Method Article
Retinal and Choroidal Thickness Changes in Populations with Helicobacter pylori Infection by Swept-Source Optical Coherence Tomography
* These authors contributed equally
In This Article
Summary
Here, we compared macular retinal and choroidal thickness in populations with and without Helicobacter pylori infection using a swept-source optical coherence tomography (SS-OCT) device, which is one of the recent milestones in the development of retinal and choroidal visualization.
Abstract
Around half of the world's population is infected with Helicobacter pylori (H. pylori), which is closely related to several ocular diseases. The study aims to evaluate the retinal and choroidal thickness changes in subjects with H. pylori infection by swept-source optical coherence tomography (SS-OCT). The ophthalmic examination and 13C-urea breath test (13C-UBT) were performed on all subjects participating in the cross-sectional study. The participants were divided into H. pylori (+) and H. pylori (−) groups depending on the 13C-UBT results. This study covered 2574 right eyes from 2574 subjects with H. pylori infection and 2574 right eyes from 2574 age- and sex-matched individuals without H. pylori infection. Out of the nine sectors of the early treatment diabetic retinopathy study (ETDRS) grid, the maximum retinal thickness was in the inner superior sector, while the minimum was in the center sector. The maximum choroidal thickness was in the inner superior sector, while the minimum was in the outer nasal sector. The choroid of each area of the ETDRS subfield in the H. pylori (+) group was significantly thicker than that in the H. pylori (−) group, but retinal thickness did not show any difference between the two groups. Increased choroidal thickness may be an early indicator of H. pylori-associated retinal or choroidal diseases.
Introduction
Helicobacter pylori (H. pylori) is a gram-negative bacterium that settles on the gastric epithelium surface and infects approximately 50% of the world's population1,2. The incidence of H. pylori infection varies from region to region, and infections are more common in societies with low socioeconomic status3. H. pylori infections are typically acquired during childhood, and a significant proportion of individuals infected with H. pylori do not exhibit any symptoms4. If left untreated, H. pylori can colonize throughout an individual's lifetime.
The main manifestations of H. pylori infection are chronic gastritis, peptic ulcer, gastric cancer, and MALT lymphoma2,5,6,7. Currently, the role of H. pylori has been shown in the pathogenesis of extra gastric pathologies, such as atherosclerosis, idiopathic thrombocytopenic purpura, urticaria, obesity, and iron-deficiency anemia8,9,10,11,12. Some studies have also demonstrated that H. pylori is associated with several eye diseases, including central serous chorioretinopathy13, blepharitis14, glaucoma15, and uveitis16.
As the tissue with the highest blood flow, the choroid provides oxygen and nutrition to the retina while removing metabolic waste17,18,19. Changes in retinal and choroidal thickness may occur due to systemic diseases18 and physiological conditions20 and may also contribute to ocular pathologies such as central serous chorioretinopathy21, diabetic retinopathy22, age-related macular degeneration23, uveitis24, glaucoma25, and myopia-related chorioretinal atrophy26. Thus, accurate measurements of retinal and choroidal thickness are of great significance for monitoring the onset and progression of the diseases. Swept-source optical coherence tomography (SS-OCT), a recent advancement in retinal and choroidal visualization, offers a noninvasive means of obtaining more precise images of the retina and choroid.
Currently, there have been limited studies examining the relationship between H. pylori infection and choroidal thickness, and the data in the literature are controversial19,27,28. Some authors have reported that choroidal thickness is related to H. pylori infection19, whereas others have not27,28. The present study aims to evaluate the associations between H. pylori infection and the thickness of the retina and choroid.
Protocol
This cross-sectional study was conducted at Huashan Hospital of Fudan University between September 2018 and April 2019. It was approved by the Institutional Review Board of Huashan Hospital, affiliated with Fudan University (No. KY2016-274). Informed consent was obtained from all participants prior to the examinations.
1. Inclusion criteria
- Collect all subjects' general conditions, including age, sex, and a history of systemic diseases (thyroid diseases, diabetes mellitus, hypertension, dyslipidemia). Inclusion criteria are as follows:
- Include participants aged 18-70 years without a history of systemic diseases noted in step 1.1.
- Collect all subjects' ocular conditions, including best-corrected visual acuity (BCVA), a history of eye diseases (retinal and choroidal diseases), and a history of ocular trauma or surgery. Inclusion criteria are as follows:
- Include participants with BCVA ≥ 20/25 Snellen.
- Include participants without a history of eye diseases.
- Include participants without a history of ocular trauma or surgery.
2. 13C-urea breath test
NOTE: Among the several noninvasive tests for the diagnosis of H. pylori infection, including stool antigen testing and serological testing, 13C-urea breath test (13C-UBT) is considered to be the gold standard with high sensitivity and specificity1,29.
- After an overnight fast, ask the participant to blow as indicated in the test kit. Collect a baseline breath sample.
- Have the subject take 75 mg of the 13C-urea substrate in a citric acid base at the 13C-UBT meal. Ask the participant again to blow 30 min after urea ingestion. Collect the breath sample.
- NOTE: This test is based on the urease produced by H. pylori present in the stomach, liberating carbon dioxide (CO2) from urea. The uptake of labeled urea leads to the generation of labeled CO2, which can subsequently be quantified in exhaled breath.
- Calculate the result expressed as the difference value between the two breath samples. Consider the test positive for H. pylori infection when the delta over baseline value was >3.5%.
NOTE: False-negative results can occur in subjects taking proton pump inhibitors (PPIs) or antibiotics that interfere with the sensitivity of 13C-UBT30. To avoid a false-negative result, PPIs and antibiotics should be stopped for at least 3 months before 13C-UBT. - Based on the 13C-UBT results, divide the participants into two groups: The H. pylori (+) group refers to those with H. pylori infection, and the H. pylori (−) group refers to those without H. pylori infection.
3. Swept-source optical coherence tomography imaging
- Open up the Power button of the SS-OCT device. Click the Radial Dia.6.0 mm Macula Overlap 4 button.
- Capture OCT images for every eye by scanning 100,000 A-scans/s between 8 a.m. and 10 a.m.
NOTE: The retinal and choroidal thickness were defined, respectively, referring to previous articles (Figure 1)31,32. - Generate thickness images of SS-OCT automatically based on the standard early treatment diabetic retinopathy study (ETDRS) subfield (Figure 2).
- Manually check each segmented layer line for every image to avoid automatic measurement errors31,32.
- Exclude the unclear OCT images due to unstable fixation or media opacity.
4. Statistical analysis
- Click on the statistical analysis software. Import the data into the software.
NOTE: SPSS was used for statistical analysis. Continuous data was summarized using the mean ± standard deviation, while categorical data was presented as frequency (percentage). - Compare continuous variables via the t-test, whereas categorical variables by the chi-square test. Define statistical significance as a p-value less than 0.05.
NOTE: The analysis included only the right eyes, which were considered as a separate study unit.
Results
A total of 2574 right eyes of 2574 subjects who tested positive for H. pylori and 2574 right eyes of 2574 individuals who tested negative for H. pylori were evaluated. Table 1 lists the baseline characteristics of the participants. The male/female ratio was 1334/1240 in both the H. pylori (+) group and the H. pylori (−) group. The mean age was 42.68 ± 10.76 years (range, 18-70 years) in both groups.
Out of the nine sectors of the ETDRS grid, the maximum retinal thickness was in the inner superior sector, while the minimum was in the center sector. The retinal thickness of each sector of the ETDRS grid showed no significant difference between the H. pylori (+) and H. pylori (−) groups (Table 2).
Out of the nine sectors of the ETDRS grid, the maximum choroidal thickness was in the inner superior sector, while the minimum was in the outer nasal sector. The choroid in each sector of the ETDRS grid in the H. pylori (+) group was significantly thicker than that in the H. pylori (−) group (Table 3).

Figure 1: Representative images of retinal and choroidal thickness. (A) Retinal thickness: between two green lines. (B) Choroidal thickness: between two green lines. Please click here to view a larger version of this figure.
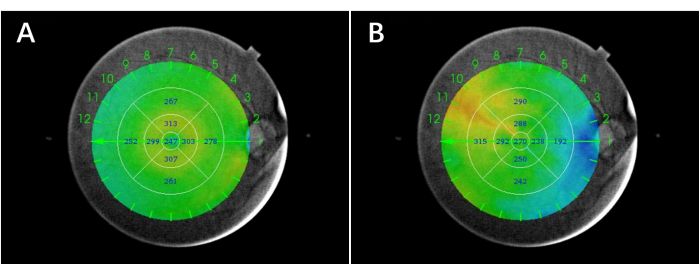
Figure 2: Representative images of the retinal and choroidal thickness in nine sectors of the ETDRS grid. (A) Retinal thickness. (B) Choroidal thickness. Please click here to view a larger version of this figure.
| Parameter | H. pylori (+) group | H. pylori (−) group | p-value |
| Patient, n | 2574 | 2574 | — |
| Eye, n | 2574 | 2574 | — |
| Gender, n (%) | 1.000a | ||
| Male | 1334 (51.8%) | 1334 (51.8%) | |
| Female | 1240 (48.2%) | 1240 (48.2%) | |
| Age, year | 42.68 ± 10.76 | 42.68 ± 10.76 | 1.000b |
| Range | 18 – 70 | 18 – 70 | |
| aChi-square test; bT-test. | |||
Table 1: Demographic characteristics
| Retinal thickness | H. pylori (+) group | H. pylori (−) group | p-value |
| n = 2574 | n = 2574 | ||
| Center, μm | 229.57 ± 19.82 | 230.55 ± 19.36 | 0. 070b |
| Inner superior, μm | 309.43 ± 18.83 | 310.25 ± 17.16 | 0. 102b |
| Inner nasal, μm | 306.14 ± 17.35 | 306.64 ± 17.14 | 0. 300b |
| Inner inferior, μm | 307.72 ± 17.45 | 308.42 ± 16.68 | 0. 146b |
| Inner temporal, μm | 299.20 ± 16.72 | 299.54 ± 16.19 | 0. 455b |
| Outer superior, μm | 277.71 ± 16.99 | 277.37 ± 16.43 | 0. 476b |
| Outer nasal, μm | 292.23 ± 15.97 | 291.71 ± 16.26 | 0. 250b |
| Outer inferior, μm | 262.14 ± 15.53 | 261.37 ± 15.97 | 0. 079b |
| Outer temporal, μm | 258.17 ± 16.12 | 257.35 ± 15.79 | 0. 066b |
| bT-test |
Table 2: Retinal thickness
| Choroidal thickness | H. pylori (+) group | H. pylori (−) group | p-value |
| n = 2574 | n = 2574 | ||
| Center, μm | 252.85 ± 72.24 | 247.54 ± 73.48 | 0. 009b |
| Inner superior, μm | 256.22 ± 72.48 | 250.44 ± 73.27 | 0. 004b |
| Inner nasal, μm | 238.35 ± 73.57 | 232.73 ± 74.37 | 0. 006b |
| Inner inferior, μm | 254.21 ± 75.84 | 249.15 ± 76.25 | 0. 017b |
| Inner temporal, μm | 250.02 ± 68.00 | 245.27 ± 67.89 | 0. 012b |
| Outer superior, μm | 248.99 ± 66.92 | 244.83 ± 66.43 | 0. 025b |
| Outer nasal, μm | 200.39 ± 72.01 | 195.36 ± 73.28 | 0. 013b |
| Outer inferior, μm | 241.87 ± 70.40 | 237.92 ± 70.76 | 0. 045b |
| Outer temporal, μm | 239.09 ± 62.55 | 235.59 ± 62.81 | 0. 045b |
| bT-test |
Table 3: Choroidal thickness
Discussion
H. pylori infection has been related to many extra gastric disorders, such as neurological, dermatological, cardiovascular, and ocular diseases33. However, the pathogenesis of H. pylori-associated ocular diseases remains elusive. We used SS-OCT to measure macular retinal and choroidal thickness in subjects with and without H. pylori infection. The results showed that the choroidal thickness was significantly increased in subjects with H. pylori infection when compared to the choroidal thickness of age- and sex-matched control subjects, whereas no difference was observed in retinal thickness between the two groups. This may suggest a correlation between H. pylori infection and choroidal thickness. Choroid may serve as a link connecting H. pylori infection and eye diseases.
There have been limited studies on the relationship between H. pylori infection and choroidal thickness. Can et al.19 compared the choroidal thickness of 25 subjects with H. pylori infection and 25 subjects without H. pylori infection in Turkey using enhanced depth imaging (EDI)-OCT instead of SS-OCT and identified H. pylori infection as a significant factor for increased choroidal thickness. Similar to this research, in our study, we observed a higher choroidal thickness in subjects with H. pylori infection. In contrast, two other studies conducted in Turkey evaluated the effect of H. pylori infection on choroidal thickness with EDI-OCT, and researchers discovered no significant difference in choroidal thickness between the subjects with and without H. pylori infection27,28. EDI-OCT may have several inherent limitations that can result in these conflicting observations. Firstly, it may not accurately detect the chorioscleral interface (CSI) in some eyes with thicker choroids due to its low penetration through the retinal pigment epithelium (RPE). In comparison with EDI-OCT, SS-OCT can detect the CSI in 100% of eyes34. Secondly, in most studies using EDI-OCT, the choroid is manually measured at only one or a few points, which can be influenced by focal thickening or thinning of the choroid, as the CSI may have an irregular shape in some eyes35,36. Thirdly, manual measurement by different subjects can lead to variations in choroidal thickness. SS-OCT has the capacity to surmount these constraints37. In this investigation, the retinal and choroidal thickness was acquired utilizing SS-OCT and subsequently averaged in accordance with the ETDRS grid through automated means, thereby ensuring confirmed reliability.
A second possible factor for the varying results can be ethnic differences among the study populations. The East Asian variant of the CagA protein exhibits greater carcinogenicity and virulence in comparison to its Western counterpart38. As a major virulence determinant, H. pylori cag PAI is associated with gastric mucosal inflammatory cell infiltration and interleukin (IL)-8 secretion38. Possibly, this explains why H. pylori-positive and H. pylori-negative participants in our study had significantly different choroidal thicknesses.
This study showed that the choroid in the H. pylori (+) group was thicker than that in the H. pylori (−) group. In the study of White et al.39, H. pylori was shown to interact with pattern recognition receptors expressed by gastric epithelial cells and thus activated inflammation. Proinflammatory cytokines, including tumor necrosis factor α, interferon γ, IL-8, IL-1β, IL-6, IL-12, CCL2-5, CCL20, and CXCL1-3, are upregulated in the infected gastric mucosa of H. pylori-infected patients. These cytokines lead to the recruitment of inflammatory cells, such as neutrophils and macrophages, and increase endothelial permeability. Moreover, virulence factors, such as lipopolysaccharide, immunoglobulin-G antibody, and cross-reactivity of anti-CagA antibodies in response to autoimmunity, may lead to endothelial dysfunction9,40. Increased choroidal endothelial permeability caused by inflammatory cytokines and endothelial dysfunction by direct virulence attack leads to the thickening of the choroid.
The blood-retina-barrier formed by the retinal capillary endothelium and RPE through well-developed tight junction proteins can prevent harmful substances from entering the eye and maintain the physiological environment for a functional retina, which may explain why the retinal thickness is not affected by H. pylori infection.
There are several limitations to the present study. First, the research is a cross-sectional study. It is not possible to observe changes in the retina and choroid during the disease or after treatment. We are currently conducting a longitudinal study with an extended cohort. Second, participants with subclinical diseases that may affect the retina and choroid were included despite all subjects being assessed in detail. Third, the findings of this study may not be generalizable to other populations, as the participants enrolled in this research are all from China.
In summary, the choroid was thicker in subjects with H. pylori infection compared with normal individuals. Increased choroidal thickness may be an early indicator of H. pylori-associated retinal or choroidal diseases.
Disclosures
None of the authors has a financial or proprietary interest in any material or method mentioned.
Acknowledgements
This study was funded by grants from the National Natural Science Foundation of China (No. 81900879) and the Science and Technology Commission of Shanghai Municipality (No. 20Y11910800).
Materials
| Name | Company | Catalog Number | Comments |
| 13C-urea breath test detector | Richen Force, Beijing, China | V505721 | |
| 13C-urea breath test kit | Richen Force, Beijing, China | H20061169 | |
| Ophthalmoscope | 66 Vision-Tech, Suzhou, China | V259204 | |
| Slit-lamp microscope | Topcon, Tokyo, Japan | 6822 | |
| SPSS software | IBM, Chicago, USA | ECS000143 | |
| Swept-source optical coherence tomography | Topcon, Tokyo, Japan | 185261 | |
| Visual chart | Yuejin, Shanghai, China | H24104 |
References
- Malfertheiner, P., et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 66 (1), 6-30 (2017).
- Suerbaum, S., Michetti, P. Helicobacter pylori infection. New England Journal of Medicine. 347 (15), 1175-1186 (2002).
- Malaty, H. M. Epidemiology of Helicobacter pylori infection. Best Practice & Research Clinical Gastroenterology. 21 (2), 205-214 (2007).
- Silva, J. M., et al. Validation of a rapid stool antigen test for diagnosis of Helicobacter pylori infection. Revista Do Instituto De Medicina Tropical De Sao Paulo. 52 (3), 125-128 (2010).
- Testerman, T. L., Morris, J. Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World Journal of Gastroenterology. 20 (36), 12781-12808 (2014).
- Wong, B. C., et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. Journal of the American Medical Association. 291 (2), 187-194 (2004).
- Biernat, M. M., et al. Antimicrobial susceptibility of Helicobacter pylori isolates from Lower Silesia, Poland. Archives of Medical Science. 10 (3), 505-509 (2014).
- Franceschi, F., et al. CagA antigen of Helicobacter pylori and coronary instability: insight from a clinico-pathological study and a meta-analysis of 4241 cases. Atherosclerosis. 202 (2), 535-542 (2009).
- De Bastiani, R., et al. High prevalence of Cag-A positive H. pylori strains in ischemic stroke: a primary care multicenter study. Helicobacter. 13 (4), 274-277 (2008).
- Huang, X., et al. Iron deficiency anaemia can be improved after eradication of Helicobacter pylori. Postgraduate Medical Journal. 86 (1015), 272-278 (2010).
- Franceschi, F., Gasbarrini, A. Helicobacter pylori and extragastric diseases. Best Practice & Research Clinical Gastroenterology. 21 (2), 325-334 (2007).
- Arslan, E., Atilgan, H., Yavasoglu, I. The prevalence of Helicobacter pylori in obese subjects. European Journal of Internal Medicine. 20 (7), 695-697 (2009).
- Casella, A. M., Berbel, R. F., Bressanim, G. L., Malaguido, M. R., Cardillo, J. A. Helicobacter pylori as a potential target for the treatment of central serous chorioretinopathy. Clinics. 67 (9), 1047-1052 (2012).
- Sacca, S. C., et al. Prevalence and treatment of Helicobacter pylori in patients with blepharitis. Investigative Ophthalmology & Visual Science. 47 (2), 501-508 (2006).
- Kountouras, J., et al. Relationship between Helicobacter pylori infection and glaucoma. Ophthalmology. 108 (3), 599-604 (2001).
- Sacca, S. C., Vagge, A., Pulliero, A., Izzotti, A. Helicobacter pylori infection and eye diseases: a systematic review. Medicine. 93 (28), 216 (2014).
- Nickla, D. L., Wallman, J. The multifunctional choroid. Progress in Retinal and Eye Research. 29 (2), 144-168 (2010).
- Tan, K. A., et al. State of science: Choroidal thickness and systemic health. Survey of Ophthalmology. 61 (5), 566-581 (2016).
- Can, M. E., et al. The association of Helicobacter pylori with choroidal and retinal nerve fiber layer thickness. International Ophthalmology. 38 (5), 1915-1922 (2018).
- Bafiq, R., et al. sex, and ethnic variations in inner and outer retinal and choroidal thickness on spectral-domain optical coherence tomography. American Journal of Ophthalmology. 160 (5), 1034-1043 (2015).
- Maruko, I., Iida, T., Sugano, Y., Ojima, A., Sekiryu, T. Subfoveal choroidal thickness in fellow eyes of patients with central serous chorioretinopathy. Retina. 31 (8), 1603-1608 (2011).
- Regatieri, C. V., Branchini, L., Carmody, J., Fujimoto, J. G., Duker, J. S. Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina. 32 (3), 563-568 (2012).
- Koizumi, H., Yamagishi, T., Yamazaki, T., Kawasaki, R., Kinoshita, S. Subfoveal choroidal thickness in typical age-related macular degeneration and polypoidal choroidal vasculopathy. Graefes Archive for Clinical and Experimental Ophthalmology. 249 (8), 1123-1128 (2011).
- Yan, H., Li, J., Zhang, J., Yang, L. Retinal and choroidal thickness in patients with uveitis. Ocular Immunology and Inflammation. 25 (2), 202-209 (2017).
- Maul, E. A., et al. Choroidal thickness measured by spectral domain optical coherence tomography: factors affecting thickness in glaucoma patients. Ophthalmology. 118 (8), 1571-1579 (2011).
- Fujiwara, T., Imamura, Y., Margolis, R., Slakter, J. S., Spaide, R. F. Enhanced depth imaging optical coherence tomography of the choroid in highly myopic eyes. American Journal of Ophthalmology. 148 (3), 445-450 (2009).
- Ucgul, A. C., et al. The evaluation of the effect of Helicobacter pylori infection on choroidal thickness. Turkish Journal of Gastroenterology. 29 (6), 636-641 (2018).
- Horozoglu, F., Sever, O., Celik, E., Mete, R., Sahin, E. Choroidal thickness of helicobacter-positive patients without central serous chorioretinopathy. Current Eye Research. 43 (2), 262-265 (2018).
- Graham, D. Y., Klein, P. D. Accurate diagnosis of Helicobacter pylori. 13C-urea breath test. Gastroenterology Clinics of North America. 29 (4), 885-893 (2000).
- Graham, D. Y., et al. Studies regarding the mechanism of false negative urea breath tests with proton pump inhibitors. American Journal of Gastroenterology. 98 (5), 1005-1009 (2003).
- Fang, D., et al. Retinal and choroidal thickness in relation to C-reactive protein on swept-source optical coherence tomography. Journal of Immunology Research. 2021, 6628224 (2021).
- Li, Q., et al. Relationships of rheumatoid factor with thickness of retina and choroid in subjects without ocular symptoms using swept-source optical coherence tomography. Journal of Immunology Research. 2021, 5547533 (2021).
- Gravina, A. G., et al. Helicobacter pylori and extragastric diseases: A review. World Journal of Gastroenterology. 24 (29), 3204-3221 (2018).
- Adhi, M., et al. Choroidal analysis in healthy eyes using swept-source optical coherence tomography compared to spectral domain optical coherence tomography. American Journal of Ophthalmology. 157 (6), 1272-1281 (2014).
- Yasuno, Y., Okamoto, F., Kawana, K., Yatagai, T., Oshika, T. Investigation of multifocal choroiditis with panuveitis by three-dimensional high-penetration optical coherence tomography. Journal of Biophotonics. 2 (6-7), 435-441 (2009).
- Chung, S. E., Kang, S. W., Lee, J. H., Kim, Y. T. Choroidal thickness in polypoidal choroidal vasculopathy and exudative age-related macular degeneration. Ophthalmology. 118 (5), 840-845 (2011).
- Zhang, L., et al. Validity of automated choroidal segmentation in SS-OCT and SD-OCT. Investigative Ophthalmology & Visual Science. 56 (5), 3202-3211 (2015).
- Yuan, X. Y., et al. Helicobacter pylori with East Asian-type cagPAI genes is more virulent than strains with Western-type in some cagPAI genes. Brazilian Journal of Microbiology. 48 (2), 218-224 (2017).
- White, J. R., Winter, J. A., Robinson, K. Differential inflammatory response to Helicobacter pylori infection: etiology and clinical outcomes. Journal of Inflammation Research. 8, 137-147 (2015).
- Franceschi, F., et al. Cross-reactivity of anti-CagA antibodies with vascular wall antigens: possible pathogenic link between Helicobacter pylori infection and atherosclerosis. Circulation. 106 (4), 430-434 (2002).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved