Method Article
Effects of Extracorporeal Membrane Oxygenation on the Coagulation System
* These authors contributed equally
In This Article
Summary
We present a protocol for establishing a long-term awake extracorporeal membrane oxygenation (ECMO) model in sheep. Special attention is given to the management and evaluation of the coagulation system during the ECMO model.
Abstract
This study aimed to investigate the effects of long-term awake extracorporeal membrane oxygenation (ECMO) on the coagulation system in a sheep model. A total of ten healthy sheep were included in the study, with 5 sheep in each group. In the veno-arterial ECMO (V-A ECMO) group, cannulation was performed in the right carotid artery and the right external jugular vein. In the veno-venous ECMO (V-V ECMO) group, a dual-lumen catheter was utilized to insert into the right external jugular vein. After initiating ECMO, the sheep were recovered from anesthesia and remained awake for 7 days. The target activated clotting time (ACT) goal was set at 220-250 s. In both groups, the actual ACT fluctuated around 250 s with the dose of heparin gradually increasing, reaching almost 60 IU/kg/min at the end of the experiments. Moreover, the activated partial thromboplastin time (APTT) and thrombin time (TT) values were significantly higher in the V-A ECMO group compared to the V-V ECMO group, despite receiving the same doses of heparin. Although laboratory test results fluctuated within a normal and reasonable range, infarct foci in the kidneys were observed in both groups at the end of the study.
Introduction
Extracorporeal membrane oxygenation (ECMO) serves as a life-saving intervention, providing cardiopulmonary support for severely ill patients. It is classified into two primary forms: veno-arterial ECMO (V-A ECMO) and veno-venous ECMO (V-V ECMO)1,2. V-A ECMO is employed for patients experiencing circulatory failure, whereas V-V ECMO is preferred for those with respiratory failure but without severe cardiovascular dysfunction3,4.
Thrombosis and bleeding are prevalent complications in ECMO patients5. The ECMO circuit exposes blood to artificial surfaces, initiating complex coagulation responses6. These processes can lead to endothelial damage and microcirculation disorders, resulting in subsequent dysfunction in vital organs7,8. Consequently, effective systemic anticoagulation management is considered crucial for ECMO patients. Despite this, there remains a lack of evidence to guide anticoagulation strategies in various ECMO-related clinical settings.
The establishment of a stable ECMO animal model can provide insights into the impact of ECMO on the body, contributing significantly to the optimization of ECMO management strategies, reduction of ECMO-related complications, and improvement of patient outcomes in clinical practice. Large animals, such as sheep and pigs, are the primary choices for establishing ECMO models due to their physiological parameters closely resembling those of humans9,10. However, previous large animal ECMO models had a maintenance time of less than 24 h, making it challenging to comprehensively evaluate the impact of ECMO on the coagulation system11. Therefore, there is a need to establish long-term ECMO large animal models to thoroughly explore the pathophysiological mechanisms of ECMO. Utilizing long-term large animal models to investigate the effects of ECMO on the coagulation system can provide more robust evidence for clinical practice.
This study aims to establish a long-term (7 days) awake V-A and V-V ECMO model in healthy sheep. The central focus of the entire model establishment and evaluation is the management of anticoagulation.
Protocol
This experimental protocol received approval from the Institutional Animal Care and Use Committee of Fuwai Hospital (no. 0101-2-20-HX(X)). All procedures adhered to the guidelines outlined in the National Institutes of Health's Guide for the Use and Care of Laboratory Animals. The experiment took place at the Beijing Key Laboratory of Pre-clinical Research and Evaluation for Cardiovascular Implant Materials, Animal Experimental Center of Fuwai Hospital (registration no. CNAS LA0009). Healthy sheep that met the required quarantine standards at the Animal Experimental Center of Fuwai Hospital were utilized in the study. Furthermore, this research followed the Animal Research: Reporting of In Vivo Experiments guidelines. Male Small Tail Han sheep with a weight range of 50-65 kg and an age range of 12-24 months (see Table of Materials) were housed in a specific pathogen-free environment with free access to food and water for at least one week before the surgery. The sheep were randomly assigned to two groups, each consisting of 5 individuals: the Veno-Arterial ECMO (V-A ECMO) group and the Veno-Venous ECMO (V-V ECMO) group. The selection of healthy sheep was justified by the necessity to precisely assess the effects of ECMO support on the organism. The equipment and reagents used in the study are listed in the Table of Materials.
1. Animal preparation
- Before the surgical procedure, fast healthy adult sheep for 48 h and deprive them of water for 12 h.
- Sterilize the surgical instruments and ECMO parameters before the surgery. Ensure the provision of personal protective equipment for all members of the surgical team, including surgical caps, disposable facemasks, clean surgical attire, shoe covers, sterile operating clothes, and sterile gloves.
- Administer a propofol injection (5 mg/kg) through the auricular vein. As previously described, fix the blood pressure cuff on the thigh and place electrocardiogram leads on all four limbs. Connect the blood pressure cuff and ECG leads to the medical monitor for ECG and non-invasive blood pressure measurement12.
- Insert a single-lumen endotracheal tube into the trachea and connect the tube to the ventilator with volume-controlled mode. Set the parameters as follows: tidal volume: 8-10 mL/kg, respiratory rate: 12-20/min, initial fraction of inspiration oxygen (FiO2): 60%.
- Administer a combination of intravenous anesthesia (propofol, 8-10 mg/kg/h) and inhaled anesthesia (isoflurane 2%-3%) for anesthesia maintenance. Provide intermittent flurbiprofen (1-2 mg/kg) intravenously for intraoperative analgesia.
- Place the sheep on the operating table and secure their limbs with a flexible cloth belt. Proceed to isolate the left carotid artery and jugular vein and insert a single-lumen central venous catheter (18 Fr) and a triple-lumen central venous catheter (7 Fr), respectively. Connect the 18 G catheter on the left carotid artery to the medical monitor for hemodynamic monitoring. Connect the 7 Fr triple-lumen central venous catheter on the left jugular vein to the infusion pump and medical monitor for simultaneous intravenous fluid administration, drug injection, and central venous pressure monitoring. Make all connections using the three-way stopcocks. Withdraw venous or arterial blood samples via three-way stopcock.
NOTE: After catheter insertion, ligate the catheter and vessel with 2-0 surgical sutures. - Expose the right jugular artery and vein. Achieve systemic anticoagulation with a bolus of heparin (120 IU/kg) through the right jugular vein.
NOTE: The target activated clotting time (ACT) for cannulation is above 250s.
2. Cannulation
- V-A ECMO circuit establishment: Insert an arterial catheter (18 Fr) through the right carotid artery to a depth of 10-15 cm and a venous catheter (24 Fr) through the right external jugular vein to the right atrium.
- V-V ECMO circuit establishment: Expose the right external jugular vein and insert a dual-lumen catheter (23 Fr) through the right external jugular vein.
NOTE: During this procedure, maintain the blood pressure and heart rate of sheep within ±20% of the baseline values. Keep the partial pressure of arterial carbon dioxide (PaCO2) between 35-40 mmHg. Ensure the partial pressure of end-tidal carbon dioxide (EtCO2) remains between 35-45 mmHg. Adjust the depth of anesthesia when encountering variations in blood pressure and heart rate in sheep. If this proves ineffective or in emergencies, consider using vasoactive drugs. Maintain PaCO2 and EtCO2 within normal values by adjusting ventilator parameters, with a primary focus on tidal volume and respiratory rate. - In V-V ECMO, pass the catheter tip through the right atrium (RA) and position it within the inferior vena cava (IVC). Direct the outflow port of the dual-lumen catheter towards the tricuspid valve (confirm position with ultrasound assistance).
NOTE: After catheter insertion, ligate the catheter and vessel with 2-0 surgical sutures. Close the skin incision using 4-0 surgical sutures.
3. Initiation of ECMO
- Connect all ECMO devices following the manufacturer's instructions (see Table of Materials). Ensure there are no leaks.
NOTE: Follow aseptic principles during the connection. - The priming solution consists of normal saline (1000 mL) with 2000 IU of heparin. After manually infusing the priming solution into the ECMO circuit and ensuring no air bubbles in the circuit, start the centrifugal pump for priming (1000-1500 rpm).
- After priming is complete, turn off the centrifugal pump. Connect the inflow catheter to the inlet of the centrifugal pump and connect the outflow catheter to the outlet of the oxygenator. Make all connections using the three-way stopcocks. Exhaust the air at the connection through the three-way stopcock. Attach the oxygen source to the ECMO system, ensuring correct oxygen flow. Then start the centrifugal pump for ECMO run. Set the initial pump flow at 2.0 L/min with a pump speed of 3000 rpm.
- Half-loop the ECMO circuit tube line around the sheep's neck to prevent displacement or kinking.
4. Postoperative management and monitoring
- Transfer the sheep to a metabolic cage and restrain the sheep appropriately after completing the operation.
NOTE: Secure the head and shoulders of the sheep with particular emphasis on preventing displacement or kinking of the cannula. Gradually reduce the depth of anesthesia. Ensure personal protection for the members of the postoperative nursing team by wearing protective gear (sterile clothes, gloves, masks, and caps). - When ensuring stable respiration and blood gas analysis results, remove the endotracheal tube.
- In the initial 24 h post-surgery, administer flurbiprofen (1-2 mg/kg) and dexmedetomidine (0.2-0.3 µg/kg·h) intravenously for sedation and pain relief.
NOTE: After the first 24 h, consider sedative and analgesic drugs if there is agitation and blood pressure fluctuation in sheep due to postoperative pain. - Inside the monitoring cage, ensure that the sheep move freely within a certain range and have unrestricted access to a suitable amount of feed and water.
- Keep a continuous real-time check on fundamental vital signs (heart rate and arterial blood pressure) along with ECMO hydraulic parameters (pump flow, pump speed, pre-pump pressure, post-pump pressure, and post-oxygenator pressure).
NOTE: Set target parameters for ECMO management (pump flow: 2.0-2.5 L/min, pump speed: 3000-3500 rpm, oxygen flow rate: 1.0-1.5 L/min with an FiO2 of 50%-80%). Adjust the above parameters according to blood gas results. - Measure blood gases and ACT every 6 h and monitor blood count, blood chemistry, and coagulation tests daily. Adjust anticoagulation strategy based on coagulation indicators.
NOTE: The target ACT goal: 220-250 s. - Adjust intravenous infusions based on fluid balance to maintain central venous pressure (CVP) between 5-12 cm H2O. Administer cefuroxime sodium (1.5 g, i.v., b.i.d.) daily for infection prevention. Conduct daily incision disinfection and closely monitor for signs of infection or bleeding.
5. Euthanasia
- After a 7-day experimental period, remove the ECMO circuit.
- Administer an intravenous injection of potassium chloride (100 mg/kg) for euthanasia under sedation with propofol (20 mg/kg).
NOTE: After euthanasia, the main organs (heart, kidney, lung, liver, brain, and intestine) were collected and visually checked for the presence of infarcts, hemorrhages, or overt damage. All organ injuries were recorded in detail. Then, the organs were cut into small pieces and fixed in 4% formaldehyde, embedded with paraffin, and divided into 4 µm sections for hematoxylin-eosin (HE) staining13. Conduct Histological examination for HE sections under a light microscope by at least two pathologists independently.
Results
A total of ten sheep were evaluated during the entire experiment, with five sheep in each group (Table 1). Following the initiation of ECMO, all sheep recovered from anesthesia and remained awake for 7 days. In both groups, ECMO flow exceeded 1.8 L/min. In the V-V ECMO group, the flow fluctuated around 1.8 L/min, while in the V-A ECMO group, it ranged from 2.3 L/min to 1.8 L/min (Figure 1A). The vital signs of each sheep remained stable. The blood lactic acid level was below 1 mmol/L between 60 h and 180 h after awakening in both groups (Figure 1B). Throughout the 7 days, the ACT level was aimed to maintain around 220-250 s, and the actual ACT fluctuated around 250 s (Figure 1C), with the dose of heparin gradually increasing, reaching almost 60 IU/kg/min at the end of the experiments (Figure 1D).
The prothrombin time (PT) for both groups ranged from 15 s to 32 s (Figure 2A). The activated partial thromboplastin time (APTT) and thrombin time (TT) in the V-V ECMO group were lower than those in the V-A ECMO group (statistically significant difference for APTT) at certain time points throughout the experiment (Figure 2B,C). The international normalized ratio in each group fluctuated around 1.5 (Figure 2D). Fibrinogen, fibrin degradation products, D-dimers, and platelets fluctuated similarly between the two groups and remained within the normal range (Figure 3).
The blood levels of creatinine and blood urea nitrogen in both groups remained within normal limits throughout the experiment, with no statistical difference between the two groups (Figure 4). Furthermore, it was observed that the fluid balance in both groups demonstrated minimal fluctuations around 0, and the CVP measurements of both groups remained within the normal range throughout the entire study (Figure 5). At the end of the experiment, sheep in both groups exhibited infarct foci in the kidneys, even though laboratory results fluctuated within normal and reasonable ranges (Figure 6 and Table 1).

Figure 1: Basic parameter monitoring. (A) ECMO flow. (B) Lac. (C) ACT. (D) Heparin dose. Lac, Lactic acid; ACT, activated clotting time; V-V ECMO, veno-venous extracorporeal membrane oxygenation; V-A ECMO, veno-arterial extracorporeal membrane oxygenation. Values: mean ± SEM. Please click here to view a larger version of this figure.

Figure 2: Results of coagulation function. (A) PT. (B) APTT. (C) TT. (D) INR. PT, prothrombin time; APTT, activated partial thromboplastin time; TT, thrombin time; INR, international normalized ratio; V-V ECMO, veno-venous extracorporeal membrane oxygenation; V-A ECMO, veno-arterial extracorporeal membrane oxygenation. An unpaired t-test was performed to compare the two groups at each time point. Values: mean ± SEM; *P < 0.05; P > 0.05 was not displayed. Please click here to view a larger version of this figure.
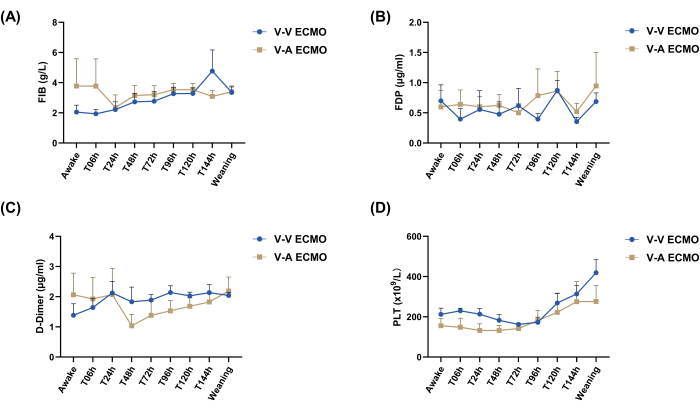
Figure 3: Fibrinogen, fibrin degradation products, D-Dimers, and platelet count in both groups. (A) FIB. (B) FDP. (C) D-Dimer. (D) PLT. FIB, fibrinogen; FDP, fibrin degradation products; PLT, platelet count; V-V ECMO, veno-venous extracorporeal membrane oxygenation; V-A ECMO, veno-arterial extracorporeal membrane oxygenation. An unpaired t-test was performed to compare the two groups at each time point. Values: mean ± SEM; P > 0.05 was not displayed. Please click here to view a larger version of this figure.

Figure 4: Kidney function monitoring. (A) Creatinine. (B) BUN. V-V ECMO, veno-venous extracorporeal membrane oxygenation; V-A ECMO, veno-arterial extracorporeal membrane oxygenation; BUN, Blood urea nitrogen. An unpaired t-test was performed to compare the two groups at each time point. Values: mean ± SEM; P > 0.05 was not displayed. Please click here to view a larger version of this figure.
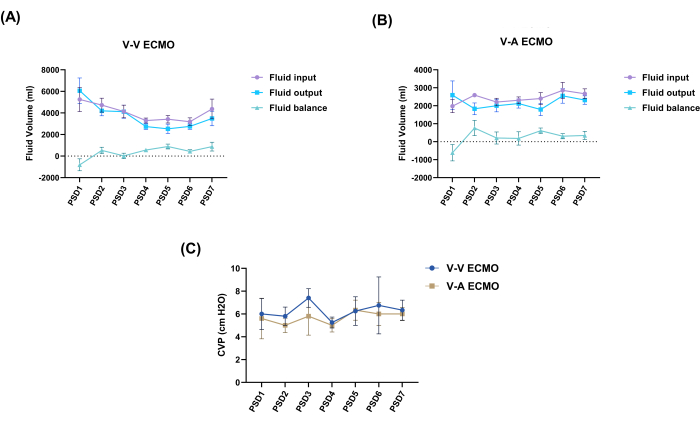
Figure 5: Dynamic changes in fluid balance and CVP values. (A) Daily fluid balance changes of V-V ECMO group. (B) Daily fluid balance changes of V-A ECMO group. (C) CVP monitoring. An unpaired t-test was performed to compare the CVP of the two groups at each time point. Values: mean ± SEM; P > 0.05 was not displayed. CVP, central venous pressure; V-V ECMO, veno-venous extracorporeal membrane oxygenation; V-A ECMO, veno-arterial extracorporeal membrane oxygenation; PSD, post-surgery day. Please click here to view a larger version of this figure.
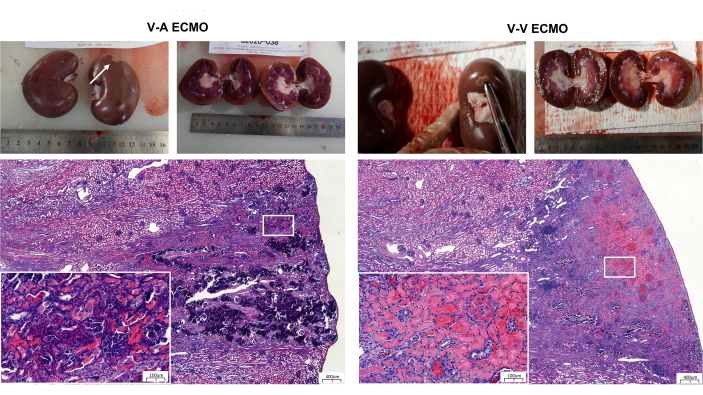
Figure 6: Renal anatomy and Hematoxylin-Eosin staining in both groups. Scale bars: 400 µm; insets: 100 µm. V-V ECMO, veno-venous extracorporeal membrane oxygenation; V-A ECMO, veno-arterial extracorporeal membrane oxygenation. Please click here to view a larger version of this figure.
| Sheep number | Sex | Weight (kg) | Renal pathological results* |
| VA1 | Male | 58 | None |
| VA2 | Male | 54 | None |
| VA3 | Male | 55 | Focal small infarction |
| VA4 | Male | 56 | Focal small infarction |
| VA5 | Male | 57 | Focal small infarction |
| VV1 | Male | 56 | None |
| VV2 | Male | 60 | None |
| VV3 | Male | 63 | None |
| VV4 | Male | 59 | None |
| VV5 | Male | 60 | Focal small infarction |
| *Renal pathological diagnosis was done by two independent pathologists to gain objective assessment. | |||
Table 1: Renal pathological results for the two groups.
Discussion
This study outlines the procedure for establishing robust, long-term survival models for V-V and V-A ECMO in sheep. All surviving animals exhibited stable vital signs, and no severe bleeding or coagulation events occurred. ECMO flow and oxygenation performance remained stable, with no major pathological injuries observed. The study provides detailed information on anticoagulation management.
Anticoagulation management plays a crucial role in ECMO perioperative care. Initially, based on previous studies, the target ACT was set at 180-220 s14,15,16,17. However, during the pre-experiment phase, fibrin deposition and thrombosis in the oxygenator (see Table of Materials) were observed, indicating that higher anticoagulation levels were necessary for the sheep. Despite the absence of significant bleeding signs and relatively stable platelet counts, the target ACT was adjusted to 220-250 s. This adjustment in anticoagulation targets highlights hematological differences between sheep and humans. Following this adjustment, fibrin deposition and thrombus formation in the oxygenator were reduced, leading to stable oxygenator performance.
Throughout the entire experiment, a trend was observed in the platelet counts of the sheep, characterized by an initial decrease followed by an increase. Remarkably, without receiving whole blood or platelet transfusions, the platelet counts recovered and surpassed the baseline level by the 7th day. The initial decline in platelet count in healthy sheep subjected to continuous heparin infusion may be attributed to heparin-induced thrombocytopenia18. The subsequent increase can be elucidated by compensatory mechanisms within the coagulation and hematopoietic systems of sheep.
Furthermore, APTT and TT values were notably higher in V-A ECMO compared to V-V ECMO when administering the same doses of heparin to healthy sheep. This observation suggests that V-A ECMO is more responsive to coagulation parameters following heparin anticoagulation. In a previous cohort study, it was noted that patients receiving V-A ECMO support required lower doses of heparin compared to those receiving V-V ECMO support to achieve similar APTT levels. Additionally, V-A ECMO patients exhibited an elevated consumption of clotting factors19. These findings collectively indicate the need for distinct considerations in coagulation management between these two ECMO modalities.
While almost all laboratory test results fluctuated within normal and reasonable ranges, infarct foci were identified in the kidneys of both groups at the experiment's conclusion. This highlights that perfusion and coagulation of vital organs remain the focal points of ECMO management, emphasizing that laboratory tests alone cannot be relied upon to assess organ status.
During the management of this model, the dose of heparin increased gradually, indicating dynamic anticoagulation for long-term ECMO management. However, the ECMO anticoagulation strategy employed for normal sheep may not be directly applicable to the clinical scenarios of critically ill patients. The complexity of critical illness and its inflammatory response can further disrupt patient hemostasis, particularly in cases of acquired hypercoagulability and hyperinflammation, such as observed in COVID-19 patients7,20. The anticoagulation strategy for ECMO in diverse clinical situations remains a crucial clinical consideration. Future detailed clinical trials are necessary to investigate ECMO anticoagulation strategies for patients with varying preoperative baseline clotting statuses and those undergoing different clinical situations (V-V and V-A models).
Several limitations persisted in this study. Firstly, a sham group was not included. Secondly, to minimize blood consumption, dynamic monitoring of inflammation markers was not conducted. Additionally, this study did not establish a disease animal model related to ECMO, and the ECMO postoperative management described in this article may differ from clinical practice.
In conclusion, the long-term awake sheep ECMO model with target ACT values between 220-250 s for anticoagulation management is shown to be feasible and stable. Besides closely monitoring coagulation function, the perfusion function of vital organs remains a focal point in ECMO management. Further experiments in the future are necessary to delve into suitable anticoagulation management strategies in disease animal ECMO models.
Disclosures
The authors have nothing to disclose.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| ACT analyzer | Hemochron, USA | Jr Signature | |
| Anaesthesia machine | Drager, Germany | Primus | |
| Arterial catheter | Edwards Lifescience, USA | 18-Fr | Provide return access into an artery for VA-EMCO |
| Blood chemistry analyzer | IDEXX Laboratories, USA | Catalyst One | |
| Blood gas analyzer | Abbott, USA | Abbott i-STAT1 | |
| Centrifugal pump | Jiangsu STMed Technologies, China | STM CP-24 I | |
| Centrifugal pump drive and console | Jiangsu STMed Technologies, China | OASSIST STM001 | |
| Coagulation test analyzer | Beijing Succeeder Technology, China | SF-8050 | |
| Complete blood count analyzer | Siemens Healthcare, Germany | ADVIA 2120i | |
| Dual-channel micro-injection pump | Zhejiang Smith Medical Instrument, China | WZS-50F6 | |
| Dual-lumen catheter | MAQUET Avalon Elite, Germany | 23-Fr | Provide return and drainage accesses into the right external jugular vein for VV-ECMO |
| Flurbiprofen | Beijing Tide Pharmaceutical Co., Ltd., China | 5ml: 50mg | |
| GraphPad software | GraphPad Software, USA | GraphPad Prism v9.0 | Statistical analysis |
| Heparin | Shanghai Shangyao No.1 Biochemical Pharmaceutical Co., Ltd., China | 2ml: 12500IU | |
| High-frequency electrosurgical | COVIDIEN, USA | Force F | |
| Multi-parameter medical monitor | Philips, Netherlands | MP60 | |
| Oxygenator kit | Medos, Germany | Hilite 7000LT | |
| Oxygenator kit | Maquet, Germany | BE-PLS 2050 | |
| Propofol | Xi’an Libang Pharmaceutical Co. Ltd, China | 20ml: 0.2g | |
| Single-lumen central venous catheter | TuoRen, China | 18Fr | Insert in left carotid artery for hemodynamic monitoring and blood sampling. |
| Small Tail Han sheep | Jinyutongfeng Commercial and Trade Co. Ltd, China | weight: 50-65 kg, age: 12-24 months | |
| Triple-lumen central venous catheter | TuoRen, China | 7Fr | Insert in left jugular vein for intravenous fluid administration, drug injection, and blood sampling. |
| Ultrasound machine | GE, USA | E9 | |
| Venous catheter | Edwards Lifescience, USA | 24-Fr | Provide the drainage access into a vein for VA-ECMO |
| Ventilator | Drager, Germany | Savina |
References
- Descamps, R., et al. Anti-Xa activity and hemorrhagic events under extracorporeal membrane oxygenation (ECMO): A multicenter cohort study. Crit Care. 25 (1), 127 (2021).
- Keller, S. P. Contemporary approaches in the use of extracorporeal membrane oxygenation to support patients waiting for lung transplantation. Ann Cardiothorac Surg. 9 (1), 29-41 (2020).
- Smith, M., et al. Duration of veno-arterial extracorporeal life support (VA ECMO) and outcome: An analysis of the Extracorporeal Life Support Organization (ELSO) registry. Crit Care. 21 (1), 45 (2017).
- Yang, L., et al. Risk factors for bloodstream infection (BSI) in patients with severe acute respiratory distress syndrome (ARDS) supported by veno-venous extracorporeal membrane oxygenation (VV-ECMO). BMC Pulm Med. 22 (1), 370 (2022).
- Arachchillage, D. J., et al. Impact of major bleeding and thrombosis on 180-day survival in patients with severe COVID-19 supported with veno-venous extracorporeal membrane oxygenation in the United Kingdom: A multicentre observational study. Br J Haematol. 196 (3), 566-576 (2022).
- King, C. S., Roy, A., Ryan, L., Singh, R. Cardiac support: Emphasis on venoarterial ECMO. Crit Care Clin. 33 (4), 777-794 (2017).
- Rajsic, S., et al. Anticoagulation Strategies during extracorporeal membrane oxygenation: A narrative review. J Clin Med. 11 (17), 5147 (2022).
- Rajsic, S., et al. The Role of Excessive anticoagulation and missing hyperinflammation in ECMO-associated bleeding. J Clin Med. 11 (9), 2314 (2022).
- Djordjevic, I., et al. Fluid management in veno-arterial extracorporeal membrane oxygenation therapy-analysis of an experimental pig model. J Clin Med. 12 (16), 5330 (2023).
- Passmore, M. R., et al. Evidence of altered haemostasis in an ovine model of venovenous extracorporeal membrane oxygenation support. Crit Care. 21 (1), 191 (2017).
- Heinsar, S., et al. Heart failure supported by veno-arterial extracorporeal membrane oxygenation (ECMO): a systematic review of pre-clinical models. Intensive Care Med Exp. 8 (1), 16 (2020).
- MohanKumar, S. M., et al. Effects of prenatal bisphenol-A exposure and postnatal overfeeding on cardiovascular function in female sheep. J Dev Orig Health Dis. 8 (1), 65-74 (2017).
- Zou, Z., et al. Naturally-occurring spinosyn A and its derivatives function as argininosuccinate synthase activator and tumor inhibitor. Nat Commun. 12 (1), 2263 (2021).
- Cianchi, G., Lazzeri, C., Bonizzoli, M., Batacchi, S., Peris, A. Echo-guided insertion of a dual-lumen cannula for venovenous extracorporeal membrane oxygenation. ASAIO J. 65 (4), 414-416 (2019).
- Conway, R. G., et al. Evaluation of an autoregulatory ECMO system for total respiratory support in an acute ovine model. Artif Organs. 44 (5), 478-487 (2020).
- McDonald, C. I., et al. The impact of acute lung injury, ECMO and transfusion on oxidative stress and plasma selenium levels in an ovine model. J Trace Elem Med Biol. 30, 4-10 (2015).
- Zhou, X., et al. Long-term support with an ambulatory percutaneous paracorporeal artificial lung. J Heart Lung Transplant. 31 (6), 648-654 (2012).
- Brodard, J., et al. COVID-19 patients often show high-titer non-platelet-activating anti-PF4/heparin IgG antibodies. J Thromb Haemost. 19 (5), 1294-1298 (2021).
- Cartwright, B., et al. Hemostasis, coagulation and thrombin in venoarterial and venovenous extracorporeal membrane oxygenation: the HECTIC study. Sci Rep. 11 (1), 7975 (2021).
- Lim, M. S., McRae, S. COVID-19 and immunothrombosis: Pathophysiology and therapeutic implications. Crit Rev Oncol Hematol. 168, 103529 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved