Method Article
Transbronchial Lung Cryobiopsy for Diagnosing Interstitial Lung Diseases and Peripheral Pulmonary Lesions - A Stepwise Approach
In This Article
Summary
Transbronchial lung cryobiopsy (TBLC) for diagnosing interstitial lung disease and peripheral pulmonary lesions is a high-yield diagnostic and safe procedure. We describe a stepwise approach to conduct TBLC for the different indications mentioned with a flexible bronchoscope, which might be helpful for novice bronchoscopists performing TBLC.
Abstract
Transbronchial lung cryobiopsy (TBLC) is an invasive procedure increasingly implemented during the last decade as an alternative to video-assisted thoracic surgery lung biopsy (SLB) for diagnosing interstitial lung diseases (ILDs). The indication for TBLC has primarily been to sub-classify a specific ILD subtype when this cannot be achieved on the basis of a preceding multidisciplinary team discussion. Although SLB is considered the gold standard for establishing a histological diagnosis, TBLC has been gradually suggested as the first-choice histological diagnostic modality in patients with unclassified ILDs due to a comparable diagnostic yield with SLB, but superior to SLB in terms of complications, including mortality. During recent years, radial endobronchial ultrasound (R-EBUS) and electromagnetic navigation bronchoscopy (ENB)-guided TBLC for peripheral pulmonary lesions have also been described as safe procedures, which may improve the diagnostic yield compared to forceps biopsies. Still, the diagnostic properties of TBLC rely on the quality of the procedure's performance. This article aims to describe the stepwise approach to conducting TBLC with a flexible bronchoscope for the different indications mentioned, which might be helpful for novice bronchoscopists performing TBLC.
Introduction
Interstitial lung diseases (ILDs) constitute a group of both acute and chronic lung diseases that affect one or more of all the lung parenchymal components forming the interstitium such as bronchi, alveoli, connective tissue, and blood- and lymphatic vessels. Despite being rare diseases, the more than 200 different subtypes of ILDs represent a heterogeneous disease category with different clinical, radiological, and cyto-histological characteristics. ILDs typically manifest as inflammation, fibrosis, or a combination of both, which are the underlying causes for the patients' usual perceived symptoms as dry cough, dyspnea on exertion, and fatigue1,2.
ILDs are categorized as idiopathic interstitial pneumonia (IIP), interstitial pneumonia of known etiology (e.g., connective tissue disease interstitial lung disease, drug-induced ILD, and work-related pneumoconiosis), granulomatous interstitial affection (e.g., sarcoidosis and hypersensitivity pneumonia), and orphan ILDs (e.g., multiple cystic lung diseases and eosinophilic pneumonia)1. This categorization and further diagnostic subtyping are fundamental to determining optimal treatment and follow-up, and allow prognostication. However, as the diagnostic puzzle may be challenging, interpretation of available clinical (including anamnesis, disposition, and potential exposures) and paraclinical information as chest high-resolution computed tomography (HRCT), lung physiology, and autoimmunology obtained on the basis of a multidisciplinary team discussion (MDD) is recommended3,4,5. If a confident MDD diagnosis is not obtainable6,7, histological sampling to increase the likelihood of a definite ILD subtype diagnosis is indicated by the use of transbronchial lung cryobiopsy (TBLC)8,9. In well-selected patients, TBLC is considered a safe invasive procedure with a diagnostic accuracy close to that of video-assisted thoracic surgery lung biopsy (SLB), which is still regarded as the histological gold standard for histological ILD diagnostics10,11,12,13,14. The TBLC procedure is performed as a systematic bronchoscopy, applying special cryoprobes for histological sampling and with recommended fluoroscopic guidance. It is recommended that TBLC is performed in tertiary ILD centers using an MDD setting and by interventional pulmonologists familiar with the management of TBLC complications, who have undergone training in a dedicated center with TBLC expertise9,10,11,15,16,17.
TBLC has also recently gained attention as a procedure to be combined with radial endobronchial ultrasound (R-EBUS) for ILD diagnostics18,19. Furthermore, TBLC has been combined with both R-EBUS and electromagnetic navigation bronchoscopy (ENB) for diagnosing peripheral pulmonary lesions (PPL) to improve the diagnostic yield when compared to conventional transbronchial forceps biopsies20,21. However, this relatively novel approach for PPL diagnostics has not yet been implemented as a standard procedure and thus, warrants further evidence in this specific area. The aim of the present report is to describe a stepwise approach to conducting TBLC with a flexible bronchoscope in a clinical setting for the indications mentioned.
Protocol
The authors come from two Danish TBLC centers (Odense University Hospital and Aarhus University Hospital) that both conduct research in accordance with the principles of the Declaration of Helsinki. Ethics approval was not necessary as the study was observational in nature. All patients included for research purposes gave written informed consent. It is important to emphasize that the described stepwise approach for TBLC conductance relates to the use of a flexible bronchoscope and is based on a combination of recommendations from international guidelines, expert statements, state-of-art reviews, and experiences from the two TBLC centers9,10,11,15,16,17,22,23,24,25.
1. PreTBLC considerations
- Ensure that TBLC is indicated, which is justified in patients in whom integration of information from HRCT, biochemistry, and autoimmunology in a preceding MDD involving pulmonologists and radiologists has not been able to establish a confident ILD diagnosis.
- Select appropriate patients by avoiding the contraindications described in Table 1.
| Relative contraindications | Absolute contraindications |
| Forced vital capacity (FVC) < 50% of predicted value | Thrombocytopenia < 50 x 109/L or INR > 1.5 |
| Diffusion capacity for carbon monoxide for the lung (DLCO) < 35% of predicted value | Uncorrected bleeding diathesis |
| Systolic pulmonary arterial pressure > 50 mmHg (e.g., estimation based on an echocardiography) | Progressive and clinical decline due to an increased risk of complications in patients with compromised pulmonary function |
| Body mass index > 35 kg/m2 |
Table 1: Contraindications for TBLC. Relative and absolute contraindications for TBLC conductance. Abbreviation: TBLC = transbronchial lung cryobiopsy.
2. PreTBLC preparation
- Review the HRCT and suggestions from the thoracic radiologist to plan from which bronchial segments (BS) histological sampling is best accessible according to radiological disease manifestation.
- Test that the system works prior to TBLC performance.
- Press the gas (carbon dioxide (CO2) or the nitrous oxide (NO)) tank capacity button on the settings panel to check the volume of the gas in the cylinder.
- Place the cryoprobe on a tray and observe the probe while pressing the pedal footswitch for 5-10 s. Look for an ice ball at the tip of the probe that will indicate it is functioning properly (Figure 1).
- Use general anesthesia (GA) or deep sedation under TBLC and consider premedication with tranexamic acid of 0.5-1 g to reduce the risk of bleeding.
- Place a special double luminal endotracheal tube (ETT) of 7.5-8.5 mm size in the trachea.
NOTE: The ETT has a main channel that allows access for the bronchoscope while the patient is ventilated and has a minor side channel that serves as a working channel for the bronchial blocker catheter.- Spray continuously with local anesthesia (e.g., lidocaine spray 10%) to reduce cough. See also step 3.5.
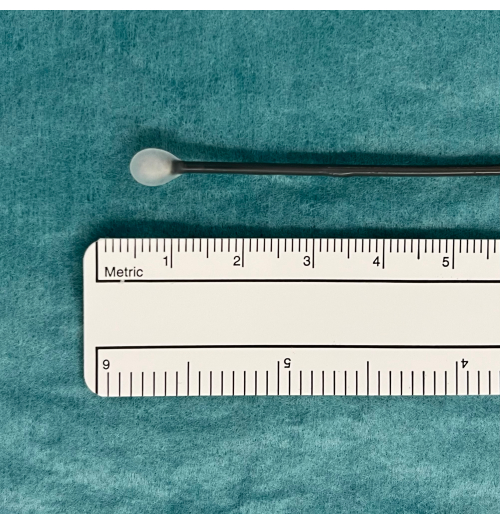
Figure 1: An ice ball as an indication of usable TBLC equipment. A pedal activates CO2 gas diffusion from the tank and induced freezing. This is tested in water where an ice ball will appear at the tip of the cryoprobe if functioning properly. Abbreviation: TBLC = transbronchial lung cryobiopsy. Please click here to view a larger version of this figure.
3. TBLC conductance
- Introduce a flexible bronchoscope through the ETT and perform the bronchoscopy procedure.
NOTE: A rigid bronchoscope is used in some TBLC centers to intubate the trachea. If the trachea is intubated by a rigid bronchoscope, a flexible bronchoscope can be passed through the rigid one.- Introduce the flexible cryoprobe through the working channel of the bronchoscope and into the selected BS.
NOTE: The cryoprobes occur as both single-use probes (1.1, 1.7, and 2.4 mm) and reusable probes (1.9 and 2.4 mm). - Use fluoroscopy to ensure that the placement of the tip of the cryoprobe is approximately 10 mm from the thorax wall corresponding to the selected BS (Figure 2).
- Introduce a bronchial blocker catheter (e.g., a Fogarty balloon) in the side channel of the double luminal ETT and place it at the selected BS ostium (Figure 3).
- Inflate the bronchial blocker catheter to evaluate the appropriateness of placement and blockage for potential bleeding occurring distally with respect to the balloon (Figure 4).
- Deflate the bronchial blocker catheter if the balloon is well placed.
- Secure the placement of the balloon by fixing the bronchial blocker catheter with a pean.
- Use small amounts of lidocaine spray or saline in the respective ETT and its side channel to reduce any friction experienced due to the introduction of the cryoprobe in the ETT and the balloon catheter in the ETT's side channel.
- When steps 3.1.1-3.1.3 are satisfactorily performed, step on the freezing pedal for 3-6 s, depending on the cryoprobe size, to exploit the Joule-Thompsons law to freeze the lung parenchymal tissue to approximately -45-79 °C for CO2 and -89 °C for NO.
- Retract the flexible bronchoscope containing the cryoprobe in one quick movement while holding the freezing pedal down to continue freezing and prevent the biopsy from falling off during the retraction.
- During the maneuver described in step 3.1.5, have a person other than the bronchoscopist keep the balloon inflated to block the biopsy site distal of the selected BS ostium to control for potential bleeding.
- Introduce the flexible cryoprobe through the working channel of the bronchoscope and into the selected BS.
- Continue step 3.1.6 until at least two biopsies from two BS from the same lobe are obtained.
- Place the biopsies in saline, and when all biopsies are obtained, fix them in formaldehyde (4%) (Figure 5).
- Send the biopsies for pathological examination prior to MDD.

Figure 2: Fluoroscopy. Fluoroscopy is used to ensure correct placement of the cryoprobe before freezing. The tip of the cryoprobe appears as the head of a drumstick (black arrowhead). Please click here to view a larger version of this figure.
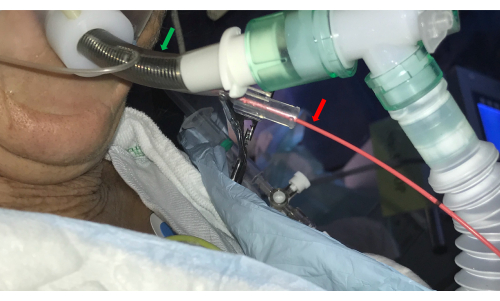
Figure 3: Endotracheal tube. A double-luminal endotracheal tube (green arrow) allows access to the airways by the bronchoscope and concurrently controls bleeding by introducing a balloon catheter in the side channel (red arrow). Please click here to view a larger version of this figure.

Figure 4: Inflation of the balloon catheter. Inflation of the balloon catheter to ensure blockage and prevent potential bleeding distal of the balloon distributing to other parts of the lobe after having performed a transbronchial lung cryobiopsy. Please click here to view a larger version of this figure.
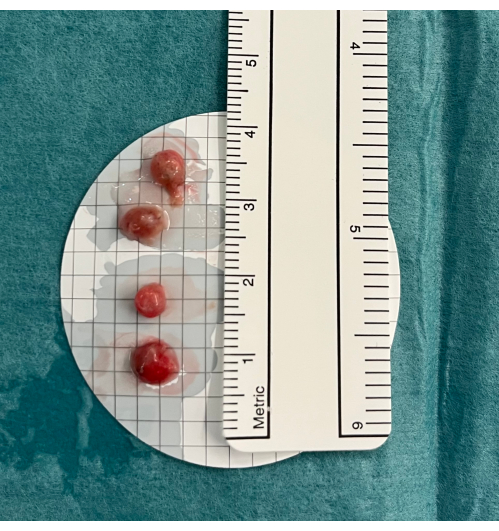
Figure 5: Biopsies. Transbronchial lung cryobiopsies are placed in cold saline before fixation in formaldehyde. Please click here to view a larger version of this figure.
4. PostTBLC procedures
- After each biopsy, reintroduce the bronchoscope into the BS and deflate the balloon to observe whether bleeding occurs.
- Reinflate the balloon if bleeding is observed (Figure 6). If the balloon is in the proper position and blocks the BS, wait for a few minutes for the bleeding to stop, and then, continue the TBLC procedure.
- If bleeding is still observed after step 4.1.1., install ice-cold saline distally to the balloon.
- In case of ongoing bleeding after step 4.1.2. or balloon failure where blood spills into other BSs, use a combination of suction, endobronchially administered ice-cold saline with or without adrenalin, and tranexamic acid.
- If the coagulated blood blocks the airways, use the cryoprobe to open up the airway again by freezing the tip of the cryoprobe into the blood clot and retracting it up through the ETT.
- If the bleeding remains uncontrolled, change the ETT to one that allows both ventilation of the non-biopsied lung and blockage of the main bronchus on the bleeding lung, and transfer the patient to an intensive care unit.
- Perform a focused lung ultrasound (FLUS) after TBLC, while the patient is still sedated to identify the indicatives of an iatrogenic pneumothorax (PTX).
- Consider insertion of a FLUS-guided pleural drain with a pigtail catheter (Fr 7-16) if FLUS observations indicate a high probability of PTX. Pleural drainage is indicated if FLUS indicates a rapidly increasing size of PTX, and especially if the clinical state of the patient is deteriorating.
- Postpone extubation by 5-10 min if FLUS reveals a small PTX and the patient is clinically stable. If the PTX size progresses hereafter or the patient becomes clinically unstable, insert a pleural drain prior to extubation.
- Extubate the patient after step 4.2 or step 4.2.2 if there is no indication of PTX or progress of the PTX size and the patient remains clinically stable.
- Observe the patient in a recovery room after TBLC. Just before the clinically stable patient goes home, ensure that late-onset PTX is not present by either repeated FLUS or a chest X-ray.
- Discuss the presentation of the histological characteristics of the biopsies at a following MDD with pulmonologists, radiologists, and pathologists in conjunction with other details to conclude an ILD subtype with high diagnostic probability.
- Inform the patient of the MDD conclusion from step 4.5 in the outpatient clinic, and plan potential treatment and follow-up.

Figure 6: Minor bleeding. If bleeding is observed after having performed a transbronchial lung cryobiopsy, in this case, minor bleeding, the balloon catheter should be kept inflated a few minutes before deflating is retried. Please click here to view a larger version of this figure.
5. TBLC in conjunction with R-EBUS and ENB for PPL diagnostics
- Navigate and confirm the location of the peripheral pulmonary lesion.
- Insert the 1.1 mm cryoprobe in the extended working channel under the guidance of fluoroscopy.
- Match the tip of the freezing probe with the position of the radial EBUS probe.
- Step on the freezing pedal for 4-8 s.
- Retract the cryoprobe through the extended working channel in one quick movement while holding the freezing pedal down to continue freezing and prevent the biopsy from falling off during the retraction.
- Keep the bronchoscope and the extended working channel in position while the cryoprobe is retracted.
- Repeat steps 5.2-5.4 until a sufficient number of biopsies are obtained.
- Handle the biopsies as described in steps 3.3 and 3.4.
Results
Based on the observations from the authors from two TBLC centers, the described stepwise procedure for TBLC with a flexible bronchoscope allowed histological sampling in well-selected Danish patients with yet undiagnosed ILD subtypes despite preceding MDD. Detailed observations from these cohorts are reported in two recently published studies23,25 and for the center of the first author summarized in Table 2.
| Biopsy location | |
| ML, n (%) | 2 (14.2) |
| RLL, n (%) | 125 (88.7) |
| LLL, n (%) | 15 (10.6) |
| Bleeding | 141 (100.0) |
| Minor, n (%) | 118 (83.7) |
| Moderate, n (%) | 23 (16.3) |
| Severe, n (%) | 0 (0.0) |
| Pneumothorax, n (%) | 21 (14.9) |
| + pleuradrain, n (%) | 14 (9.9) |
| 30-day mortality, n (%) | 2 (1.4) |
| Histological diagnosis, n (%) | 101 (75.2) |
| MDD-based diagnosis, n (%) | 124 (87.9) |
Table 2: TBLC observations from one Danish TBLC center. A total of 141 was due to patients having TBLC performed in more lobes. Data are expressed as numbers (n and N) and percentages (%). The observations are extracted from Davidsen et al.23, and in agreement with Creative Common License (https://creativecommons.org/licenses/by/4.0/). Abbreviations: LLL = left lower lobe; ML = middle lobe; RLL = right lower lobe; OUH = Odense University Hospital; TBLC = transbronchial lung cryobiopsy; MDD = multidisciplinary team discussion.
We used general anesthesia and relaxation to reduce patient-related procedure intolerance (e.g., cough), but also importantly, make TBLC conductance easier for the interventional bronchoscopist, and allow the introduction of a double luminal ETT. Reviewing the HRCT gave us a reliable impression of radiologic ILD manifestations and the site where TBLC should be appropriately performed. Intending to achieve the highest diagnostic yield from TBLC. For both TBLC cohorts23,25, the predominant biopsy site was the right lower lobe in 88.0-88.7% of the cases. By using fluoroscopy, we took into account caution during probe placement regarding approximation to pleura and pulmonary vessels to reduce the risk of procedure-related complications.
In addition, the Fogarty balloon allowed us to manage and control potential bleeding from the biopsy site, ensuring that bleeding would not spread to other BS. For the Odense and Aarhus cohorts, moderate bleeding (defined as a need for cold saline water and/or Fogarty balloon required for more than 2 min and/or use of supplemental tranexamic acid) was observed with a prevalence of 16.3% and 21.0%, respectively. As default, we went for at least four biopsies permitting us to obtain acceptable, high diagnostic yields on the basis of MDD of 87.9% and 82.0% from the Odense and Aarhus cohorts, respectively. FLUS was used as a postTBLC procedure to identify potential PTX and was detected in 4/19 (21%) cases from Odense and in 19/70 (27%) cases from Aarhus24. The prevalence of pleural drain-dependent PTX was 14.9% in Odense and 20.0% in Aarhus. The 90-day mortality from Odense and Aarhus was 1.5% and 0.4%, respectively.
Following the described steps in protocol section 5, tissue samples from patients with PPL were obtained from the targeted lesion without removing the bronchoscope and repeating the navigation process. The biopsies collected with the 1.1 mm cryoprobe were smaller than the biopsies required for ILD diagnostics. However, the smaller biopsies allowed for the retraction of the probe with biopsies through the extended working channel and simultaneously maintain the position of the bronchoscope. The risk of bleeding was expected to be lower due to minor biopsies, and it was easier to safely manage bleeding with the bronchoscope in the BS. This process allowed us to perform TBLC without GA, ETT, or the use of an endobronchial blocker.
Discussion
Regardless of the indication for TBLC, its diagnostic properties rely on the quality of the procedure's performance and the selected criteria for undergoing the procedure. This emphasizes the recommendation of implementing a formal and certified training program to acquire the competences required to perform a standardized TBLC procedure. Despite the fact that no official TBLC education is currently obtainable, the recent European Respiratory Society guideline on TBLC for ILD suggests that interventional pulmonologists performing TBLC should undergo formal training to increase the diagnostic yield and reduce the risk of complications, while also being able to manage these in terms of, for example, bleeding and iatrogenic PTX 9. At present, such a training program has neither been defined nor developed due to a lack of evidence. However, several experts within the field of TBLC have unanimously recommended the procedure to be performed in specialized centers with knowledge of ILDs and the technical aspects of TBLC, including the management of complications, and with access to MDD and thoracic surgical expertise8,11,17,26,27.
The procedure assumes that certain preprocedure criteria are considered to ensure histological sampling in the safest way and diminish the risk for potential procedure-related complications17. However, selection criteria for TBLC may differ slightly across centers (e.g., diffusion of the lung for carbon monoxide (DLCO) ranging from 35% to 40% pred.) resulting in some cohorts being more vulnerable to even minor complications than others23,25. Moreover, the selection of procedure-related variables (e.g., anesthetic approach, probe size, and freezing time) may vary and bias the interpretation of observations related to complications and mortality when comparing across different study cohorts. Again, this calls for standardization.
Patients' pulmonary anatomy differs and in patients where the bronchi and segment bronchi tend to bend sharply, this may challenge appropriate probe placement according to the biopsy site chosen preprocedure. In such cases, several attempts at probe placement may be done, sometimes necessitating choosing another biopsy site. This well-known problem is due to relatively rigid probes. Conversely, although the balloon catheter has a removable stylet, it is quite mobile and challenging to navigate with. In this case, the bronchoscope could be used, by itself or with the cryoprobe a bit extended from it, to guide or press the balloon into the selected BS. In difficult cases, it can sometimes be helpful to use biopsy forceps to gently direct the bronchial blocker into the right BS or to gently bend it prior to insertion, especially if targeting the upper lobes. Care must be taken while advancing the cryoprobe due to a risk of PTX if the stiff tip of the cryoprobe is pushed distally. Further advancing must be stopped if resistance is felt, which warrants the introduction of the cryoprobe under direct fluoroscopic guidance to avoid penetration of the visceral pleura. Precautions against bleeding, however, are also crucial, especially due to an increased risk of PTX associated with increasing probe size and the number of biopsies28.
Although SLB is considered the gold standard for ILD diagnostics, more recent studies have established consistent evidence that TBLC is a safe and cost-effective outpatient intervention procedure with respect to complications such as bleeding and PTX and with high diagnostic agreements between SLB and TBLC in the assessment of ILD13,27,29,30,31. This was also concluded in a systemic review, which led to the recommendation of TBLC as an alternative ILD diagnostic modality to SLB in the recently updated guideline on idiopathic pulmonary fibrosis (IPF)27,32. Additionally, TBLC favors SLB regarding 30-day postprocedure mortality (i.e., 0.6% for TBLC vs. 1.7% for SLB)8. Accordingly, TBLC is increasingly being regarded as the first-choice histological sampling method for well-selected patients with unclassifiable ILD in tertiary ILD centers with expertise in TBLC and access to ILD MDDs8,12,13,23,25,31,33. These indications are also the ones experienced in our two TBLC centers, and where our observations on complications, mortality, and diagnostic yields parallel the findings from a recent systematic review and meta-analysis evaluating diagnostic yield and safety from 43 studies8. To further support the use of TBLC, some studies have suggested R-EBUS-guided TBLC as an additional tool to especially target ground-glass opacity lesions in patients suspected of ILD19. Although the combination of these two modalities in ILD diagnostics may be more time-consuming than TBLC alone, observations indicate not only a significantly reduced bleeding risk by concurrent use of R-EBUS, but also R-EBUS as a valuable aid for the bronchoscopist to obtain representative biopsies, with diagnostic accuracies reported to be up to 92.5%18,19,34. While the results from adding R-EBUS to TBLC seem promising, these, however, require further investigation in comparative trials34.
Due to the demographic development, there is already an observed increased incidence of ILD calling for an increased access and need for TBLC in the diagnostic process35, which again undermines the requirement of the correct indications for and knowledge on the TBLC procedure and its systematic conductance9,17,26. In a pilot-study, Kronborg-White et al. have previously shown that combining ENB and TBLC for ILD diagnostics is feasible; however, solid evidence that this may increase the diagnostic yield and reduce further invasive diagnostics is lacking36. Confocal laser endomicroscopy (CLE) is a high-resolution modality that can be applied through a bronchoscope to visualize fibrotic areas compatible with fibrotic ILD and may replace fluoroscopy in the future. One study has concluded that CLE is a promising real-time alternative to fluoroscopy during the TBLC procedure, but this has not been further investigated in prospective studies with respect to the improved quality of biopsies and reduced prevalence of complications37. Further, immunohistochemistry and genomic classifier (GC) testing, added to TBLC using tissue transcriptomic analysis by RNA sequencing, has shown promising results to distinguish histological usual interstitial pneumonia (UIP) from non-UIP with high specificity38,39,40. The availability of GC regarding the characterization and prediction of UIP seems to increase the diagnostic confidence of progressive fibrotic ILD (e.g., IPF)39,41. Future such novel histological techniques and modalities to improve diagnostic yields of ILD are thus expected to be further developed42,43.
For targeting PPL, the utility of R-EBUS- and ENB-guided TBLC has recently been described as a safe procedure. Although there are few publications, studies indicate that the use of TBLC increases the diagnostic yield compared to using forceps alone20,21,44. Data on safety are still lacking, although one study using the method described in this article reported no PTX and only mild bleeding with no need for intervention20. Overall, this area is in its infancy due to the lack of evidence and requires further elucidation.
In summary, TBLC is gradually suggested as the first-choice histological procedure for ILD subtype diagnostics in well-selected patients in whom a confident diagnosis has not been attainable on the basis of preceding clinical and paraclinical information. This recommendation is due to TBLC's high diagnostic yield and safety profile with a low prevalence of complications and mortality compared to SLB. The procedure should be done by interventional pulmonologists with expertise in TBLC after being certified. However, to date, a certified TBLC training program has not been developed. The use of TBLC for sampling PPL has very little evidence supporting its use. However, the few studies published so far suggest an increased diagnostic yield compared to forceps and an excellent safety profile. The limited and unpublished experiences from our center suggest that TBLC is feasible and can be combined with existing modalities such as ENB and R-EBUS already used for PPL location in a bronchoscopy setting without access to GA. However, the procedure is still experimental, and further evidence regarding diagnostic yield and safety is highly warranted.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors would like to acknowledge the personnel from the Departments of Thoracic Surgery and Anesthesiology at the Bronchoscopy Ward at Odense University Hospital, Denmark, for their help with the preparation of the figures for this article.
Materials
| Name | Company | Catalog Number | Comments |
| "Chimney" for tube | |||
| CO2 gas bottle adapter | |||
| CO2 gas tank | Erbe | ||
| Endoscopy column | |||
| Endotracheal tube, size 7.5-8.5 mm | Erbe | ||
| Erbecryo pedal footswitch | Erbe | ||
| Erbecryo2 workstation | Erbe | ||
| Flexible bronchoscope | |||
| Flexible gas hose | Mediland | ||
| Flexible single use cryoprobe, OD 1.1 mm | Erbe | ||
| Flexible single use cryoprobe, OD 1.7 mm | Erbe | ||
| Flexible single use cryoprobe, OD 2.4 mm | |||
| Fluoroscope | |||
| Fogarty balloon catheter | |||
| Formalin glasses in closed system | |||
| NaCl incl. cold NaCl | |||
| Pean for fixating Fogarty balloon | |||
| Sterile disposable cup | |||
| Sterile suction tube | |||
| Sterile tweesers | |||
| Syringe for Fogarty balloon inflation/deflation | |||
| Table bag for flouroscope | |||
| Three way tap for Fogarty balloon syringe | |||
| Tracheal suction | |||
| Ultrasound machine | Erbe | ||
| Valve for biopsy chanel | |||
| Valve to suction duct |
References
- Travis, W. D., et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. American Journal of Respiratory and Critical Care Medicine. 188 (6), 733-748 (2013).
- Ruaro, B., et al. Editorial: Pulmonary fibrosis: One manifestation, various diseases. Frontiers in Pharmacology. 13, 1027332 (2022).
- Lamas, D. J., et al. Delayed access and survival in idiopathic pulmonary fibrosis: a cohort study. American Journal of Respiratory and Critical Care Medicine. 184 (7), 842-847 (2011).
- Tomassetti, S., Piciucchi, S., Tantalocco, P., Dubini, A., Poletti, V. The multidisciplinary approach in the diagnosis of idiopathic pulmonary fibrosis: a patient case-based review. European Respiratory Review. 24 (135), 69-77 (2015).
- Walsh, S. L. F., et al. Multicentre evaluation of multidisciplinary team meeting agreement on diagnosis in diffuse parenchymal lung disease: a case-cohort study. Lancet Respiratory Medicine. 4 (7), 557-565 (2016).
- Ryerson, C. J., et al. A standardized diagnostic ontology for fibrotic interstitial lung disease. An International Working Group perspective. American Journal of Respiratory and Critical Care Medicine. 196 (10), 1249-1254 (2017).
- Cottin, V., et al. Integrating clinical probability into the diagnostic approach to idiopathic pulmonary fibrosis: An International Working Group perspective. American Journal of Respiratory and Critical Care Medicine. 206 (3), 247-259 (2022).
- Rodrigues, I., et al. Diagnostic yield and safety of transbronchial lung cryobiopsy and surgical lung biopsy in interstitial lung diseases: a systematic review and meta-analysis. European Respiratory Review. 31 (166), 210280 (2022).
- Korevaar, D. A., et al. European Respiratory Society guidelines on transbronchial lung cryobiopsy in the diagnosis of interstitial lung diseases. European Respiratory Journal. 60 (5), 2200425 (2022).
- Colella, S., Haentschel, M., Shah, P., Poletti, V., Hetzel, J. Transbronchial lung cryobiopsy in interstitial lung diseases: best practice. Respiration. 95 (6), 383-391 (2018).
- Hetzel, J., et al. Transbronchial cryobiopsies for the diagnosis of diffuse parenchymal lung diseases: expert statement from the Cryobiopsy Working Group on safety and utility and a call for standardization of the procedure. Respiration. 95 (3), 188-200 (2018).
- Ravaglia, C., Poletti, V. Transbronchial lung cryobiopsy for the diagnosis of interstitial lung diseases. Current Opinion in Pulmonary Medicine. 28 (1), 9-16 (2022).
- Troy, L. K., et al. Diagnostic accuracy of transbronchial lung cryobiopsy for interstitial lung disease diagnosis (COLDICE): a prospective, comparative study. Lancet Respiratory Medicine. 8 (2), 171-181 (2020).
- Ruaro, B., et al. Transbronchial lung cryobiopsy and pulmonary fibrosis: A never-ending story. Heliyon. 9 (4), e14768 (2023).
- Lentz, R. J., Argento, A. C., Colby, T. V., Rickman, O. B., Maldonado, F. Transbronchial cryobiopsy for diffuse parenchymal lung disease: a state-of-the-art review of procedural techniques, current evidence, and future challenges. Journal of Thoracis Disease. 9 (7), 2186-2203 (2017).
- Maldonado, F., et al. Transbronchial cryobiopsy for the diagnosis of interstitial lung diseases: CHEST Guideline and Expert Panel Report. Chest. 157 (4), 1030-1042 (2020).
- Avasarala, S. K., Wells, A. U., Colby, T. V., Maldonado, F. Transbronchial cryobiopsy in interstitial lung diseases: State-of-the-art review for the interventional pulmonologist. Journal of Bronchology Interventional Pulmonology. 28 (1), 81-92 (2021).
- Abdelghani, R., Thakore, S., Kaphle, U., Lasky, J. A., Kheir, F. Radial Endobronchial Ultrasound-guided Transbronchial Cryobiopsy. Journal of Bronchology Interventional Pulmonology. 26 (4), 245-249 (2019).
- Inomata, M., et al. Utility of radial endobronchial ultrasonography combined with transbronchial lung cryobiopsy in patients with diffuse parenchymal lung diseases: a multicentre prospective study. BMJ Open Respiratory Research. 8 (1), e000826 (2021).
- Benn, B. S., Gmehlin, C. G., Kurman, J. S., Doan, J. Does transbronchial lung cryobiopsy improve diagnostic yield of digital tomosynthesis-assisted electromagnetic navigation guided bronchoscopic biopsy of pulmonary nodules? A pilot study. Respiratory Medicine. 202, 106966 (2022).
- Ankudavicius, V., Miliauskas, S., Poskiene, L., Vajauskas, D., Zemaitis, M. Diagnostic yield of transbronchial cryobiopsy guided by radial endobronchial ultrasound and fluoroscopy in the radiologically suspected lung cancer: A single institution prospective study. Cancers. 14 (6), 1563 (2022).
- Ravaglia, C., et al. Transbronchial lung cryobiopsy in diffuse parenchymal lung disease: Comparison between biopsy from 1 segment and biopsy from 2 segments - diagnostic yield and complications. Respiration. 93 (4), 285-292 (2017).
- Davidsen, J. R., Skov, I. R., Louw, I. G., Laursen, C. B. Implementation of transbronchial lung cryobiopsy in a tertiary referral center for interstitial lung diseases: a cohort study on diagnostic yield, complications, and learning curves. BMC Pulmonary Medicine. 21 (1), 67 (2021).
- Laursen, C. B., et al. Lung ultrasound assessment for pneumothorax following transbronchial lung cryobiopsy. ERJ Open Research. 7 (3), 00045-2021 (2021).
- Kronborg-White, S., et al. Integration of cryobiopsies for interstitial lung disease diagnosis is a valid and safe diagnostic strategy-experiences based on 250 biopsy procedures. Journal of Thoracic Disease. 13 (3), 1455-1465 (2021).
- Barisione, E., et al. Competence in transbronchial cryobiopsy. Panminerva Medica. 61 (3), 290-297 (2019).
- Raghu, G., et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. American Journal of Respiratory and Critical Care Medicine. 205 (9), e18-e47 (2022).
- Ravaglia, C., et al. Diagnostic yield and risk/benefit analysis of trans-bronchial lung cryobiopsy in diffuse parenchymal lung diseases: a large cohort of 699 patients. BMC Pulmonary Medicine. 19 (1), 16 (2019).
- Hernandez-Gonzalez, F., et al. Cryobiopsy in the diagnosis of diffuse interstitial lung disease: yield and cost-effectiveness analysis. Archivos de Bronconeumología. 51 (6), 261-267 (2015).
- Cooley, J., et al. Safety of performing transbronchial lung cryobiopsy on hospitalized patients with interstitial lung disease. Respiratory Medicine. 140, 71-76 (2018).
- Hetzel, J., et al. Transbronchial cryobiopsy increases diagnostic confidence in interstitial lung disease: a prospective multicenter trial. European Respiratory Journal. 56 (6), 1901520 (2020).
- Kheir, F., et al. Transbronchial lung cryobiopsy in patients with interstitial lung disease: a systematic review. Annals of the American Thoracic Society. 19 (7), 1193-1202 (2022).
- Walscher, J., et al. Transbronchial cryobiopsies for diagnosing interstitial lung disease: real-life experience from a tertiary referral center for interstitial lung disease. Respiration. 97 (4), 348-354 (2019).
- Gnass, M., et al. Transbronchial lung cryobiopsy guided by radial mini-probe endobronchial ultrasound in interstitial lung diseases - a multicenter prospective study. Advances in Respiratory Medicine. 88 (2), 123-128 (2020).
- Ma, X., et al. Global and regional burden of interstitial lung disease and pulmonary sarcoidosis from 1990 to 2019: results from the Global Burden of Disease study 2019. Thorax. 77 (6), 596-605 (2022).
- Kronborg-White, S., et al. A pilot study on the use of the super dimension navigation system for optimal cryobiopsy location in interstitial lung disease diagnostics. Pulmonology. 29 (2), 119-123 (2021).
- Wijmans, L., et al. Confocal laser endomicroscopy as a guidance tool for transbronchial lung cryobiopsies in interstitial lung disorder. Respiration. 97 (3), 259-263 (2019).
- Kheir, F., et al. Using bronchoscopic lung cryobiopsy and a genomic classifier in the multidisciplinary diagnosis of diffuse interstitial lung diseases. Chest. 158 (5), 2015-2025 (2020).
- Renzoni, E. A., Poletti, V., Mackintosh, J. A. Disease pathology in fibrotic interstitial lung disease: is it all about usual interstitial pneumonia. Lancet. 398 (10309), 1437-1449 (2021).
- Chaudhary, S., et al. Interstitial lung disease progression after genomic usual interstitial pneumonia testing. European Respiratory Journal. 61 (4), 2201245 (2023).
- Raghu, G., et al. Use of a molecular classifier to identify usual interstitial pneumonia in conventional transbronchial lung biopsy samples: a prospective validation study. Lancet Respiratory Medicine. 7 (6), 487-496 (2019).
- Kheir, F., et al. Use of a genomic classifier in patients with interstitial lung disease: a systematic review and meta-analysis. Annals of American Thoracic Society. 19 (5), 827-832 (2022).
- Glenn, L. M., Troy, L. K., Corte, T. J. Novel diagnostic techniques in interstitial lung disease. Frontiers in Medicine. 10, 1174443 (2023).
- Kim, S. H., et al. The additive impact of transbronchial cryobiopsy using a 1.1-mm diameter cryoprobe on conventional biopsy for peripheral lung nodules. Cancer Research and Treatment. 55 (2), 506-512 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved