Method Article
Heterotopic Auxiliary Whole Liver Rat Transplant Model Utilizing a Hepaticoureterostomy for Allograft Rejection Studies
In This Article
Summary
The rat heterotopic auxiliary liver transplant protocol described here offers a practical investigational tool for exploring mechanisms of hepatic allograft rejection. This model helps to alleviate the surgical hurdles and animal stress of orthotopic liver transplantation in rats.
Abstract
Small animal transplant models are indispensable for organ tolerance studies investigating feasible therapeutic interventions in preclinical studies. Rat liver transplantation (LTx) protocols typically use an orthotopic model where the recipients' native liver is removed and replaced with a donor liver. This technically demanding surgical procedure requires advanced micro-surgical skills and is further complicated by lengthy anhepatic and lower body ischemia times. This prompted the development of a less complicated heterotopic method that can be performed faster with no anhepatic or lower body ischemia time, reducing post-surgery stress for the recipient animal.
This heterotopic LTx protocol includes two main steps: excising the liver from the donor rat and transplanting the whole liver into the recipient rat. During the excision of the donor liver, the surgeon ligates the supra-hepatic vena cava (SHVC) and hepatic artery (HA). On the recipient side, the surgeon removes the left kidney and positions the donor liver with the portal vein (PV), infra-hepatic vena cava (IHVC), and bile duct facing the renal vessels. Further, the surgeon anastomoses the recipient's renal vein end to end with the IHVC of the liver and arterializes the PV with the renal artery using a stent. A hepaticoureterostomy is utilized for biliary drainage by anastomosing the bile duct to the recipient's ureter, permitting the discharge of bile via the bladder.
The average duration of the transplantation was 130 min, cold ischemia duration was around 35 min, and warm ischemia duration was less than 25 min. Hematoxylin and eosin histology of the auxiliary liver from syngeneic transplants showed normal hepatocyte structure with no significant parenchymal alterations 30 days post-transplant. In contrast, 8-day post-transplant allogeneic graft specimens demonstrated extensive lymphocytic infiltration with a Banff Schema rejection activity index score of 9. Therefore, this LTx method facilitates a low morbidity rejection model alternative to orthotopic LTx.
Introduction
Small animal LTx is an invaluable model for investigating mechanisms of liver rejection. Heterotopic auxiliary liver transplantation with portal vein arterialization (HALT-PVA) in rats was introduced in 1968 by Lee and Edgington1when they reported using a recipient's renal vein and artery to re-vascularize a grafted auxiliary liver. Subsequently, Hess et al.2 enhanced the protocol with the mitigation of functional competition between the native and auxiliary livers by reducing the native and donor liver size along with reconstructing the donor bile duct connection, resulting in long-term graft survival. Further refinements were made with the introduction of cuff anastomosis3,4, and Schleimer et al.5 determined the optimal stent diameter for regulating blood flow to obtain physiological portal flow and avoid hyper- or hypo-perfusion of the graft. Other investigators developed significant alterations to the method by using the splenic6 or common iliac7 artery for graft blood supply, while some developed models that used only venous blood8 or only arterial blood via the hepatic artery9 to supply the auxiliary liver graft.
The present study hypothesized that functional competition from the native liver would not interfere with allograft rejection, so we developed a protocol based on the flow-regulated Schleimer model10 that did not include any size reduction of the native or auxiliary liver. The left side of the recipient was selected to locate the graft because it provided optimal orientation between the recipient's renal and donor liver vessels. Initially, we attempted biliary reconstruction via hepaticoduodenostomy but these trials simply confirmed Schleimer's assertion that "biliary drainage is the Achilles heel of liver transplantation"10. This prompted the development of a new technique where the bile duct is anastomosed end-to-end using a stent with the recipients' ureter, permitting the discharge of bile via the bladder. A noteworthy benefit of using a hepaticoureterostomy is that graft liver functionality can be monitored daily by observing the urine; a bile-producing liver graft colors the urine a bright yellow. Figure 1 represents the schematic overview of the HALT-PVA method.
An important advantage of heterotopic over orthotopic rat LTx relates to the absence of any anhepatic or total lower body ischemia time, which permits quicker and easier recoveries for heterotopic recipients. Additionally, LTx immunological studies utilizing orthotopic methods often rely on severe rejection or death of the recipient as an experimental endpoint, which is not the case with heterotopic transplants, where the animal remains healthy even if the allograft stops functioning due to rejection. Both of these features of the heterotopic method support principles of the international 3R's initiative (Replacement, Reduction, and Refinement)11, which promotes a framework for minimizing the pain, suffering, and distress experienced by research animals and improving their welfare.
The HALT-PVA model reported here is a practical and reliable method for investigating the mechanisms of hepatic allograft rejection in preclinical studies. This useful experimental technique helps overcome the considerable surgical demands and animal stress of orthotopic LTx in rats. In the future, we intend to use this method to investigate the mechanisms of acute immune rejection while exploring novel targets and therapeutic strategies to suppress hepatic allograft rejection.
Protocol
Animals were bred and housed in specific pathogen-free conditions in the animal care facilities at the University of Wisconsin (UW)-Madison Institute for Medical Research in accordance with institutional guidelines. The study protocol (No. M006022) was approved by the Institutional Animal Care and Use Committee at the UW School of Medicine and Public Health, and all animals were treated ethically.
1. Animals
- Use adult Lewis female rats weighing 205-235 g and Lewis males weighing 250-280 g as donor rats. Use adult male Lewis and Brown Norway rats weighing 365-420 g as recipients.
- Perform syngeneic transplants by transplanting Lewis donors into Lewis recipients, while the allogeneic transplants utilized Lewis donors transplanted into Brown Norway recipients.
- Perform all surgeries by two persons using a dual-head microscope.
2. Auxiliary liver donor procurement procedure
- Anesthetize the donor rat with 5% isoflurane inhalation in an induction chamber. Record the weight of the rat and shave the abdomen with an electric clipper.
- Position the rat in a supine position on a heated surgery pad with its nose in an anesthesia cone and immobilize the limbs with tape. Disinfect the abdomen with 75% alcohol and lower the isoflurane to 2%.
- Make a longitudinal midline skin and muscle incision from the pubis to the xiphoid using scissors. Near the mid-point of the longitudinal incision, extend it laterally to the left and right, then install retractors on both sides of the abdominal wall and the xiphoid process.
- Using moist cotton swabs, retract the intestines to the left side of the abdomen while using spring scissors to cut the gastro ligaments attached to the liver, then immobilize the intestines under moistened gauze. Cover the liver with a small piece of sterile gauze moistened with warm saline.
- Use moist cotton swabs to retract the liver and dissect the falciform, triangular, hepatogastric, and hepatoduodenal ligaments. Next, cauterize with bipolar forceps and divide the para-esophageal vessels between the left lateral and anterior caudate lobe.
- Using angle forceps or needle holders, dissect behind the SHVC inferior to the diaphragm, then pass a 5-0 silk suture under the SHVC and loosely tie a double knot for later use.
- Retract the inferior right lateral lobe upward, cut the ligament, and immobilize under moistened gauze. Isolate the IHVC from the retroperitoneal tissue down to the right renal vein and ligate the right adrenal vein with a 6-0 silk suture as close to the IHVC as possible. Divide this vein later when the graft is removed.
- Using a 27 G hydro-dissection needle (Figure 2A), dissociate the PV from surrounding connective tissue and separate it from the pyloric and splenic veins by ligating and dividing them using a 7-0 silk suture.
- Isolate, ligate with 6-0 silk suture, and divide the common hepatic artery close to where it passes beneath the PV.
- Ligate the bile duct with a 5-0 silk suture at the branching of the gastroduodenal artery while preserving the fat tissue around the bile duct as much as possible; in particular, avoid separating the bile duct from the proper hepatic artery while keeping the overall length as short as is practical.
- Using spring scissors, make a small incision in the wall of the bile duct proximal to the ligation. Insert a 0.0215" diameter by 5 mm long polyimide tubing stent into the bile duct lumen and secure it with a 6-0 silk suture, leaving one end of the suture long for later use. Divide the bile duct by cutting between the 5-0 and 6-0 ligations.
- Mark the top side of the IHVC and PV with a surgical dye pen to help align the vessels during anastomosis, then clamp the portal vein with a microvessel clamp as distal to the liver as possible.
- Insert a 20 mL syringe with a 26 G needle into the PV proximal to the micro clamp and perfuse the liver with 10-15 mL of ice-cold heparinized saline; simultaneously, divide the IHVC using spring scissors as close to the right renal vein as possible.
- Excise the liver using spring scissors by dissecting the PV proximal to the micro clamp, tightening the 5-0 suture previously placed around the SHVC, and dissecting the diaphragm to transect the intrathoracic vena cava.
- Continue by dissecting the remaining ligaments at the back of the liver and divide the previously ligated adrenal vein. Place the excised liver in cold saline on ice.
3. Auxiliary liver recipient transplant procedure
- Anesthetize the recipient rat with 5% isoflurane inhalation in an induction chamber. Record the weight of the rat and shave the abdomen with an electric clipper.
- Position the rat in a supine position on a heated surgery pad with its nose in an anesthesia cone and immobilize the limbs with tape. Apply an eye lubricant, disinfect the abdomen with 75% alcohol, and lower the isoflurane to 2%.
- Using scissors, make a longitudinal midline skin and muscle incision from the pubis to the xiphoid, then install retractors on both sides of the abdominal wall.
- Using moist cotton swabs, retract the intestines to the right side of the abdomen and cover them with moistened gauze. Apply another moist gauze to cover the stomach, spleen, and liver, exposing the left kidney and renal vessels.
- Using a 27 G hydro-dissection needle and blunt tip forceps, separate the left renal vein from the renal artery, carefully removing fat and connective tissue from both vessels.
- Isolate the gonadal and adrenal veins, and use 6-0 silk to ligate them proximal to the renal vein temporarily. Cauterize, using bipolar forceps, all micro side branches isolating the renal vein and artery between the aorta/VC and the kidney.
- Mobilize and ligate the ureter with a 6-0 silk suture at the inferior pole. Mark the renal vein and artery with a surgical dye pen to help orientate the vessels during anastomosis and ensure there are no twists.
- Clamp the renal artery and the renal vein with a microvessel clamp as close to the aorta and VC as possible. Transect the renal artery with spring scissors just past the vessel bifurcation and divide the renal vein about halfway between the VC and kidney. Mobilize the left kidney from the surrounding connective tissue and remove it.
- Flush both vessels with heparinized saline using a 27 G hydro-dissection needle to remove all remaining blood.
- With spring scissors, cut a small fish mouth opening in the fork of the renal artery bifurcation to make a funnel-shaped opening and insert an 8 mm stent cut from a 26 G catheter (Figure 2B). Secure the stent with 6-0 silk suture, leaving one end of the suture long for later use.
- Introduce the donor liver and position it with the PV, IHVC, and bile duct facing the recipient's left renal vein and artery. Using a 9-0 nylon suture, install two stay sutures on opposite sides of the IHVC-renal vein connection.
- Compare the width of the vessels and make a small fish mouth incision using spring scissors into the face of the renal vein until it has a similar width to the donor's IHVC (Figure 2C).
- Using a 9-0 nylon suture, anastomose the liver IHVC end-to-end to the renal vein with 9 or 10 running sutures across both front and back walls of the vessel. Alternatively, use a cuff method to complete this anastomosis3,4.
- Confirm that placement of the renal artery is beneath the IHVC (Figure 2D), and insert the stent previously placed in the renal artery into the liver portal vein and secure with a 6-0 silk suture, leaving one end of the suture long to attach to the opposite thread on the artery. Draw the ends together to hold each from sliding off the stent.
- Remove the micro clamp on the renal vein first, then remove the micro clamp on the renal artery.
- During reperfusion of the liver, use gauze and cotton swabs to apply light pressure around the anastomosis region until a patent anastomosis is achieved (Figure 2D). Remove the temporary ligations previously placed on the adrenal and gonadal veins.
- Carefully mobilize approximately 10 mm down the end of the left ureter from surrounding connective tissue, leaving a significant amount of fat tissue attached. With spring scissors, make a small incision in the wall of the ureter proximal to the 5-0 ligation previously placed.
- Insert the polyimide stent previously attached to the bile duct into the small incision made in the ureter wall. Secure with a 6-0 silk suture and tie one end together with the long thread on the bile duct side of the stent, drawing both ends firmly together.
- Return the intestines back to their natural position (Figure 2E), irrigate with 2-3 mL of saline, and close the abdomen in two layers using 3-0 silk running sutures.
- Inject 0.1 mg/kg buprenorphine subcutaneously, place the recipient in a clean, heated cage, and monitor recovery for 1-2 h before returning the animal to the animal housing facility.
4. Post-surgical follow-up
- Starting on day 2 post-surgery, inject allogeneic transplant recipients with heparin (1 IU/g) subcutaneously daily.
- Starting on day 2 post-surgery, inject syngeneic transplant recipients with heparin (1 IU/2 g) subcutaneously every other day.
Results
Presently, 29 pairs of rats have been used to establish the HALT-PVA protocol, 17 syngeneic transplants, and 12 allogeneic transplants. The syngeneic transplanted livers survived to their designated 8 or 30-day experimental endpoint with a 70% success rate, while allogeneic transplanted livers survived to their designated 3 or 8-day endpoints with a 50% success rate. Failures include rats that died due to surgical complications and auxiliary livers that failed even when the recipient survived.
The average duration of the operation was 130 min, with a cold ischemia time of about 35 min, and a warm ischemia time of less than 25 min. Provided there were no intra-operative complications, the recipients woke and became active within 10-20 min, began drinking and ambulating within 1 h and 24 h later, they behaved like normal healthy rats.
Syngeneic liver grafts demonstrated excellent color and bile duct patency 30 days post-transplant (Figure 3), while Hematoxylin and eosin (H&E) histology showed normal hepatocyte structure with no significant parenchymal alterations at both the 8-day and 30-day time points (Figure 4A,B). After only 3 days, histology of the allogenic transplants showed significant portal inflammation (Figure 4C), while the 8 day allogenic grafts demonstrated acute cellular rejection with extensive lymphocytic infiltration (Figure 4D).
Representative allogeneic and syngeneic LTx specimens were evaluated by a board-certified liver pathologist, and rejection was scored using the Banff Schema rejection activity index (RAI)12. Pathological evaluation determined no rejection present, with a Banff RAI of 0, in both 8-day and 30-day syngeneic transplants, while 8-day allografts were severely rejected with a Banff RAI score of 9 (Table 1).
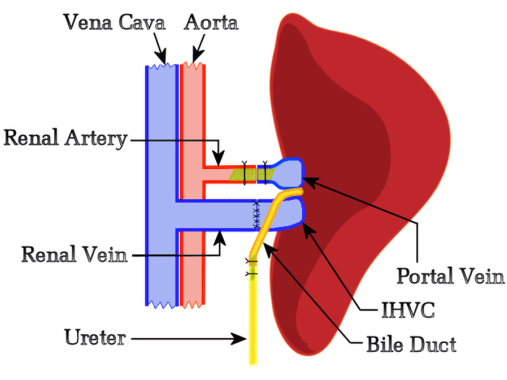
Figure 1: Schematic diagram of the rat HALT-PVA procedure. The PV is arterialized with the left renal artery using a stent, the IHVC is anastomosed end-to-end to the left renal vein, and the bile duct is attached to the ureter using a stent. Please click here to view a larger version of this figure.
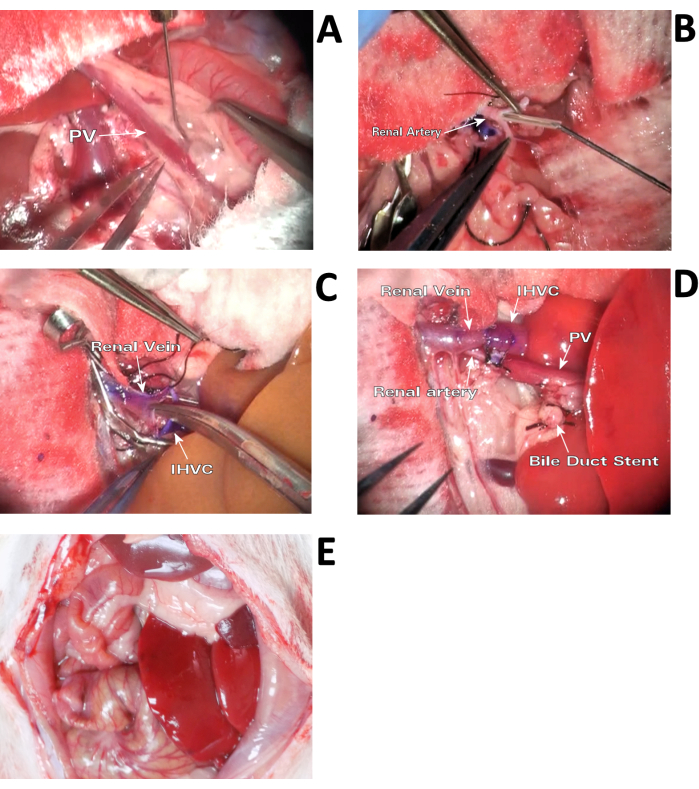
Figure 2: Surgical procedure. (A) Hydro-dissection of PV. Dissociate the PV from surrounding connective tissue with a 27 G hydro-dissection needle. (B) Inserting arterial stent. The arterial stent is inserted into a small funnel-shaped opening cut in the fork of the renal artery bifurcation. (C) Widening the renal vein. In order to match the larger width of the donor IHVC for anastomosis, a small fish mouth cut is made into the face of the recipient's renal vein after two stay sutures are in place. (D) Perfused liver graft immediately after anastomosis. Positioning the renal artery and PV connection beneath the renal vein and IHVC is crucial for preventing thrombosis of the graft. (E) Rat HALT-PVA in situ. The auxiliary liver is positioned against the left wall of the abdomen before returning the intestines. Please click here to view a larger version of this figure.
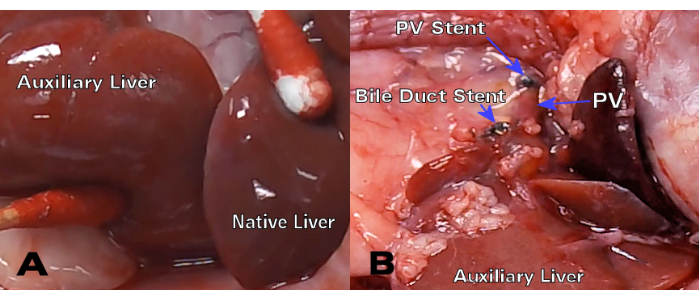
Figure 3: Syngeneic HALT-PVA 30-day post-transplant. (A) After 30 days, the auxiliary liver has a similar color and texture to the native liver, while (B) the bile duct and PV stent remain patent and unrestricted. Please click here to view a larger version of this figure.
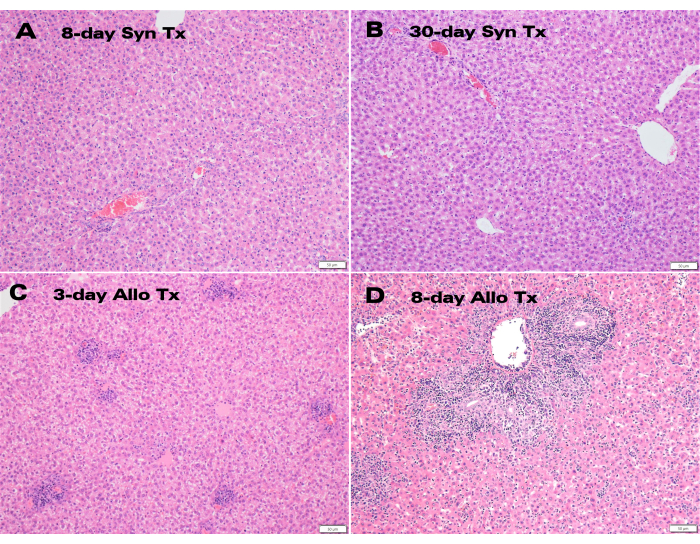
Figure 4: Histology of auxiliary liver grafts. H&E staining of (A) 8-day and (B) 30-day post-transplant syngeneic grafts exhibiting normal hepatocyte structure together with (C) 3-day and (D) 8-day allogeneic grafts displaying portal inflammation and lymphocytic infiltration. Scale bar: 50 µm. Please click here to view a larger version of this figure.
| Allogeneic Tx's | Portal Inflammation | Bile Duct Injury | Venous Endo Inflammation | Banff RAI |
| 3-day (LT-34) | 2 | 2 | 3 | 7 |
| 3-day (LT-38) | 1 | 0 | 0 | 1 |
| 8-day (LT-20) | 3 | 3 | 3 | 9 |
| 8-day (LT-40) | 3 | 3 | 3 | 9 |
| Syngeneic Tx's | ||||
| 8-day (LT-13) | 0 | 0 | 0 | 0 |
| 8-day (LT-19) | 0 | 0 | 0 | 0 |
| 30-day (LT-28) | 0 | 0 | 0 | 0 |
| 30-day (LT-32) | 0 | 0 | 0 | 0 |
Table 1: Banff schema. Rejection activity index (RAI) of allogeneic and syngeneic rat LTx's.
Discussion
Liver transplantation is the only treatment option for patients with end-stage liver disease, with almost 9,000 LTxs performed yearly in the US13. Unfortunately, immunological rejection is seen in up to 25% of LTx recipients, and this rejection is detrimental to the transplanted organ and patient14,15. To improve outcomes after LTx, the development of innovative models to study organ rejection and implement strategies to decrease rejection are required.
Compared to orthotopic rat liver transplantation, the present heterotopic model is remarkably easy to establish. The degree of technical difficulty is best calculated by considering the single most difficult step in the procedure, which is the end-to-end anastomoses of the IHVC to the renal vein. Our personal preference for this connection is to utilize traditional suturing while others find a cuff system more convenient3,4; either way, anyone comfortable with end-to-end anastomosis of a 3 mm vein by any method has all the technical skills needed to accomplish this procedure. Having access to a dual head microscope, we prefer to do these surgeries with two people; however, this is not a requirement for success with this protocol as it has been performed solo as well.
The primary postoperative complication of this model is thrombosis of the graft liver which is provoked by three things. First, the arterial stent itself can induce thrombosis if the ends of the stent have any burrs or irregularities that cause eddy turbulence in the blood flow. The stent should be cut with a sharp razor, not with scissors, then inspected under the microscope to ensure there are no imperfections. Second, if the renal artery/PV stent anastomosis is positioned on top of the renal vein/IHVC anastomosis, it will restrict blood flow and induce thrombosis. The final placement of the arterial stent should be beneath the IHVC, see Figure 2D. Third, post-surgical treatment with the anti-coagulant Heparin is essential for preventing thrombosis of allogeneic transplants, while a lower dose of heparin is also helpful for eliminating the risk of thrombosis in the syngeneic grafts.
The lower survival rate for the allogeneic transplants compared to the syngeneic grafts reported here reflects the pro-thrombotic nature of the rejecting auxiliary liver, which triggers thrombosis of the arterial stent. Several attempts were required to find a heparin dosing scheme that could prevent the stent from thrombosing in allogeneic transplants. Initially, of the first 6 allograft recipients to survive surgery, 50% of the auxiliary livers failed due to thrombosis, but following an increase in heparin dosing, the next, final three allogeneic transplants survived with no thrombosis. Moving forward, having determined an effective heparin dose, we expect the success rate of allogeneic transplants to increase significantly. Likewise, most of the syngeneic failures occurred early in development, and we expect the success rate of syngeneic transplants to improve as well.
We used the acute rejection model of Lewis to Brown Norway LTx because the Brown Norway to Lewis strain combination does not reject16. It is noteworthy that when orthotopic transplant methods are utilized with this rejection model, and liver rejection occurs, the recipient animals suffer severe morbidity as they become depressed, inactive, and stop eating before dying within 14 days16. However, with this heterotopic LTx model, death is not used as an endpoint, and morbidity does not occur; the animal remains healthy and active for the duration of the experiment even when the auxiliary liver is completely rejected. Undoubtedly, this heterotopic LTx model contributes significantly to minimizing the pain, suffering, and distress experienced by the recipient animals.
Recent advances in normothermic ex vivo liver perfusion (NEVLP) are an exciting advancement in the way livers are stored prior to clinical transplantation17,18,19. During NEVLP, the donor liver resumes physiologic activity allowing therapeutic interventions prior to transplantation20,21. NEVLP is also being increasingly used to assess the viability of marginal organs (livers from older or obese donors or livers procured from donors after cardiac death)22,23. Although exciting, only a handful of labs have been able to transplant rat livers after NEVLP24,25. This is likely due to the surgical stress on the animal and the high technical demand of preparing the liver for both NEVLP and transplantation. In contrast, the heterotopic LTx operative technique outlined in this manuscript is less technically demanding and causes less stress on the animal. As such, it may be a viable option for small animal models of NEVLP and subsequent transplantation.
In conclusion, we present an alternative, low-morbidity liver transplant model that may be beneficial for future transplant rejection studies.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by the National Institute of Health (NIH) K08AI155816, awarded to DA.
Materials
| Name | Company | Catalog Number | Comments |
| 3-0 Silk Suture | Ethicon | C013D | |
| 5-0 Silk ties | Fine Science Tools | 18020-50 | |
| 6-0 Silk ties | Fine Science Tools | 18020-60 | |
| 7-0 Silk ties | Teleflex | 103-s | |
| 9-0 Polyamide Suture | AROSurgical | T05A09N10-13 | Black |
| Bipolar Cautery | Codman & Shurtleff Inc. | P.H. 234 | |
| Buprenorphine HCL | Hospira | 409201232 | |
| Forceps, Adson-Brown | Fine Science Tools | 11627-12 | 12.5 cm |
| Forceps, Angled Dumont | Fine Science Tools | 11253-25 | Medical #5/45 11 cm |
| Forceps, Suture Tying | Fine Science Tools | 18025-10 | 10 cm |
| Heparin Sodium Injection, USB | Fresenius Kabi | 504015 | 10,000 USP units per 10 mL |
| Hydrodissection Cannula | Ambler Surgical | 1021E | 27 G |
| Isoflurane | Dechra Vet. Products | 17033-091-25 | |
| I.V. Catheter | Kendall | 2619PUR | 26 G x 3/4" |
| Magnetic Retraction System | Fine Science Tools | 18200-50 | |
| Micro Clamps | Fine Science Tools | 18055-05 | 6 mm |
| Micro Clamps | Fine Science Tools | 18055-06 | 4 mm |
| Micro Clamp Applicator | Fine Science Tools | 18057-14 | 14 cm |
| Micro Needle Holder | S&T | C-14 | 14 cm |
| Microscope | Zeiss | Universal S3 | Dual head |
| Ophthalmic Ointment | Puralube | 14590500 | |
| Polyimidi Tubing | Cole Parmer | 95820-04 | OD 0.0215", ID 0.0195", wall 0.0010" |
| Saline | Baxter | 281324 | 0.9% Sodium Chloride |
| Surgical Spring Scissors | S&T | SDC-15 | Blunt 14 cm |
| Surgical Spring Scissors | Fine Science Tools | 15021-15 | Vannas 14 cm |
References
- Lee, S., Edgington, T. S. Heterotopic liver transplantation utilizing inbred rat strains. Am J Pathol. 52 (3), 649-669 (1968).
- Hess, F., Jerusalem, C., Van der Heyde, M. N. Advantages of auxiliary liver homotransplantation in rats. Arch Surg. 104, 76-80 (1972).
- Marni, A., Ferrero, M. Heterotopic liver grafting in the rat. A simplified method using cuff techniques. Transplantation. 39 (3), 329-331 (1985).
- Kobayashi, E., et al. Auxiliary heterotopic liver transplantation in the rat: a simplified model using cuff technique and application for congenitally hyperbilirubimemic Gunn rat. Microsurgery. 18 (2), 97-102 (1998).
- Schleimer, K., et al. Auxiliary liver transplantation with flow-regulated portal vein arterialization offers a successful therapeutic option in acute hepatic failure--investigations in heterotopic auxiliary rat liver transplantation. Transpl Int. 19 (7), 581-588 (2006).
- Qiao, J., Han, C., Zhang, J., Wang, Z., Meng, X. A new model of auxiliary partial heterotopic liver transplantation with liver dual artery supply. Exp Ther Med. 9 (2), 367-371 (2015).
- Li, J., Ren, J., Zhang, J., Meng, X. A. Modified kidney-sparing portal vein arterialization model of heterotopic auxiliary liver transplantation increases liver IL-6, TNF-α, and HGF levels and enhances liver regeneration: an animal model. BMC Surg. 2, 281-292 (2022).
- Ono, Y., et al. Regeneration and cell recruitment in an improved heterotopic auxiliary partial liver transplantation (APLT) model in the rat. Transplantation. 101 (1), 92-100 (2017).
- Wang, J., et al. Auxiliary partial liver grafting in rats: effect of host hepatectomy on graft regeneration, and review of literature on surgical technique. Microsurgery. 22 (8), 371-377 (2002).
- Schleimer, K., et al. Heterotopic auxiliary rat liver transplantation with flow-regulated portal vein arterialization in acute hepatic failure. J Vis Exp. (91), e51115 (2014).
- Prescott, M. J., Lidster, K. Improving quality of science through better animal welfare: the NC3Rs strategy. Lab Animal. 46 (4), 152-156 (2017).
- . Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 25 (3), 658-663 (1997).
- Kwong, A. J., et al. OPTN/SRTR 2020 Annual Data Report: Liver. Am J Transplant. 22, 204-309 (2022).
- Nacif, L. S., et al. Late acute rejection in liver transplant: a systematic review. Arq Bras Cir Dig. 28 (3), 212-215 (2015).
- Levitsky, J., et al. Acute rejection increases risk of graft failure and death in recent liver transplant recipients. Clin Gastroenterol Hepatol. 15 (4), 584-593 (2017).
- Gong, J., Cao, D., Chen, Y., Li, J., Gong, J., Zeng, Z. Role of programmed death ligand 1 and Kupffer cell in immune regulation after orthotopic liver transplantation in rats. Int Immunopharmacol. 48, 8-16 (2017).
- Ceresa, C. D. L., Nasralla, D., Coussios, C. C., Friend, P. J. The case for normothermic machine perfusion in liver transplantation. Liver Transpl. 24 (2), 269-275 (2018).
- Nasralla, D., et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 557 (7703), 50-56 (2018).
- Markmann, J. F., et al. Impact of portable normothermic blood-based machine perfusion on outcomes of liver transplant: The OCS liver PROTECT randomized clinical trial. JAMA Surg. 157 (3), 189-198 (2022).
- Goldaracena, N., et al. Anti-inflammatory signaling during ex vivo liver perfusion improves the preservation of pig liver grafts before transplantation. Liver Transpl. 22 (11), 1573-1583 (2016).
- Carlson, K. N., et al. Interleukin-10 and transforming growth factor-beta cytokines decrease immune activation during normothermic ex vivo machine perfusion of the rat liver. Liver Transpl. 27 (11), 1577-1591 (2021).
- Ig-Izevbekhai, K., Goldberg, D. S., Karp, S. J., Foley, D. P., Abt, P. L. Immunosuppression in donation after circulatory death liver transplantation: Can induction modify graft survival. Liver Transpl. 26 (9), 1154-1166 (2020).
- Kageyama, S., et al. Ischemia-reperfusion Injury in allogeneic liver transplantation: A role of CD4 T cells in early allograft injury. Transplantation. 105 (9), 1989-1997 (2021).
- Oldani, G., et al. The impact of short-term machine perfusion on the risk of cancer recurrence after rat liver transplantation with donors after circulatory death. PLoS One. 14 (11), e0224890 (2019).
- Schlegel, A., Graf, R., Clavien, P. A., Dutkowski, P. Hypothermic oxygenated perfusion (HOPE) protects from biliary injury in a rodent model of DCD liver transplantation. J Hepatol. 59 (5), 984-991 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved