Method Article
Exosome Isolation after in vitro Shock Wave Therapy
In This Article
Summary
Exosomes are released upon in vitro application of shockwaves. Here, we describe how to apply shockwaves on cultured endothelial cells and subsequently isolate exosomes for further investigation.
Abstract
Shock wave therapy is routinely applied in orthopedic indications including tendinopathies such as lateral epicondylitis (tennis elbow) and Achilles tendinitis (heel spurs) as well as non-healing wounds and bones. Despite different pathologies, the combination of an angiogenic and an anti-inflammatory effect of shock wave therapy leads to regeneration in soft tissue and bones. In over 30 years of clinical application, no side effects were observed. Furthermore, basic research even revealed regenerative effects on ischemic myocardium.
In a previous work we could show that the mechanical stimulus of cultured cells is translated via an exosome release into a biological response. However, the exact mechanism remains to be elucidated. Mechanical coupling is crucial when applying shock wave therapy as even small air bubbles can absorb shock waves. The previously described water bath method is a valid method to guarantee adequate and reproducible shock wave application in vitro.
We were able to develop a feasible and replicable protocol to isolate exosomes from cultured cells after shock wave application. Thereby we demonstrate a possibility to study underlying mechanisms of mechanotransduction as well as the regenerative and angiogenic potential of shock wave released exosomes.
Introduction
Shock waves (SW) are sound pressure waves that appear in nature when a high amount of energy is released in a short period of time (e.g., thunder with lightning). In clinical routine, shock waves are used in lithotripsy to disintegrate kidney stones without relevant side effects for over 30 years1,2. By incident, a thickening of the iliac bone was observed in X-rays after kidney stone disintegration. This observation provided the basis for research in bone healing disorders and lead to treatment in long-bone non unions3,4,5.
Indications for shock wave therapy were expanded and today the method is in routine clinical use in orthopedic indications including tendinopathies such as lateral epicondylitis (tennis elbow) and Achilles tendinitis (heel spurs)6,7. Furthermore, basic research demonstrated a high angiogenic potential of shock wave therapy (SWT). Therein, a study showed an increase in angiogenic growth factors such as VEGF (vascular endothelial growth factor), PIGF (placental growth factor) and FGF (vascular endothelial growth factor) upon SWT followed by angiogenesis8.
To investigate a possible beneficial role of shock wave induced angiogenesis in other pathologies, we applied SW therapy in an animal model of ischemic heart disease9,10. After a regenerative effect in the ischemic myocardium could be demonstrated, we could identify the indispensable role of the innate immune receptor TLR 3 (Toll like receptor 3) upon shock wave therapy11,12. Further studies showed that the mechanical stimulus of SW therapy is translated into a biological signal via exosome release. Compared to exosomes physiologically released from endothelial cells, exosomes released upon SWT contain an increased cargo of angiogenic microRNA. Injected into ischemic myocardium, SWT released exosomes induced regeneration13.
Since air absorbs SW, perfect coupling between the applicator and the cell culture flasks is crucial. A standardized water bath represents a feasible method to apply SWT in vitro and a reproducible experimental setup. In order to avoid a reflection and consequently interference of waves, a wedge-shaped absorber destructs the primary waves running to the back of the water bath. For this reason, we recommend applying shock waves in vitro using the described water bath only.
In this protocol we describe the application of shock waves in vitro to release angiogenic exosomes into the supernatant. This protocol provides the possibility to investigate the role of exosomes in mechanotransduction and is the basis for further investigation of exosome release upon SWT.
Protocol
Human umbilical vein endothelial cells were obtained from caesarean sections at the Department of Gynecology. Therefore, written informed consent of patients was obtained. Permission was given from the ethics committee of Innsbruck Medical University (no. UN4435).
NOTE: Work under a sterile laminar flow hood to avoid contamination.
1. 24 h before the experiment
- Prepare exosome free endothelial growth medium by ultra-centrifuging fetal bovine serum (FCS) at 4 °C and 120,000 x g overnight.
- Coat a 75 cm² cell culture flask using 10% gelatin.
- Seed human endothelial cells in the cell culture flask using endothelial growth medium.
Experiment day:
NOTE: Pre-warm 4 L of ddH2O, exosome free FCS, Endothelial Cell Growth Basal Medium and PBS to 37 °C.
2. Prepare the shock wave therapy application
- Prepare exosome free endothelial growth medium by adding supplements from the commercial kit (e.g., Bullet Kit) and adding ultracentrifuged exosome free FCS instead of common FCS to endothelial growth medium.
- Fill a water bath with approximately 3.5 L of 37 °C prewarmed ddH2O.
- Connect the SW applicator to the water bath.
- Define treatment parameters (energy flux density, impulse frequency) on the SW device. We recommend 500 impulses at an energy flux density of 0.07 mJ/mm² and a frequency of 5 Hz.
3. Shock wave application
- Survey cell viability by morphological appearance and the density of endothelial cells via a microscope using a 100x total magnification. Use flasks with high confluence for an increased amount of exosomes only.
- Carefully replace endothelial cell medium with approximately 250 mL of PBS to completely fill the flask and repress all residual air.
- Use parafilm or airtight caps to seal the flask.
- Hold the cell culture flask in a vertical position inside a water bath with the cell covered side opposite the SW device. Make sure the distance of flask to the applicator is in line with the indicated marking. For the Orthowave 180c device, use a distance from 10 cm.
- Utilize 250 impulses of SW therapy to the lower half of the cell culture flask.
- Turn the flask 180° and utilize 250 impulses to the upper half of the cell culture flask.
- Subsequent remove the flask sealing and replace PBS with 15 mL of endothelial growth medium with exosome free FCS.
- Culture therapized cells in an exosome free medium for an appropriate time for the experiment setup. The highest amount of exosomes can be gathered 4 h after SW therapy (Figure 1).
NOTE: In the sham operated group, subject cell culture flasks to same treatment (see section above) without utilizing SW therapy.
4. Isolation of exosomes
- Transfer the supernatant into a collection tube and centrifuge at 4 °C and 300 x g for 10 min. This step removes all cells in the supernatant.
- For removal of cell debris, transfer the supernatant to a new tube and centrifuge at 4 °C and 3,000 x g for 20 min.
- Strain the supernatant through a 200 nm filter into a centrifugal tube to remove apoptotic bodies.
- Centrifuge filtered supernatant at 4 °C with 120,000 x g for 70 min. Discard the supernatant and resuspend exosome pellet with 300 µL of PBS.
- Since the exosomes pellet is not visible after ultracentrifugation, vortex the ultracentrifugation tube for 30 seconds to make sure the exosome pellet is resuspended. Adjust the volume of PBS for the experimental needs and further experiments.
- Store resuspended exosomes at -80 °C.
NOTE: The protocol can be pause after transferring the supernatant into a collection tube or before ultracentrifugation by freezing the supernatant at -80 °C.
5. Pitfalls
- Make sure that the SW applicator is connected tightly to the water bath. An incomplete connection can lead to a leaky water bath.
- Ensure that there is an accurate distance from the flask to the applicator to utilize the same amount of energy flux density to different flasks.
- Mark the cell culture flasks that were subjected to SW therapy and the cell culture flasks that were the control samples.
- Dry flasks before putting them back into incubator to avoid contamination.
Results
Using the described protocol, we subjected human endothelial cells (HUVECs) as well as human coronary artery endothelial cells (CAECs; PromoCell) to shock wave therapy (e.g., Orthowave 180c). Released vesicles were quantified via nano tracking analysis (NTA). Thereby, we could observe an increase in microvesicle release upon SW therapy (Figure 1A,B). Imaging of HUVECs released vesicles via transmission electron microscopy revealed the characteristic 100 nm size of the exosomes (Figure 1C) as well as the typically described cup shaped form (Figure 1D). Since flow cytometer analysis revealed the presence of exosome protein markers CD9, CD81 and CD63 there can be no doubt that HUVECs released microvesicles are exosomes (Figure 1 E-G).
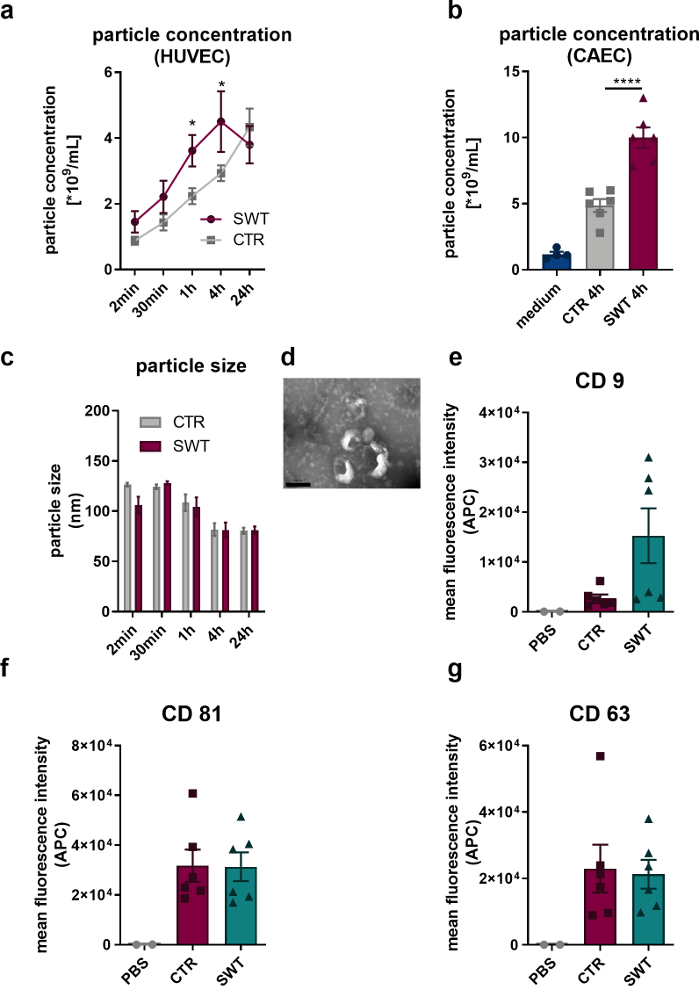
Figure 1: Exosome release upon shock wave therapy in vitro. (A) HUVECs released microvesicles were quantified via NTA in a time course manner after SW therapy. Data are expressed as means ± SEM. *P<0.05. n=3–6. (B) Increased levels of microvesicles were found in supernatant of CAECs 4 hours after SW therapy. Data are expressed as means ± SEM. ****P < 0.0001. n=4–6. (C-D) NTA revealed typical size and transmission electron microscopy revealed typical exosome shape. n=3–5. Scale bar = 200 nm. (E-G) Released microvesicles express exosome markers CD 9, CD 63 and CD 81. Data are expressed as means ± SEM. n=6. Statistical comparisons between two groups: Student’s t-test, multiple groups: one-way ANOVA with Tukey post hoc analysis. Modified from Gollmann-Tepeköylü et al.13. Please click here to view a larger version of this figure.
Discussion
In multiple basic research works, the regenerative effect of the shock wave therapy could be demonstrated and is routinely applied in orthopedic indications3,4,5. In diverse animal models, the regenerative effect on the ischemic myocardium could be demonstrated and lead to the initiation of the CAST-HF trial9,10. This randomized controlled trial aims to evaluate the benefit of direct cardiac shock wave therapy applied additionally during coronary artery bypass surgery14. The indispensable role of exosomes upon shock wave therapy could be demonstrated in a previous work13. However, the exact mechanism of exosome release as well as the exact cargo of released exosomes remains unclear. To study the release as well as the regenerative effect of released exosomes, we established a feasible and standardized protocol to obtain exosomes upon shock wave therapy.
To our knowledge, this is the first protocol describing a method to isolate exosomes upon shock wave therapy. A water bath is necessarily needed for the protocol to guarantee adequate SW application, as it avoids air absorption15 . Thereby, all exosomes isolated with this protocol arise from endothelial cells treated with the same amount of energy flux density.
Since we only have experience in producing exosomes from endothelial cells, we cannot recommend an exact experiment setup for experiments with other cell lines. However, we are convinced that mechanotransduction via exosomes upon SW therapy plays a crucial role in other cell types as well. Regardless, further investigations must prove this theory. When working with other cell lines besides endothelial cells, we would recommend a pilot trail. While working with endothelial cells, we found it necessarily to coat the flasks carefully before seeding cells. Otherwise, cells will detach upon SW therapy. We believe this also applies to other cell lines.
Moreover, an accurate connection between the applicator and the water bath, as well as knowing the exact distance from applicator to the cell culture flask, is important for comparable results. Since the source (e.g., electrode tips in an electro-hydraulic system) located inside the applicator is positioned differently in diverse SW applicators, be aware that the distance between the applicator and the cell culture flasks differs with different SW devices. Furthermore, we recommend using adherent cells only, since in a suspension culture there is no constant distance between the applicator and each cell. A limitation of this protocol is the need for an ultracentrifuge. However, this method avoids the purchase of exosome isolation kits.
Altogether, this protocol provides a standardized method to study the role of extracellular vesicle release upon shock wave therapy and might therefore be a crucial tool for the understanding of molecular SW effects.
Disclosures
JH and MG are shareholders of Heart Regeneration Technologies GmbH, an Innsbruck Medical University spin-off aiming to promote cardiac shock wave therapy (www.heart-regeneration.com). All other authors have nothing to disclose.
Acknowledgements
This study was supported by an unrestricted AUVA research grant to JH and CGT.
Materials
| Name | Company | Catalog Number | Comments |
| Cell culture flasks | Cellstar | 658170 | 75 cm2 |
| Collection tubes – Falcon Tube | Corning | 352070 | |
| Endothelial Cell Growth Medium-2 BulletKit | Lonza | CC-3162 | |
| Endothelial Cell Growth Basal Medium | Lonza | CC-3121 | |
| Fetal bovine serum | Sigma | F0804 | |
| Parafilm | Pechiney | PM996 | |
| Phosphat buffered saline | gibco | 14190-904 | |
| Shockwave applicator | MTS | Orthowave 180c |
References
- Pearle, M. S., et al. Shock-wave lithotripsy for renal calculi. The New England Journal of Medicine. 367 (1), 50-57 (2012).
- Chaussy, C., Brendel, W., Schmiedt, E. Extracorporeally Induced Destruction of Kidney Stones By Shock Waves. Lancet. 316 (8207), 1265-1268 (1980).
- Valchanou, V. D., Michailov, P. High energy shock waves in the treatment of delayed and nonunion of fractures. International Orthopedics. 15 (3), 181-184 (1991).
- Haupt, G., Haupt, A., Ekkernkamp, A., Gerety, B., Chvapil, M. Influence of shock waves on fracture healing. Urology. 39 (6), 529-532 (1992).
- Moretti, B., et al. Bone healing induced by ESWT. Clinical Cases in Mineral and Bone Metabolism. 6 (2), 155-158 (2009).
- Romeo, P., Lavanga, V., Pagani, D., Sansone, V. Extracorporeal shock wave therapy in musculoskeletal disorders: A review. Medical Principles and Practice. 23 (1), 7-13 (2014).
- Schaden, W., et al. Shock Wave Therapy for Acute and Chronic Soft Tissue Wounds: A Feasibility Study. Journal of Surgical Research. 143 (1), 1-12 (2007).
- Holfeld, J., et al. Low energy shock wave therapy induces angiogenesis in acute hind-limb ischemia via VEGF receptor 2 phosphorylation. PLoS One. 9 (8), 1-7 (2014).
- Zimpfer, D., et al. Direct epicardial shock wave therapy improves ventricular function and induces angiogenesis in ischemic heart failure. Journal of Thoracic and Cardiovascular Surgery. 137 (4), 963-970 (2009).
- Holfeld, J., et al. Epicardial shock-wave therapy improves ventricular function in a porcine model of ischemic heart disease. Journal of Tissue Engineering and Regenerative Medicine. 10, 1057-1064 (2016).
- Holfeld, J., et al. Toll-like receptor 3 signaling mediates angiogenic response upon shock wave treatment of ischemic muscle. Cardiovascular Research. 109 (2), 331-343 (2016).
- Tepeköylü, C., et al. Shockwaves prevent from heart failure after acute myocardial ischemia via RNA/protein complexes. Journal of Cellular and Molecular Medicine. 21 (4), 791-801 (2017).
- Gollmann-Tepeköylü, C., et al. miR-19a-3p containing exosomes improve function of ischemic myocardium upon shock wave therapy. Cardiovascular Research. , (2019).
- Pölzl, L., et al. Safety and efficacy of direct Cardiac Shockwave Therapy in patients with ischemic cardiomyopathy undergoing coronary artery bypass grafting (the CAST-HF trial), Study protocol for a randomized controlled trial. Trials. 21 (1), 1-10 (2020).
- Holfeld, J., et al. Shock wave application to cell cultures. Journal of Visualized Experiments. (86), e51076 (2014).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved