., Taniuchi, Y. Detection of nitrogenase in individual cells of a natural population of Trichodesmium using immunocytochemical methods for fluorescent cells. Journal of Oceanography. 65, 427-432 (2009).
- Orcutt, K. M., Ren, S., Gundersen, K. Detecting proteins in highly autofluorescent cells using quantum dot antibody conjugates. Sensors. 9 (9), 7540-7549 (2009).
- Bobrow, M. N., Harris, T. D., Shaughnessy, K. J., Litt, G. J. Catalyzed reporter deposition, a novel method of signal amplification: Application to immunoassays. Journal of Immunological Methods. 125, 279-285 (1989).
- Bobrow, M. N., Shaughnessy, K. J., Litt, G. J. Catalyzed reporter deposition, a novel method of signal amplification. II. Application to membrane immunoassays. Journal of Immunological Methods. 137, 103-112 (1991).
- Bobrow, M. N., Litt, G. J., Shaughnessy, K. J., Mayer, P. C., Conlon, J. The use of catalyzed reporter deposition as a means of signal amplification in a variety of formats. Journal of Immunological Methods. 150, 145-149 (1992).
- Piccini, C., Fabre, A., Lacerot, G., Bonilla, S. Combining immunolabeling and catalyzed reporter deposition to detect intracellular saxitoxin in a cyanobacterium. Journal of Microbiol Methods. 117, 18-21 (2015).
- Chen, Y. B., Zehr, J. P., Mellon, M. Growth and nitrogen fixation of the diazotrophic filamentous nonheterocystous cyanobacterium Trichodesmium sp. IMS 101 in defined media: evidence for a circadian rhythm. Journal of Phycology. 32, 916-923 (1996).
- Ohki, K. Intercellular localization of nitrogenase in a non-heterocystous cyanobacterium (Cyanophyte), Trichodesmium sp. NIBB1067. Journal of Oceanography. 64, 211-216 (2008).
- Schindelin, J., et al. Fiji: an open-source platform for biological-image analysis. Nature Methods. 9 (7), 676-682 (2012).
- McKnight, P. E., Najab, J. Mann-Whitney U Test. The Corsini Encyclopedia of Psychology. , 1 (2010).
- Steel, R. G. D. Some rank sum multiple comparison tests. Biometrics. 17, 539-552 (1961).
- M, S. H. K., Lovric, M. Standard Deviation. International Encyclopedia of Statistical Science. , (2014).
- Gross, A. J., Sizer, I. W. The oxidation of tyramine, tyrosine, and related compounds by peroxidase. Journal of Biological Chemistry. 234 (6), 1611-1614 (1959).
- Eling, T. E., Thompson, D. C., Foureman, G. L., Curtis, J. F., Hughes, M. F. Prostaglandin H synthase and xenobiotic oxidation. Annual Review of Pharmacology and Toxicology. 30, 1-45 (1990).
- Clutter, M. R., Heffner, G. C., Krutzik, P. O., Sachen, K. L., Nolan, G. P. Tyramide signal amplification for analysis of kinase activity by intracellular flow cytometry. Cytometry Part A. 77 (11), 1020-1031 (2010).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved