Method Article
Extracting the Cochlea from a Human Temporal Bone: A Cadaveric Protocol
In This Article
Summary
This article presents a reliable method for extracting the human cochlea from the cadaver temporal bone by drill-out while following distinct anatomical landmarks.
Abstract
The extraction of the cochlea from a cadaver human temporal bone may be required for different studies of the inner ear. For histological evaluations, the inner ear must be extracted from the temporal bone to facilitate histologic processing; likewise, some micro-computed tomography devices are too small to accommodate the complete temporal bones; additionally, the image quality can be enhanced when the cochlea is isolated.
The inner ear is located within the petrous part of the temporal bone. The inner ear can be divided into the osseous labyrinth or otic capsule and the membranous labyrinth inside the otic capsule. Furthermore, the inner ear can be divided into the vestibular system (the semicircular canals and the vestibule) and the cochlea. The appreciation of the location and orientation of the cochlea within the temporal bone is difficult, as it is embedded within bony structures and thus cannot be directly visualized. Nevertheless, there are distinct anatomical structures that can help guide the process to allow a reliable drill-out of the cochlea. The landmarks in the posterior parts of the cochlea are the facial nerve, semicircular canals, and the vestibule. In the middle, the inferior borders of the cochlea are identified by the round window and the basal turn of the cochlea. In the anterior border, one encounters the carotid artery; the landmark for the superior border is the genicular ganglion (GG) of the facial nerve. The medial structures are determined by the locations of the internal auditory canal, the superior semicircular canal, and the canal of the internal carotid artery.
In this article, we present a method for extracting the cochlea reliably out of the temporal bone by drill-out while following several anatomical landmarks.
Introduction
The inner ear is a delicate organ that provides us with the sense of hearing and balance. The inner ear is located at the base of the skull in the petrous part of the temporal bone (TB). The TB encases several crucial anatomical structures that twist and turn inside the bone. Thus, the TB forms a challenging anatomical entity to comprehend1. Rask-Andersen et al., in their review2,discuss the history of cochlea research and understanding of its microstructures.
The inner ear includes the semicircular canals, the vestibulum, and the cochlea. The three semicircular canals and the vestibulum form the vestibular system where the balance receptors are located3,4. The cochlea is a shell-like structure that is connected to the vestibulum. The cochlea converts the mechanical sound waves into neural signals. The normal cochlea makes two and a half turns and ends at the apex of the cochlea. The average length of the cochlea duct is 37.6 mm; however, there is considerable variety between individuals5. In addition, the inner ear can be divided into the osseous labyrinth (otic capsule), formed by the bony margins of the inner ear, and the membranous labyrinth inside the otic capsule. The nerve from the inner ear passes through the internal acoustic canal (IAC) and divides into the vestibular nerve fibers and the cochlear nerve. The cochlear nerve is formed from the axons of the spiral ganglion neurons that lie in the modiolus of the cochlea3. The facial nerve (FN) makes its route also through the IAC; it passes superiorly along the cochlea, making a tight, approximately 110° turn back and downwards through the middle ear until it leaves the TB through the mastoid cavity from the foramen stylomastoid at the base of the skull1,4. There are several other significant structures in the proximity of the cochlea, e.g., the ossicles (malleus, incus, and stapes) in the middle ear, the carotid artery (ICA) (which arises inferior to the cochlea and then makes a turn medially at the level of the basal turn of the cochlea), the middle fossa plate (tegmen) and the bulbus jugulare which is inferior to the middle ear. The approach to the cochlea during surgery is generally performed through the air cells of the mastoid process. The largest air cell in the mastoid cavity is called the antrum, which communicates with the middle ear via aditus. The size and organization of these mastoid air cells vary significantly, even in terms of "normal anatomy". The prominence of the lateral semicircular canal is usually present on the floor of the antrum. The anatomy of the temporal bone with the many landmarks is presented in Figure 1 and Figure 2. The radiological anatomy of TB from the middle fossa view is presented in Figure 3.
The extraction of the cochlea from a human temporal bone may be required for different investigations of the inner ear. In histological studies, the inner ear is usually extracted from the temporal bone to facilitate its histological processing; likewise, some micro-computed tomography (micro-CT) devices have a relatively limited space to accommodate the sample and may not cope with the complete temporal bones; additionally, the image quality can be enhanced when the cochlea has been isolated6,7,8,9. Especially in developing and testing new cochlear implant electrode arrays, histological processing and/or micro-CT are conducted to determine the intra-cochlear position of the electrode9,10,11,12. In addition, histology can be performed with reduced consumption of processing solutions when the sample is small.
Nonetheless, the extraction of the cochlea requires a profound understanding of the surrounding structures, especially when the aim is to avoid excess bone in the sample. At first impressions, the anatomy of TB might seem hard to comprehend. However, ultimately, the anatomical structures inside the TB serve as boundaries around the cochlea, which can be exploited during the extraction. The accidental opening of the cochlea may lead to traumatic damage to the delicate structures inside this tissue and thus, in a flawed sample which may lead to the need to discard that cochlea.
This article presents a method for extracting the whole cochlea reliably out of the temporal bone by drill-out while watching for the following anatomical landmarks.
Protocol
This study used temporal bones (TB) gathered from the human cadaver subject post-mortem. The study was granted Institutional approval and fulfilled the Helsinki Declaration for the ethical use of human material. Approval for cadaver temporal bones was granted to the Kuopio University Hospital by the Finnish National Supervisory Authority for Welfare and Health (NRO: 9202/06.01.03.01/2013), and the study was conducted following Finnish regulations and laws. All of the bones were gathered anonymously during medical autopsy.
1. Removal of the soft tissue from the TB surface
- Remove the soft tissue from the TB surface with a surgical blade and raspatory.
- Carry out the rest of the operation under a surgical microscope and a high-speed surgical drill with an irrigation system. Use ear instruments, such as a micro-dissector, for dissection.
NOTE: The bur blades needed are a 5 mm cutting bur, a 3 mm rough diamond bur, and a 2 mm fine diamond bur. While smaller diamond burs may be used to open critical and small structures, generally, the 2 mm fine diamond bur will be adequate for this procedure.
2. Mastoidectomy (Figure 4)
- Open the cortex and identify the tegmen with the high-speed surgical drill (e.g., 5 mm cutting bur).
- Follow the tegmen and the superior and posterior side of the external ear canal (EEC) to the antrum with the 5 mm cutting bur.
- Open the antrum and identify the corpus of the incus through the opening of the antrum to the middle ear. Depending on the anatomy, do this with a 5 mm cutting bur or a 3 mm rough diamond bur.
- Identify the prominence of the lateral semicircular canal (LSC).
- Use the short leg of the incus as a landmark for identifying the facial nerve (FN) posterior to the EEC.
- Drill alongside the FN towards the mastoid tip until a space is opened inferior to the EEC.
- Drill open the sinodural angle and the sigmoid sinus in the posterior part of the mastoid cavity to create more space for removing the cochlea. Anatomical landmarks and the cutting lines are presented in Figure 5.
3. Posterior tympanotomy, removal of the posterior parts of the EEC, and exposing the middle ear
- Open the facial recess between the facial nerve and the external ear canal with a 2 mm diamond bur. Identify the chorda tympani between the FN and the EEC. For cochlea extraction, remove the chorda tympani.
- After opening the posterior tympanotomy, identify the incudostapedial joint and open the joint.
- Cut the stapedial tendon with a micro-blade or micro-scissors to avoid dislocation of stapes during the inferior cut of the extraction (step 6.3, the stapedial muscle is drilled and dissected off).
- Drill the buttress away and remove the incus. Open the incudostapedial joint to avoid the dislocation of the stapes' footplate and open the vestibule through the oval window (step 3.2).
- Remove the posterior part of the bony external ear canal with the malleus, tympanic membrane, and skin of the external ear canal in order to create more space. Remove the soft tissue with suction and a micro-dissector.
NOTE: Cut the tensor tympani tendon prior to removing the malleus to facilitate the removal.
4. Removal of the tissues that are posterior to the cochlea (Figure 5A-C and Figure 6)
- Drill the posterior alongside the posterior side of the tympanic section of the FN following the posterior crus of the LSC and the posterior semicircular canal (PSC). The semicircular canals lead into the vestibulum, which is the anterior border for the drilling for the removal of the posterior parts of the cochlea.
- Continue the drilling towards the meatus of the internal auditory canal (IAC).
- Form a cutting line at the middle fossa using the superior semicircular canal (SSC) as a landmark and follow it through the tegmen. Open the SSC from a superior approach through the middle fossa, as the prominence of the SSC can often be identified at the middle fossa. Expose the genicular ganglion (GG) of FN with a diamond drill to serve as a landmark for the SSC from the middle fossa (see step 6.2).
- Continue drilling vertically towards the tip of the mastoid, starting from the vestibulum. The round window is a landmark for identifying the cochlea´s basal turn in the middle ear and acts as the limiting anterior border.
- Open the posterior cut inferiorly until below the level of the cochlea´s basal turn.
5. Removal of the lateral and inferior parts of the temporal bone (Figure 5E)
- Identify the round window, the basal turn of the cochlea, and the canal of the internal carotid artery (ICA) anteriorly to the cochlea's basal turn. Observe the ICA at the anterior of the middle ear, medially to the opening of the eustachian tube.
- Drill into the floor of the middle ear in parallel to the basal turn connecting the cut between the ICA and the cut for the posterior to the FN below the vestibulum.
- Use scissors or a microblade to cut the FN to avoid drilling it away extensively as the FN serves as a good landmark for the orientation during the removal of the cochlea and after the cochlea has been removed.
NOTE: The stapedial muscle is cut with the FN as it has been exposed in the inferior cut. - Continue the drilling to create a space under the basal turn to make it easier to conduct the inferior cut.
NOTE: Depending on the individual anatomy of the TB, the bulbus jugulare may be opened during the drilling of the middle ear floor. - Drill anterolaterally to the ICA to open the mandibular joint.
- Once the mandibular joint is opened, identify the ICA and basal turn. Then remove the lateral parts of the TB without any risk of damaging the cochlea.
- Cut the superior parts at the roof of the middle ear by drilling open the tegmen from the level of the middle ear all the way to the sigmoid sinus.
NOTE: Usually, it is easy to create the opening at the level of the SSC.
6. Removal of the anterior and inferior parts of the TB (Figure 5D,E)
- Drill open the ICA through the middle ear. The ICA makes a turn medially anterior to the cochlea; thus, this turn serves as the anterior border for the cochlea.
- Continue drilling superior from the turn of the ICA, parallel to the route of the ICA. Keep continuing the drilling medially following the course of the ICA and remove any excess bone both superiorly and inferiorly to the ICA.
NOTE: Do not extend the drilling posterior of the ICA in order to avoid damaging the cochlea. - Drill inferiorly alongside the basal turn of the cochlea, approximately 1-1.5 cm for the cut at the posterior parts.
- For the inferior cut, leave a suitable safety margin between the basal turn and the drilling to ensure the preservation of the cochlea.
NOTE: if needed, any excess bone here can be honed away carefully after removing the cochlea.
7. Removal of medial parts of TB (Figure 5F)
- Follow the facial towards the GG at the roof of the middle ear.
- Expose the GG with a 3 mm rough diamond drill and continue drilling through the tegmen to the middle fossa.
- Drill open the GG from the middle fossa and follow alongside the FN proximal to GG to the IAC.
- Use the IAC, GG, and SSC as landmarks to form a cut-line medial to the cochlea. The IAC forms an approximately 70° angle with the basal turn of the cochlea. Perform the drilling from the proximal parts of the IAC to the proximal parts of the horizontal segment of the ICA.
- Connect the medial line by first exposing the IAC to the inferior cut.
8. Finalizing the extraction
- Use the fine diamond bur (e.g., 3-5 mm bur) to carefully hone excess bone around the cochlea if needed for downsizing the sample.
- Observe carefully the color of the bone during the trimming; if the bone starts to change to become translucent, then stop drilling.
- There should now be a 1.5 cm x 1.5 cm piece containing the intact cochlea. Maintain the orientation by using FN, stapes, and RW.
Results
When successful, the cochlea is extracted from the temporal bone without the need to open the perilymph compartment of the cochlea. In a negative case, there is an opening inside the cochlea and damage to the tissue's membranous labyrinth.
This extraction method has been used in 36 cadaveric TBs during our cochlear implant electrode investigations (Table 1). In 33 TBs, the extraction has been successful without causing any damage to the cochlea. In two out of 36 TBs, the samples had to be discarded due to the obvious movements of the cochlear implant electrode array during the extraction. However, the cochlea had been preserved intact. In one TB, the cochlea was accidentally opened just behind the vestibulum during the extraction procedure. One of the studies has been published9; the remaining investigations are ongoing.
As a major limitation, there is no comparative data for this method. In addition, there is a rather small sample presented in this paper from the previous research, which may not reflect the true effectiveness of the method.
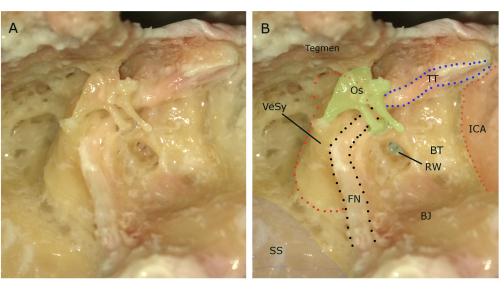
Figure 1: Anatomy of the temporal bone after removal of the mastoid air cells and the exposure of the middle ear and internal carotid artery. (A) Plain view without name tags. (B) Highlighted structures with name tags. Ossicles left intact. Abbreviations: SS: sigmoid sinus, FN: facial nerve, VeSy: vestibular system, Os: ossicles, Tegmen: Tegmen/middle dura plate, TT: tensor tympani, RW: round window, BT: bony prominence of the basal turn of the cochlea, BJ: bulbus jugulare, ICA: canal of the internal carotid artery. Please click here to view a larger version of this figure.
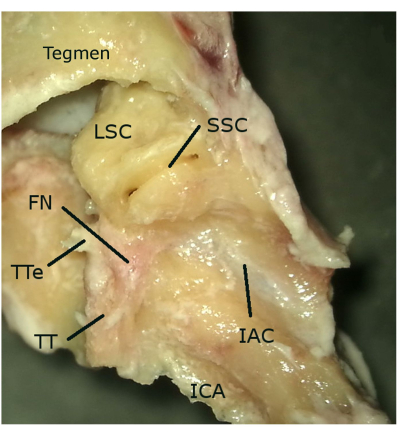
Figure 2: View from the middle fossa. Abbreviations: SSC: superior semicircular canal, FN: facial nerve, IAC: internal acoustic canal, EEC: external ear canal, ICA: internal carotid artery. Please click here to view a larger version of this figure.
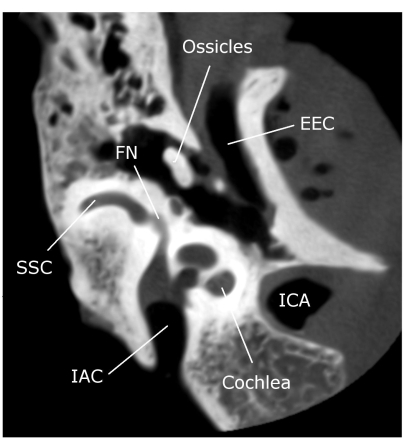
Figure 3: Axial view of the CB-CT image temporal bone. Abbreviations: SSC: superior semicircular canal, FN: facial nerve, IAC: internal acoustic canal, EEC: external ear canal, ICA: internal carotid artery. Please click here to view a larger version of this figure.

Figure 4: Partial mastoidectomy with posterior tympanotomy. Abbreviations: SSC: superior semicircular canal, LSC: lateral semicircular canal, PSC: posterior semicircular canal, PT: posterior tympanotomy, EEC: external ear canal, Ch. Tymp.: chorda tympani, FN: facial nerve, SS: sinus sigmoideus. Please click here to view a larger version of this figure.
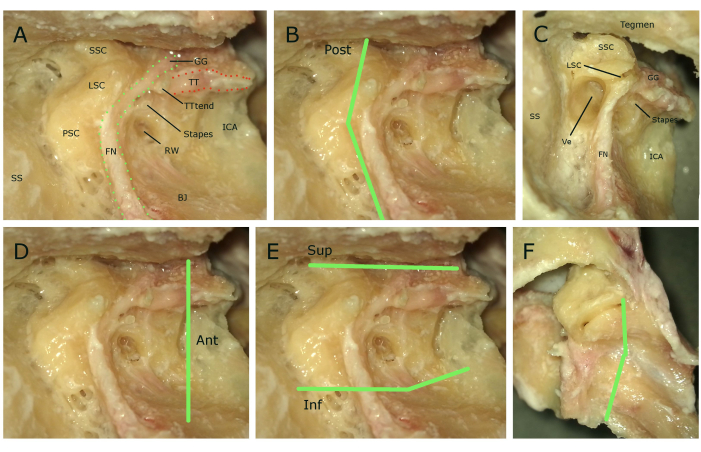
Figure 5: Cutting lines. (A) Anatomy of the temporal bone after removal of the mastoid air cells and exposing the middle ear and internal carotid artery without the ossicles. Structures highlighted with name tags. (B) Posterior cutting line. (C) Opened vestibular system during the posterior cutting. (D) Anterior cutting line. (E) Superior and inferior cutting lines. (F) Medial cutting line from the middle fossa view (see landmarks from Figure 2). Abbreviations: SSC: superior semicircular canal, LSC: lateral semicircular canal, PSC: posterior semicircular canal, SS: sinus sigmoideus, FN: facial nerve, GG: genicular ganglion, BJ: bulbus jugulare, RW: round window, TTtend: tensor tympani tendon, TT: tensor tympani, ICA: internal carotid artery, Ve: vestibulum. Please click here to view a larger version of this figure.
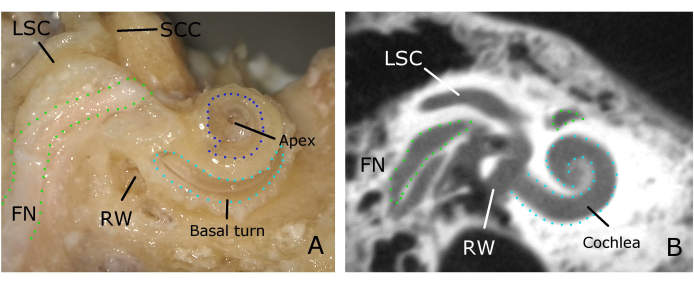
Figure 6: Different views of the cochlea. (A) Cochlea drilled open with the view to the basilar membrane. (B) The cochlea viewed from a cadaver TB scanned with cone-beam computed tomography (CBCT). Please click here to view a larger version of this figure.
| Mean (mm) | Max (mm) | Min (mm) | |
| A-Measure | 9.4 | 10.1 | 8.4 |
| B-Measure | 6.8 | 7.5 | 6.2 |
| Total Number | Successful Extraction | Unsuccessful Extraction | |
| Number of TBs | 36 | 33 | 3 |
Table 1: Size of the cochlea and success of the removal procedures using the described method. "A-measure" refers largest distance from the round window to the lateral wall, and "B-measure" refers to the perpendicular distance of the cochlea determined as proposed by Escudé et al.13. "Max" refers to the largest value, and "min" to the smallest value of measurements.
Discussion
There are several landmarks to be followed for removing the cochlea, so this procedure can be done systematically if the anatomy in the TB is normal (no malformations of the TB). The most critical parts of the removal procedure are the inferior margin and medial proportion between the IAC and the ICA. We recommend maintaining a slightly greater margin and, if necessary, precisely honing any excess bone after extracting the block. It is important to avoid opening the cochlea as it might cause trauma to its delicate inner structures and lead to an unsuccessful sample.
For later analyses (e.g., histology), the orientation of the cochlea can be determined utilizing the FN remnant, stapes, and round window. Additional routes for entry of histological solution may be created by opening the round window membrane and removing the stapes; this means that fixation solutions can enter more evenly inside the cochlea and provide better samples for histological analysis. Furthermore, if the vestibular organ has been removed, the vestibule can serve as an opening into the cochlea. Freshly frozen TBs can be used in cochlear implant electrode studies, where the main aim is generally to test the atraumatic properties of a new electrode or insertion technique6,7. If one wishes to perform more detailed research, e.g., at the cellular level from the inner ear, the sample should be gathered as quickly as possible in order to avoid post-mortem tissue damage, especially at the cellular level. Multiple factors may influence the sample's condition (such as the cause of death, the condition of the cochlea before death, and possible head injuries). However, as time is one of these factors that can actually be influenced, the sample should be gathered within 24 h after death14. Although the tissue may have undergone some post-mortem decay at this stage, generally, it should still be good enough to allow further analysis.
If the samples are gathered for cochlear implant research, then during the removal process, care should be taken not to manipulate the electrode array during the extraction. Careless drilling near the electrodes may lead to a change in their position or even may displace the array completely.
While the extraction of the cochlea is practically always performed for research purposes, it also represents an excellent way to let novice otosurgeons learn and understand the surgical anatomy of TB. Therefore when performed as described, it is an informative, practical exercise.
Pinhasi et al. presented a similar method for extracting cochlea for DNA analysis in archeological samples15. Like the approach used in this study, Pinhasi et al. also performed their tissue navigation by exploiting the anatomical landmarks inside the temporal bone during the cochlea removal. In contrast to this protocol, Pinhasi et al. used sandblasting to eradicate the tissues surrounding the cochlea. A high-speed surgical drill is usually more accessible for medical researchers, as it is an essential surgical tool applied in the training and the performance of ear surgery. One additional option for gathering the cochlea would be the so-called "plug in" method, where the cochlea is removed in a larger block and then, if necessary, honed down with the high-speed drill. Although obtaining the smaller block from the TB might be faster, the honing part of the protocol might be harder since there will be fewer recognizable landmarks in the comparatively larger block.
Recently, Vaisbuch et al.14 presented a different approach compared to the method used in this study on collecting fresh inner ear tissue for research purposes from organ donors. In contrast to our approach, Vaisbuch et al. aimed to gather the soft tissues of the cochlea in three parts (basal, mid, and apical turn), not the whole cochlea as one single block. The trans-canal approach has certain advantages when gathering cochlea samples from organ donors where the surrounding organs are still intact, and this limits the approach angles that can be utilized compared to those available when working with cadaveric TB. The advantage of ears from organ donors is that the soft tissues, with their delicate cell structures, can be obtained before significant cell death starts to occur. Thus the fine microscopic structures remain in the best possible condition. The approach described in this study is more feasible with cadavers and when the whole cochlea is required for the analysis, as when investigating the siting of cochlear implant electrodes.
As mentioned above, there is no comparative data regarding this method presented here. The need for the precise extraction of the cochlea has arisen from the limitations in sample processing during preclinical cochlear implant research. Up until now, the extraction method has not been evaluated as an independent research undertaking.
Disclosures
The authors report no conflict of interest.
Acknowledgements
Matti Iso-Mustajärvi receives research grants from the Finnish government research funding (VTR), the Instrumentarium Science Foundation, the North Savo Regional Grant, and the Finnish Society of Ear Surgery. Aarno Dietz receives research grants from the Academy of Finland (Grant No. 333525) and the North Savo Regional grant.
Materials
| Name | Company | Catalog Number | Comments |
| Drillblades for Drill | N/A | See below. Drillblades should be suitable for your drill system | |
| High speed surgical drill | Medtronic | https://www.medtronic.com/us-en/healthcare-professionals/products/neurological/powered-surgical-instruments/midas-rex-mr8.html | There are numerous providers from various different cateories for surgical drills. The one with irrigation system is recommended (e.g.,Stryker, Bbraun, Medtronic, etc.) |
| Operating Microscope | Zeiss | https://www.leica-microsystems.com/products/surgical-microscopes/ | Microscope for microsurcigal preparation of the temporal bone. Higly recommended microscopes include Zeiss, Leica, etc. |
| Temporal Bone holder | Stortz | N/A | Cup to fixate the temporal bone while drilling |
References
- Francis, H. W., Niparko, J. K. . Temporal Bone Dissection Guide. , (2016).
- Rask-Andersen, H., et al. Human cochlea: anatomical characteristics and their relevance for cochlear implantation. The Anatomical Record: Advances in Integrative Anatomy and Evolutionary Biology. 295 (11), 1791-1811 (2012).
- Yost, W. A. . Fundamentals of Hearing, An Introduction. , (2006).
- Putz, R., Pabst, R. . Sobotta-Atlas of Human Anatomy: Head, Neck, Upper Limb, Thorax, Abdomen, Pelvis, Lower Limb. , (2006).
- Wurfel, W., Lanfermann, H., Lenarz, T., Lenarz, T., Majdani, O. Cochlear length determination using cone beam computed tomography in a clinical setting. Hearing Research. 316, 65-72 (2014).
- Avci, E., Nauwelaers, T., Lenarz, T., Hamacher, V., Kral, A. Variations in microanatomy of the human cochlea. The Journal of Comparative Neurology. 522 (14), 3245-3261 (2014).
- Biedron, S., Prescher, A., Ilgner, J., Westhofen, M. The internal dimensions of the cochlear scalae with special reference to cochlear electrode insertion trauma. Otology & Neurotology. 31 (5), 731-737 (2010).
- Lane, J., Witte, R., Driscoll, C., Camp, J., Richard, A. Imaging microscopy of the middle and inner ear: Part I: CT microscopy. Clinical Anatomy. 17 (8), 607-612 (2004).
- Sipari, S., et al. Cochlear implantation with a novel long straight electrode: The Insertion results evaluated by imaging and histology in human temporal bones. Otology & Neurotology. 39 (9), e784-e793 (2018).
- Iso-Mustajärvi, M., et al. A new slim modiolar electrode array for cochlear implantation: A radiological and histological study. Otology & Neurotology. 38 (9), e327-e334 (2017).
- Teymouri, J., Hullar, T., Holden, T., Chole, R. Verification of computed tomographic estimates of cochlear implant array position: a micro-CT and histologic analysis. Otology & Neurotology. 32 (6), 980-986 (2011).
- Postnov, A., et al. High resolution micro-CT scanning as an innovative tool for evaluation of the surgical positioning of cochlear implant electrodes. Acta Otolaryngologica. 126 (5), 467-474 (2006).
- Escudé, B., et al. The size of the cochlea and predictions of insertion depth angles for cochlear implant electrodes. Audiology & Neurootology. 11 (suppl 1), 27-33 (2006).
- Vaisbuch, Y., et al. Surgical approach for rapid and minimally traumatic recovery of human inner ear tissues from deceased organ donors. Otology & Neurotology. 43 (4), e519-e525 (2022).
- Pinhasi, R., Fernandes, D., Sirak, K., Cheronet, O. Isolating the human cochlea to generate bone powder for ancient DNA analysis. Nature Protocols. 14 (4), 1194-1205 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved