Method Article
Establishment of a Minimally Invasive Rat Model of Pulmonary Embolism Using Autologous Blood Clots
In This Article
Summary
A detailed methodology for establishing a minimally invasive rat model of pulmonary embolism using autologous blood clots is described. Additional methods for quantifying the infarcted area and visualizing the pulmonary arterial tree are also provided.
Abstract
Pulmonary embolism (PE) is one of the leading causes of cardiovascular death, resulting in a significant socioeconomic burden. Although current treatments primarily focus on anticoagulation and thrombolysis, there is an urgent need for a better understanding of its pathophysiology and the development of new treatment strategies. Animal models play a crucial role in understanding PE and developing new therapies for the disease, with rodents commonly used due to ethical and cost considerations. However, existing rodent models for PE are limited by a lack of standardized procedures, which hampers reproducibility and cross-study comparisons. This study aims to establish a minimally invasive rat model of PE using autologous blood clots. The model features a minimally invasive blood sampling technique, a standardized thrombus generation procedure, and minimally invasive vein access. Additionally, protocols for quantifying infarcted areas and visualizing the pulmonary arterial tree are provided. These procedures aim to improve the reliability of rodent models for studying PE progression and facilitate the development of novel treatments.
Introduction
Pulmonary embolism (PE) is a leading cause of in-hospital death and the third most frequent cause of cardiovascular death. Despite its high incidence, prevention and prompt diagnosis remain challenging1,2. Anticoagulation and thrombolytic therapies are critical in treating PE, yet a deeper understanding of disease progression and novel therapeutic approaches is essential for improving disease management3.
In modern biomedical research, animal models play a pivotal role in elucidating the mechanisms of human diseases and developing new therapies4,5. Mice, rats, hamsters, and rabbits are frequently used in PE modeling due to ethical considerations and cost-effectiveness6,7,8,9,10. PE modeling approaches generally fall into three categories: in vivo thrombus formation, in vitro blood clot injection, and the administration of non-thrombotic particles. The choice of animal species and modeling technique is determined by the specific research objectives, as no single model suits all purposes. For instance, studies focused on exploring new thrombolytic therapies often employ models involving autologous blood clots instead of non-thrombotic particles.
Current methods for modeling PE in rodents face challenges due to the lack of detailed, standardized methodologies. This affects key processes such as blood sampling, blood clot formation, and subsequent embolization, all of which are crucial for ensuring reproducible results across studies. Additionally, there is a significant gap in the ability to quantify the embolized area and accurately map the distribution of emboli post-embolization. Addressing these shortcomings is essential for advancing the reliability and utility of rodent models in PE research.
In this study, detailed protocols for establishing a rat model of PE using autologous blood clots are described. This model features a minimally invasive blood sampling technique, a standardized thrombus generation procedure, and minimally invasive vein access. Additionally, protocols for quantifying infarcted areas in the lungs and visualizing the pulmonary arterial tree are provided, which may facilitate further research discoveries.
Protocol
All animal experiments were conducted with the approval of the Animal Care and Use Committee of the Chinese Academy of Medical Sciences & Peking Union Medical College (approval number: IRM/2-1ACUC-2311-015). Male Sprague-Dawley rats, 6 weeks old and weighing around 250 g, were used in this study. The animals were housed in a specific pathogen-free environment with ad libitum access to a balanced chow diet and water. They were kept under a 12-h light/dark cycle at a room temperature of 22 °C ± 2 °C. The animals were allowed to adapt to the environment for 1 week before undergoing any surgical procedures. The details of the reagents and equipment used in this study are listed in the Table of Materials.
1. Equipment and materials
- Anesthesia equipment: Use a gas anesthesia machine for small animals and isoflurane.
- Surgical materials: Prepare cotton swabs, iodophor disinfectant, a stereo microscope, a shaver, medical tape, a 1 mL syringe with a 27 G needle, 5 mL syringe, 10 mL syringe, normal saline, gauze, 8-0 nylon threads, 6-0 nylon thread with a suture needle, micro hemostatic clamps, micro tweezers, and fine scissors.
- Other materials: Obtain 19 G and 18 G dispensing needles compatible with medical syringes, a silicone tube with an inner diameter of 1.2 mm and a length of 10 cm, an infusion tube with an inner diameter of 3.0 mm and a length of 20 cm, a 1.5 mL centrifuge tube, a pipette, and pipette tips.
- Self-made thrombus generator and blood clot injector (Figure 1): Connect the silicone tube to the 19 G dispensing needle attached to a 1 mL syringe to create the thrombus generator. Connect one end of the infusion tube to the 18 G dispensing needle and attach the other end to a 5 mL syringe to create the clot injector.
2. Autologous blood clot preparation
- Anesthesia
- Place the animal inside the anesthesia induction chamber and administer a mixture of 4% isoflurane with room air at a flow rate of 2 L/min for 2-3 min (following institutionally approved protocols).
- Position the animal on the operation platform in a supine position. Maintain anesthesia by administering 2.5% isoflurane at a flow rate of 0.4 L/min. Test the pedal reflex to ensure adequate anesthesia is achieved.
- Extend both forelimbs horizontally from the body, and extend the hindlimbs to adequately expose the groin area. Secure the animal in this position using medical tape (Figure 2A).
- Blood sampling through the cranial vena cava (CVC)
- Remove the hair around the manubrium using a shaver and hair removal cream. Disinfect the skin with iodophor.
- Insert the 27 G needle attached to the 1 mL syringe vertically to a depth of 8 mm to puncture either the left or right CVC, as shown in Figure 2A.
NOTE: The body surface projection of the CVC is 5 mm lateral to the manubrium. Both sides can be used, and the choice does not affect the following steps. - Hold the syringe and gently pull back the plunger to create negative pressure within the barrel. Gradually withdraw the needle until blood flows in, then maintain that position until 0.3 mL of blood is collected (Figure 2B).
- Blood clot preparation
- Push the collected blood into a 1.5 mL centrifuge tube, taking care not to produce bubbles.
- Draw the blood into the silicone tube of the self-made thrombus generator until the blood column is 10 cm in length.
NOTE: Perform these two steps quickly, as coagulation will initiate and propagate in the centrifuge tube, lowering the quality of the harvested blood clots. - Wait for 30 min until the blood is fully thrombosed. During this time, prepare blood columns for other animals in the same way.
- Flush the thrombosed blood column into a Petri dish containing normal saline, and wash the thrombus column with normal saline three times (Figure 2C).
- Use fine scissors to cut the thrombus column into uniform blood clots, each 4 mm in length (Figure 2D). Draw 10-20 clots into the self-made injector pre-filled with 2 mL of normal saline.
NOTE: The length of the blood clots should not exceed 1 cm, or they will become trapped in the right ventricle.
3. Vein access preparation
- Exposing the superficial epigastric vessels
- Anesthetize and fix the animal as described in step 2.1.
- Shave the hair surrounding the left groin area. Apply hair removal cream to these areas, wait for 1 min, and then wipe off the cream with gauze. Disinfect the skin with iodophor.
- Make a 5 mm skin incision above the origin of the superficial epigastric vessels in the groin area. Usually, the origin of the superficial epigastric vessels can be traced by observing a tiny pulsation in the groin area.
- Using a 10x magnification stereo microscope, separate a thin layer of subcutaneous fat to expose the superficial epigastric vessels (Figure 3A).
- Isolating the superficial epigastric vein
- Carefully isolate the superficial epigastric vein from the artery, nerve, and fat tissue. Perform this both proximally, up to the point where it drains into the femoral vein, and distally, down to the first vein bifurcation.
- Ligate the distal side with 8-0 thread and clamp the thread with a micro-clamp. Control the proximal side with an 8-0 thread loop attached to another micro-clamp (Figure 3B).
- Make a tiny transverse incision at the distal side of the isolated vein. Control the proximal side to avoid bleeding. Flush the remaining blood in the vein lumen with normal saline. Once the vein is clear, use micro tweezers to enlarge the venous incision.
4. Embolization
- Vein cannulation
- Gently insert the needle of the self-made injector into the vein through the vein incision. Secure the needle with a third clamp to prevent it from slipping out of the vein.
- Embolization procedure
- Slowly push the plunger to administer a small amount of normal saline. The fullness of the vein without resistance indicates unobstructed vein access (Figure 3D).
- Continue pushing the plunger to administer clots into the femoral vein through the superficial epigastric vein access. Control the embolization speed at 5 emboli per minute. Monitor the respiration of the animal; emerging and exacerbating dyspnea indicates successful embolization.
- After injecting the predefined amount of blood clots, withdraw the needle and ligate the proximal side of the superficial epigastric vein.
- Suture the incision and place the animal in a warm box. Transfer the animal to its cage once it has awakened.
5. Quantification of infarcted area
- Euthanasia and cardiac perfusion
- Add 25,000 U of heparin sodium to 250 mL of normal saline and mix thoroughly. Connect the bottle to an infusion device and hang the bottle upside down at a height of 80 cm. Close the valve of the infusion device.
- Euthanize the animals with an overdose of isoflurane (following institutionally approved protocols).
- Cut the ribs with large scissors and open the chest, avoiding damage to the lungs and large vessels.
- Insert the needle of the infusion device into the left ventricle. Open the valve of the infusion device to allow heparinized saline infusion. Use scissors to cut the inferior vena cava and the left appendage to allow drainage. Control the perfusion speed at 50 mL/min.
NOTE: During this procedure, normal lung tissue will turn white, while the embolized zone remains rusty. - After completing the perfusion with heparinized saline, puncture the pulmonary artery trunk through the right ventricle using a 10 mL syringe filled with a 4% paraformaldehyde solution. Fix the lung tissue by performing 4% paraformaldehyde perfusion through the lungs for a total volume of 30 mL.
- Introducing an index called Infarction Ratio
- After fixation, excise the lungs, heart, trachea, esophagus, thymus, and large vessels as a whole from the spine and diaphragm11.
- Carefully remove the esophagus, thymus, and fat tissue from the initial mass, leaving only the heart, lungs, and large vessels.
- Submerge the remaining part in an adequate volume of 4% paraformaldehyde solution for at least 48 h.
- Use an analytical balance to weigh the entire lungs. Use fine scissors to separate the infarcted part of the lungs and weigh the infarcted tissue with the same balance. Calculate the Infarction Ratio using the following equation:

i is the weight of the infarcted tissue, w is the weight of the entire lungs, I represents the Infarction Ratio. - After quantifying the infarcted area, trim the paraformaldehyde-fixed lung tissue for paraffin embedding11.
6. Visualization of pulmonary arterial tree
- Pulmonary artery casting
- Euthanize the animal and perform cardiac perfusion as described in step 5.1.
- Add an equal quantity (by weight) of diluent to the silicone casting compound, blend the mixture, and then add 5% (by weight or volume) of the curing agent. Blend the mixture again.
- Draw 1 mL of the mixture into a 1 mL syringe, exhaust the air, and insert the needle into the pulmonary artery trunk through the right ventricle. Secure the needle to the pulmonary artery to prevent slippage and backflow.
- Gently press the plunger to allow the silicone casting mixture to fill the pulmonary arterial tree. The normal lung areas will be dyed by the casting mixture, while the infarcted areas remain rusty red.
NOTE: Pulmonary artery casting should only be applied to intact lung tissue. Typically, 0.6-0.8 mL of the casting mixture is sufficient to fill the pulmonary arterial tree. Over-injection of the casting mixture can lead to pulmonary vein casting, which impacts the visualization of the pulmonary arterial tree. The casting mixture should be used within 20 min after adding the curing agent, as it will crystallize beyond this time. - Withdraw the syringe after casting perfusion, ligate the pulmonary artery trunk, and harvest the lungs, heart, and large vessels as described in step 5.2. Place the whole mass into a Petri dish and store it in a 4 °C refrigerator overnight to allow the casting mixture to crystallize.
- Clearing and visualization
- Remove the heart and large vessels from the lungs with scissors, then clean off the curing mixture debris from the lung surface.
- Immerse the lungs sequentially in 25%, 50%, 75%, 95%, and 100% ethyl alcohol solutions, each for 24 h. This procedure will completely dehydrate the lungs.
- Immerse the dehydrated lungs in methyl salicylate for 24 h. After this clearing process, the pulmonary arterial tree will be visible, and the emboli can also be seen, as methyl salicylate does not clear hemoglobin (Figure 4B,D).
Results
Symptoms and pathology of the PE model
During embolization, the rats experienced shortness of breath, and the thorax showed widened fluctuations. Nearly all the animals survived the pulmonary embolism episode when fewer than 10 cm of blood clots were administered (14 out of 15 modeled animals). After being returned to their cages, the animals curled up in corners and showed reduced interest in food and water. However, these symptoms resolved quickly, and within several hours, the animals behaved normally. There was no significant difference in weight between baseline and 24 h after embolization (341.8 ± 10.4 vs. 343.3 ± 8.5, p = 0.25).
Gross pathology revealed that the embolized (infarcted) areas were wedge-shaped, mostly located in the lower part of the lungs, with clear borders. The emboli were found at the apex of the infarcted areas, blocking the first and secondary branches of the pulmonary artery. Microscopic pathology using hematoxylin-eosin staining confirmed embolization of a pulmonary artery branch (Figure 4F).
Blood clot load and infarcted area
In a preliminary investigation of the impact of blood clot load on lung infarction, we tested both low blood clot load (6 cm thrombus column, 15 clots) and high blood clot load (10 cm thrombus column, 25 clots). As expected, a higher load of emboli resulted in a larger area of lung infarction (Figure 5A-C).
Intrinsic fibrinolysis within 24 h
Compared to lung samples taken immediately after embolization, the lung samples collected 24 h after embolization showed markedly diminished infarcted areas, indicating a strong intrinsic fibrinolysis potential in rats (Figure 5D).
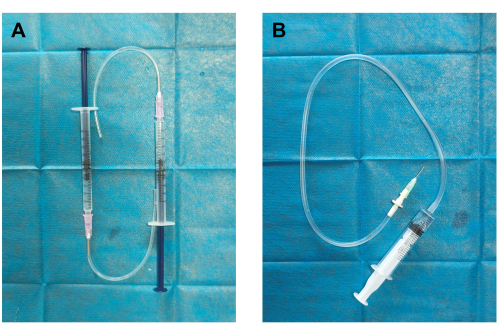
Figure 1: Construction of thrombus generator and blood clot injector. (A) The thrombus generator was assembled by connecting a silicon tube to an 18 G dispensing needle (12 mm outer diameter) and a 1 mL syringe pre-filled with 0.5 mL normal saline. (B) The blood clot injector was constructed by connecting a 19 G dispensing needle (0.84 mm inner diameter, 1.08 mm outer diameter) to an infusion tube (clot container) and a 5 mL syringe pre-filled with 2 mL normal saline. Please click here to view a larger version of this figure.
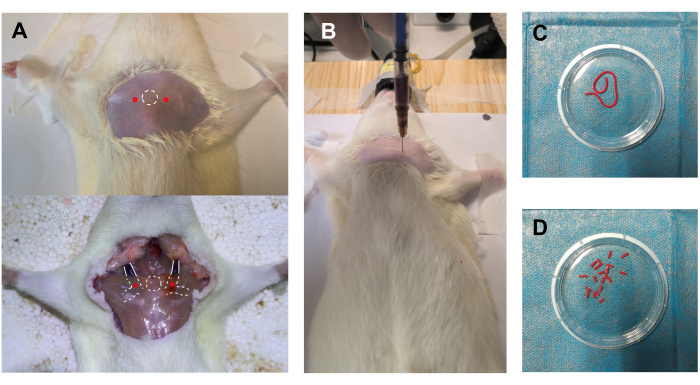
Figure 2: Blood sampling and clot preparation. (A) The rat was anesthetized and fixed in a supine position. The white circle with a dotted line indicates the manubrium, serving as a body surface marker. The red dots next to the white circle indicate the puncture sites for accessing the bilateral cranial vena cavas (CVCs). The lower part of the figure shows an autopsy illustration of the relative positions of the manubrium, CVCs, and puncture sites. (B) Successful blood sampling through the right CVC. (C) Thrombosed blood column being flushed out of the self-made thrombus generator. (D) Blood clots derived from the thrombosed blood column. Please click here to view a larger version of this figure.

Figure 3: Vein access and embolization. (A) Exposure of the superficial epigastric vessels through a 5 mm skin incision, with the blue arrow indicating the superficial epigastric vein and the red arrow indicating the superficial epigastric artery. (B) Isolation of the superficial epigastric vein from surrounding tissue and control of the vein with nylon threads. (C) The self-made injector containing blood clots. (D) Cannulation of the vein with the dispensing needle of the self-made injector and administration of blood clots through the vein access. Please click here to view a larger version of this figure.

Figure 4: Pathology of pulmonary embolism. (A) Normal lungs after cardiac saline perfusion and 4% paraformaldehyde fixation. (B) Normal lungs visualized by silicone casting mixture and methyl salicylate clearing, showing a patent pulmonary artery tree and microvasculature. (C) Lungs immediately after embolization with autologous blood clots, with marked areas indicating lung infarction due to embolization. (D) Diseased lungs visualized by silicone mixture casting and methyl salicylate clearing, with black arrows indicating emboli obstructing pulmonary artery branches and white dashed circles indicating their affected areas, which are infarcted. (E) Dissection of the left lung lobe showing emboli (red arrows) in the pulmonary artery branches. (F) Microscopic images of blood clot embolization in a pulmonary artery branch using Hematoxylin and eosin staining. Scale bar: 200 µm. Please click here to view a larger version of this figure.
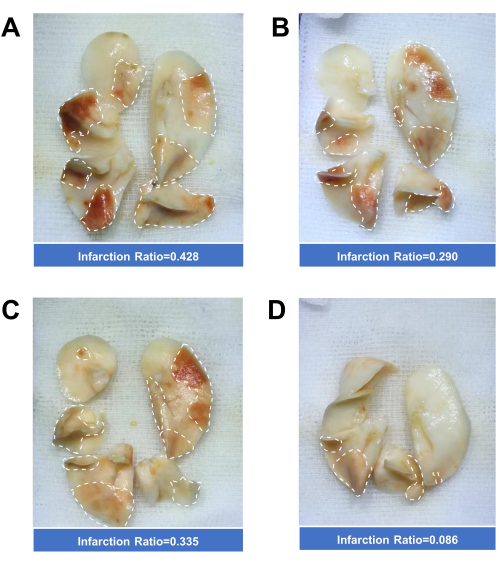
Figure 5: Quantification of lung Infarction Ratio. (A) Lungs immediately taken from a rat after embolization with 10 cm of blood clots (total length). (B) Lungs immediately taken from a rat after embolization with 6 cm of thrombus. (C) Lungs immediately taken from a rat after embolization with 8 cm of blood clots. (D) Lungs taken from a rat 24 h after embolization with 8 cm of blood clots. Please click here to view a larger version of this figure.
Discussion
In this study, a minimally invasive rat model of PE using autologous blood clots was successfully established. Once mastered, this modeling procedure can be completed within 30 min. The model effectively captures key features of clinical PE, as confirmed by pathological examinations. Consequently, it offers a valuable tool for elucidating the hemodynamic changes and pathogenesis of complications following PE, developing new diagnostic biomarkers and therapeutic targets, and testing novel anti-thrombotic treatments.
Significant efforts have been made over several decades to develop a PE animal model, yet no single method has been identified as optimal for modeling PE12. In this article, the modified PE model exhibits several distinct features: (1) Drawing blood from the central venous catheter (CVC): In rodents, the CVC connects with the confluence of the subclavian and internal jugular veins and extends caudally into the thoracic cavity13. The left and right CVCs are nearly symmetric. The CVC has a larger lumen than the jugular veins, is situated close to the manubrium, and lies beneath the pectoralis major. These anatomical characteristics allow for precise vein puncture, larger blood sampling volumes, and quick hemostasis, resulting in a high success rate. Additionally, drawing blood from the CVC is less susceptible to contamination compared to other blood sampling routes, and complications such as hematoma, thrombosis, and pneumothorax are less likely. Hemostasis is typically achieved automatically or quickly with pressure. (2) Injection through the superficial epigastric vein: Typically, the external jugular veins are used for blood clot injection to induce PE6,8,9, and the skin incision for exposure can be minimal. However, cannulation of the external jugular vein often leads to vein occlusion, which can negatively impact subsequent experiments because the external jugular vein is the main cranial outflow in rats after birth14, and cannulation of the other external jugular vein is needed for right ventricle pressure measurement6,8,10. In this modified model, sacrificing the superficial epigastric vein has minimal impact on the body. Additionally, this route for blood clot injection mimics clinical PE, where most emboli originate from veins of the lower extremities. The precise location of the superficial epigastric artery is crucial for a small incision. The pulsation of the superficial epigastric artery can usually be noted on the groin's skin surface, aiding in vessel identification. (3) Customized embolus burden: The embolus burden is determined by the diameter and total length of the blood column. The thrombus generator used in this study allows for standard and customized embolus load preparation. Researchers can adjust these parameters based on their research needs. In this case, the embolus load induces noticeable symptoms and signs but is less likely to result in death. It is generally safe if less than 10 cm of blood clots are injected. If apnea occurs, the injection should be stopped immediately. The experimenter should then hold the animal's chest with fingers placed on the lateral and anterior chest wall and perform rhythmic chest compressions; the animal's respiration may recover after these maneuvers. (4) Lung perfusion and quantification of infarcted areas: This study introduces detailed lung perfusion techniques for better visualization of lung infarction following PE induction. Based on this, an index to quantify the infarcted area, termed the infarction ratio, was developed. The current results indicate that this index may respond to embolus load and intrinsic thrombolysis, suggesting its potential usefulness for cross-group comparisons in studies investigating new anti-thrombotic therapies.
Compared to PE modeling, modeling chronic thromboembolic pulmonary hypertension (CTEPH), a severe long-term complication of PE, is more challenging due to the high fibrinolytic activity in rodents. Potential solutions include repeated embolization and the administration of tranexamic acid (TXA), a fibrinolysis inhibitor7,10,12,15. However, vein access damage and the rapid plasma clearance of TXA continue to make CTEPH modeling difficult. The model and accompanying visualization protocols presented here may provide insights into establishing a reliable CTEPH model.
The limitations of this manuscript should also be addressed. First, there is a lack of hemodynamic measurements for this model, such as right ventricle pressure and echocardiography. However, this does not negate the validity of the model, as it has been pathologically validated. Researchers may refer to other literature for detailed methodologies on hemodynamic measurements16. Secondly, a treatment group to assess drug responses in this PE model was not included, such as the administration of warfarin and urokinase, which could have further demonstrated the model's utility.
In conclusion, a modified rodent model of PE characterized by its minimally invasive approach has been successfully established. Methods for quantifying infarcted areas and visualizing the pulmonary arterial tree have also been provided. This model holds promise for addressing critical questions regarding the prevention and treatment of PE complications.
Disclosures
The authors declare no conflict of interest.
Acknowledgements
This study is supported by a grant from Wu Jieping Medical Foundation (320.6750.19089-36).
Materials
| Name | Company | Catalog Number | Comments |
| Analytical balance | METTLER TOLEDO | MA55/A | None |
| Dispensing needle | Jinrong electronics | None | 19 G and 18 G |
| Fine scissors | Stronger | XGJ1300 | None |
| Heparin sodium salt | Solarbio | 01-08-9041 | 140U/mg |
| Isoflurane | RWD | R510-22-10 | None |
| Methyl salicylate | Macklin | M813577 | AR, 99% |
| Micro clamp | JZ | W40160 | None |
| Micro tweezers | Stronger | XGN1310 | None |
| Silicone casting compound | Flow Tech | MV-130 | None |
| Sprague-Dawley rats | Vital River | SD-IGS | None |
| Stereo microscope | Murzider | MSD204 | None |
References
- Götzinger, F., et al. Interventional therapies for pulmonary embolism. Nat Rev Cardiol. 20 (10), 670-684 (2023).
- Falster, C., et al. Comparison of international guideline recommendations for the diagnosis of pulmonary embolism. Lancet Haematol. 10 (11), e922-e935 (2023).
- Konstantinides, S. V., et al. ESC guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 41 (4), 543-603 (2020).
- Domínguez-Oliva, A., et al. The importance of animal models in biomedical research: Current insights and applications. Animals (Basel). 13 (7), 1223 (2023).
- Mukherjee, P., Roy, S., Ghosh, D., Nandi, S. K. Role of animal models in biomedical research: A review. Lab Anim Res. 38 (1), 18 (2022).
- Tang, Z., et al. Gene expression profiling of pulmonary artery in a rabbit model of pulmonary thromboembolism. PLoS One. 11 (10), e0164530 (2016).
- Deng, C., et al. Expression of tissue factor and forkhead box transcription factor o-1 in a rat model for chronic thromboembolic pulmonary hypertension. J Thromb Thrombolysis. 42 (4), 520-528 (2016).
- Runyon, M. S., Gellar, M. A., Sanapareddy, N., Kline, J. A., Watts, J. A. Development and comparison of a minimally-invasive model of autologous clot pulmonary embolism in Sprague-Dawley and Copenhagen rats. Thromb J. 8, 3 (2010).
- Li, S. Q., et al. Comparative proteomic study of acute pulmonary embolism in a rat model. Proteomics. 7 (13), 2287-2299 (2007).
- Deng, C., et al. Role of FOXO1 and apoptosis in pulmonary vascular remolding in a rat model of chronic thromboembolic pulmonary hypertension. Sci Rep. 7 (1), 2270 (2017).
- Morton, J., Snider, T. A. Guidelines for collection and processing of lungs from aged mice for histological studies. Pathobiol Aging Age Relat Dis. 7 (1), 1313676 (2017).
- Karpov, A. A., Vaulina, D. D., Smirnov, S. S., Moiseeva, O. M., Galagudza, M. M. Rodent models of pulmonary embolism and chronic thromboembolic pulmonary hypertension. Heliyon. 8 (3), e09014 (2022).
- Picazo, M. G., Benito, P. J., García-Olmo, D. C. Efficiency and safety of a technique for drawing blood from the hamster cranial vena cava. Lab Anim (NY). 38 (6), 211-216 (2009).
- Szabó, K. The cranial venous system in the rat: Anatomical pattern and ontogenetic development. Ii. Dorsal drainage. Ann Anat. 177 (4), 313-322 (1995).
- Li, C. Y., et al. The effects and mechanism of ginsenoside rg1 on myocardial remodeling in an animal model of chronic thromboembolic pulmonary hypertension. Eur J Med Res. 18 (1), 16 (2013).
- Sullivan, D. M., Watts, J. A., Kline, J. A. Biventricular cardiac dysfunction after acute massive pulmonary embolism in the rat. J Appl Physiol (1985). 90 (5), 1648-1656 (2001).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved