Method Article
Image-Guided Optical Coherence Tomography to Assess Structural Changes in Rodent Retinas
* These authors contributed equally
In This Article
Summary
The protocol presented here details the procedures of data collection and data analysis for image-guided optical coherence tomography (OCT) and demonstrates its application in multiple rodent models of ocular diseases.
Abstract
Ocular diseases, such as age-related macular degeneration, glaucoma, retinitis pigmentosa, and uveitis, are always accompanied by retinal structural changes. These diseases affecting the fundus always exhibit typical abnormalities in certain cell types in the retina, including photoreceptor cells, retinal ganglion cells, cells in the retinal blood vessels, and cells in the choroidal vascular cells. Noninvasive, highly efficient, and adaptable imaging techniques are required for both clinical practice and basic research. Image-guided optical coherence tomography (OCT) satisfies these requirements because it combines fundus photography and high-resolution OCT, providing an accurate diagnosis of tiny lesions as well as important changes in the retinal architecture. This study details the procedures of data collection and data analysis for image-guided OCT and demonstrates its application in rodent models of choroidal neovascularization (CNV), optic nerve crush (ONC), light-induced retinal degeneration, and experimental autoimmune uveitis (EAU). This technique helps researchers in the eye field to identify rodent retinal structural changes conveniently, reliably, and tractably.
Introduction
Ocular diseases affecting the fundus always exhibit typical abnormalities in certain cell types in the retina, such as photoreceptor cells, retinal ganglion cells, cells in the retinal blood vessels, and cells in the choroidal blood vessels, which may subsequently influence the visual acuity of patients1. To avoid irreversible visual impairment, timely diagnoses and appropriate treatments are required1. Optical coherence tomography (OCT) has been widely used in the clinic to evaluate a range of ocular diseases, including age-related macular degeneration, retinitis pigmentosa, glaucoma, uveitis, and retinal detachment, among others2,3,4. This kind of noninvasive, highly efficient, and adaptable imaging technique is also needed for the timely evaluation of the disease conditions in experimental animals5,6,7,8,9,10.
Image-guided optical coherence tomography (OCT) uses interferometry to produce cross-sectional images of animal retinas at 1.8 µm longitudinal resolution and 2 µm axial resolution. It has at least three advantages in the investigation of retinal architectural changes2,3,4,5,6,7,8,9,10. First, it is a noninvasive technique that allows researchers to dynamically follow the location of interest in the same animal retina5,6,7,8,9,10. Second, this trait substantially reduces the sample size for every experiment3. Meanwhile, it saves considerable time and effort in research projects2,3,4,5,6,7,8,9,10. Third, image-guided OCT acquires colorful fundus images while capturing OCT images, thus providing accurate and reliable results for users.
This manuscript describes the procedures of image collection and data analysis for image-guided OCT and elaborates on its application in mouse and rat models of choroidal neovascularization (CNV)11,12, optic nerve crush (ONC)13,14,15,16, light-induced retinal degeneration17,18,19,20,21, and experimental autoimmune uveitis (EAU)22,23. With this versatile technique, researchers can capture high-resolution OCT images as well as fundus images conveniently and efficiently.
Protocol
All the animal procedures conformed to the Association for Research on Vision and Ophthalmology's statement on the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee of Wenzhou Medical University (WMU). The rats and mice were given free access to water and food with an environmental light intensity of 18 lux on a 12 h dark/light cycle.
1. Preparation of the ocular animal models
- Mouse laser-induced choroidal neovascularization (CNV) model11,12
- Anesthetize 4-week-old female mice (C57BL/6J background) with ketamine and xylazine, and dilate the pupils with tropicamide phenylephrine eye drops following step 3.2. Consider the mouse properly anesthetized when no movement is detected after pinching the toe.
- Engage the light-source box, the software, and the laser box (wavelength: 532 nm), with the output energy adjusted to 100 mW and the duration to 100 ms.
- Place the mouse on the experimental platform and adjust the position of the mouse and the platform until the view of the mouse fundus is clear.
- Press the Laser ON button on the screen of the laser box and adjust the focus of the red laser reference spot. Move the laser reference spot and adjust it to one to two papillary diameters away from the optic disc. Operate the foot pedal to cause laser damage.
- Conduct an immediate check to determine whether a vaporizing bubble occurred immediately after the hit, which is a sign of successful laser damage. Generate three to five laser spots for each eye.
- Mouse optic nerve crush (ONC) model13,14,15,16
- Anesthetize female mice (C57BL/6J background) at postnatal day (P) 21 to P35 following step 3.2. Consider the mouse properly anesthetized when no movement is detected after pinching the toe.
- Place the mouse under a surgical microscope, and incise the conjunctiva of one eye using spring scissors, ensuring the size of the conjunctiva incision is approximately 1 mm.
- Expose the intraorbital optic nerve, and crush with fine forceps for 5 s at 0.5 mm away from the optic disc. While doing this, gently deflect the orbital muscles and the other tissues, and place them aside. Avoid damage to any blood vessels in the surgical eye.
NOTE: Be careful, as the eyeball will swing when touching or plucking the white bunchy optic nerve. - Apply ophthalmic ointment postoperatively to avoid corneal dryness.
- Mouse light-induced retinal degeneration (LIRD) model17,18,19,20,21
- Preparations before the experiment: Enclose the mouse cages (30 cm x 18 cm x 13 cm) with aluminum foil and an iron mesh cover. Place each cage in a paper box (52 cm x 35 cm x 30 cm) with a white light on the top.
- Dark-adapt 6-week-old male mice (BALB/c) overnight in the dark room, and then dilate the eyes using tropicamide phenylephrine eye drops in each eye. Fully dilate the pupil until 3/4 of the cornea area is not covered by the iris; this often takes no more than 3 min.
- Illuminate the mice with 10,000 lux white light for 2 h. Keep the mice in the dark overnight postoperatively, and then return to normal dark-light environments. To ensure an effective light stimulus, keep one mouse in the cage at all times.
- Rat experimental autoimmune uveitis (EAU) model
- Preparations before the experiment: Emulsify 2.5 mg of hIRBP161-180 in complete Freund's adjuvant (1:1 weight/volume) with 2.5 mg/mL Mycobacterium tuberculosis H37Ra22,23.
- Inject 100 µL of emulsion subcutaneously into the left footpad of each Lewis male rat (about 180 g).
2. OCT module setup
- Connect the optical fiber and the OCT control cable between the OCT scan head and the OCT engine (Figure 1A). Align the notch on the fiber with a slot on the connector, and gently insert the tip until it is seated (Figure 1B).
NOTE: Before connecting the control cable, turn the power switch of the OCT engine OFF. The optical fiber is very fragile, so avoid both ends of the fiber tip (Figure 1C). Assembling and disassembling the OCT scan head and the fiber/cable should be avoided if this module is under frequent use. - Thread the mouse or rat OCT objective lens to the front of the machine body (Figure 1A). Place the OCT scan head onto the OCT lens, with the M-R focus indicator facing away from the machine body (Figure 1A).
- Secure the OCT scan head with two thumbscrews: one on the lens and one on the camera body. Fix the scan head tightly.
- Turn on the main power switch, followed by the camera light, and then the OCT software.
NOTE: The camera needs some time to boot up for the computer to find it.
3. Animal preparation for OCT experiments
- At 10 min before the OCT experiments, instill tropicamide phenylephrine eye drops in the animal's eyes, and wipe away excess eye drops using a clean towel.
- At 5 min before the experiment, inject 200 µL of the anesthetic solution into the mouse, and inject 2.0 mL into the rat intraperitoneally. Consider the mouse/rat properly anesthetized when no movement is detected after pinching the toe.
NOTE: The anesthetic solutions were composed of ketamine (12 mg/mL) and xylazine (1 mg/mL for the mouse and 2 mg/mL for the rat) in saline; typically, 10 µL of anesthetic solution per 1 g of animal body mass is applied. The solutions were stored at 4 °C for a maximum of 2 weeks. - Once anesthesia is administered, lubricate the cornea with gel ointment to avoid ocular surface dryness.
4. Image-guided OCT Imaging
NOTE: The software interface was divided into three parts: brightfield image, OCT control tabs, and OCT display (Figure 2).
- When looking at the brightfield image, type the annotation of the animal information if needed.
- Toggle the Beam Position Controls ON or OFF using the check box to capture a brightfield image with or without the line overlay on the image.
- To place the scanning beam more precisely, use the Position Nudge arrows for fine control to direct it up, down, left, or right. Choose the thickness values and color, and choose an angle for the beam targeting the location of interest (e.g., thick, black, and 0). Drag the gain value to 14 db.
- Go to the OCT control tabs. From the menu, select File/File_Settings, and then create a path to where the OCT and fundus images will be saved.
- Specify the scan type, size, and animal (rat or mouse); choose Eye, left or right, and Imaging Retina.
NOTE: OCT can capture line, circle, and 3D volume scan types at full, half, quarter, and eighth sizes. - Set the image controls. Adjust the values: 60 for the contrast, 100 for the gamma , and 0 for the brightness.
- Place the mouse on the experimental platform and adjust the position of the mouse and platform until the view of the mouse fundus is clear. Finely adjust the position to localize the optic disc in the center.
- Adjust the reference arm to 850 for mice and 830 for rats at the beginning, and then finely adjust the position by clicking the <-1 or +1> buttons. Adjust the OCT image horizontally, and then move the OCT image to the top 1/3 of the full view.
- Move the polarization slider to adjust the brightness of the signal through the retina, if needed.
- After observing a clear and stable OCT image as well as the fundus image, set the number of frames (usually 20, 50, 80, or 100), and then press Average.
NOTE: It takes several seconds to capture the OCT images. A larger number of frames means the image capture takes longer to finish but produces a higher-quality captured image. - Press Save to save the OCT and fundus images, and then click Restart to capture another sample.
NOTE: The animals were allowed to recover on a 37 °C heating plate before awakening. The animals were not left unattended until they had regained sufficient consciousness to maintain sternal recumbency. The animals that had undergone treatment were not returned to the raising system of other animals until fully recovered.
5. Thickness measurement and quantitative analysis
NOTE: This OCT has built-in analysis software. OCT images can be segmented and analyzed using this software (Figure 3).
- Open the analysis software and open an OCT image that needs to be analyzed.
- Choose the number of layers, and then press Get Initial Layers (Figure 3A). The software will draw the layers automatically.
- Click the Pencil icon, and then move the dots on the target layer to finely adjust the layer (Figure 3A, B). Modify all the layers carefully one by one.
NOTE: Adding more layers is doable. Click on the Plus icon, draw several dots over the OCT image, and then press the Tick icon. A new layer will be created. - Once fine adjustments of the layers are completed, export the detailed values (CSV format) and different image types of the segmented OCT (Figure 3A).
NOTE: It is possible to export different image types, including layers, thickness, thickness map, layers without OCT, and screen grabs. The value in the middle that overlaps with the optic nerve of every image needs to be abandoned and set as zero since there is no retinal layer there.
Results
Image-guided OCT can be used to monitor the development of the laser spot in laser-induced choroidal neovascularization (CNV) in mice. As shown in Figure 1, the newborn blood vessels passed through Bruch's membrane as well as the retinal pigment epithelium (RPE) layer and formed a fibrotic scar after laser injury11,12. This lesion spot could be captured under either full-size scanning (Figure 4A) or half-size scanning (Figure 4B). The half-size scan is recommended because it provides enlarged pictures and makes the observation more focused on the laser spot. Of course, monitoring a wider time window including 0 days, 3 days, 7 days, and 14 days is possible if needed by the study.
In the mouse optic nerve crush (ONC) model, dynamic changes in the thickness of the ganglion cell complex (GCC), which is composed of the retinal nerve fiber layer (RNFL), ganglion cell layer (GCL), and inner plexiform layer (IPL), were measured. Using the circle scanning type (Figure 5A), a degenerative feature of the ganglion cells was observed in the OCT images (Figure 5B,C). The thickness of the GCC was approximately 69-74 µm in the untreated control mouse retina, whereas the thickness decreased to 50-57 µm at 7 days post-crush (dpc) and to 43-49 µm at 14 dpc (Figure 5C).
This kind of layer thickness analysis is also applicable to the outer retina. An example is measuring the thicknesses of the mouse outer retina in the model of light-induced retinal degeneration (LIRD) by OCT using full-size scanning (Figure 6). Before light damage, the outer retina displayed clear layers, including the outer plexiform layer (OPL), outer nuclear layer (ONL), photoreceptor inner segment (IS), and photoreceptor outer segment (OS; Figure 6A). However, 1 day after light damage, a blurred outcome was observed in the outer retina of the OCT image, which may have been caused by the immune response and cell death19,20. By then, the outer retina was still thicker than the inner retina (composed of the GCC and the inner nuclear layer; Figure 6B). Over time, it became thinner than the inner retina on day 5 and day 7, and its color reversed from red to blue (indicated by the heat map scale; Figure 6B).
In an experimental autoimmune uveitis (EAU) rat model, the inflammation characteristics of the retina and vitreous were surveyed. Image-guided OCT could produce a fundus image and the structure of the retina and vitreous (Figure 7) simultaneously, thus providing an easy way to evaluate the severity of the disease condition. Compared to the control rats (Figure 7A), the EAU rats (Figure 7B) exhibited severe vasculitis, the presence of vitreous cells, optic disk swelling, and retinal folds.
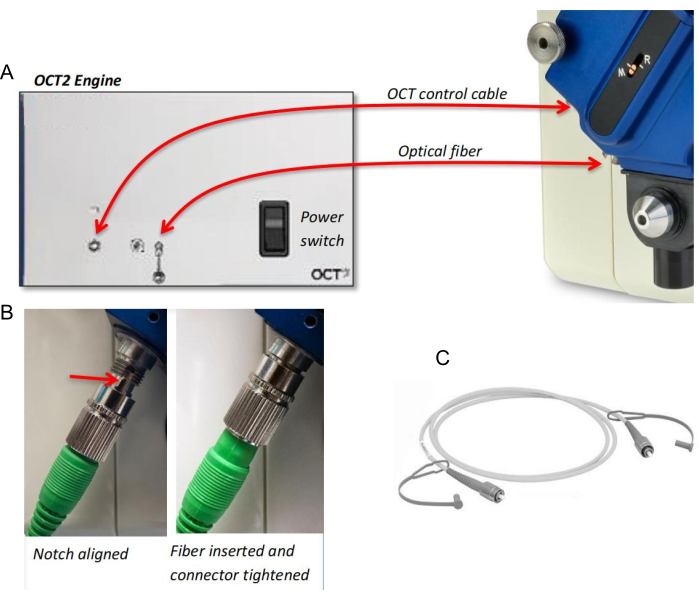
Figure 1: The hardware of the OCT module. (A) Connection of the optical fiber and the OCT control cable between the OCT scan head and the OCT engine. (B) Connection of the optical fiber to the OCT scan head. (C) Optical fiber. Please click here to view a larger version of this figure.

Figure 2: The imaging software of image-guided OCT. The software interface is divided into three parts: brightfield image, OCT control tabs, and OCT display. Please click here to view a larger version of this figure.
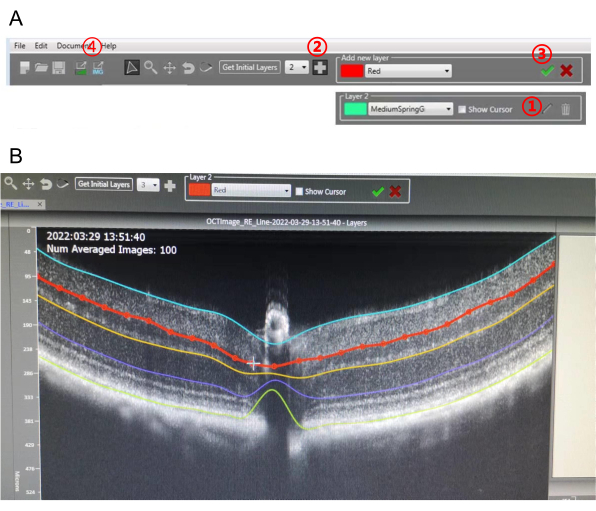
Figure 3: The software for image-guided OCT layer segmentation. (A) Toolbar displayed in the software.  Pencil icon;
Pencil icon;  + icon;
+ icon;  tick icon;
tick icon;  export icon. (B) Fine adjustment of the newly added layers on the OCT image. Please click here to view a larger version of this figure.
export icon. (B) Fine adjustment of the newly added layers on the OCT image. Please click here to view a larger version of this figure.
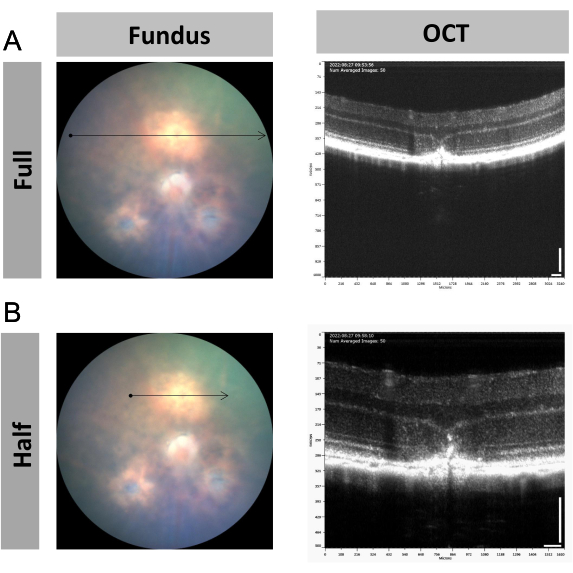
Figure 4: Representative figures of the retinal structural changes in the mouse laser-induced choroidal neovascularization (CNV) model. (A,B) Representative fundus and OCT images of the location of interest with CNV using (A) full-size and (B) half-size scanning at 7 days post laser injury. Scale bar = 100 µm; n = 1. Please click here to view a larger version of this figure.

Figure 5: Representative figures of the retinal structural changes in the mouse optic nerve crush (ONC) model. (A-C) Representative images of (A) OCT (scanning type: circle) and fundus, (B) OCT with layers, and (C) OCT thickness maps at day 7 and day 14 post-crush. Abbreviation: dpc = days post crush. Scale bar = 100 µm; n = 1. Please click here to view a larger version of this figure.
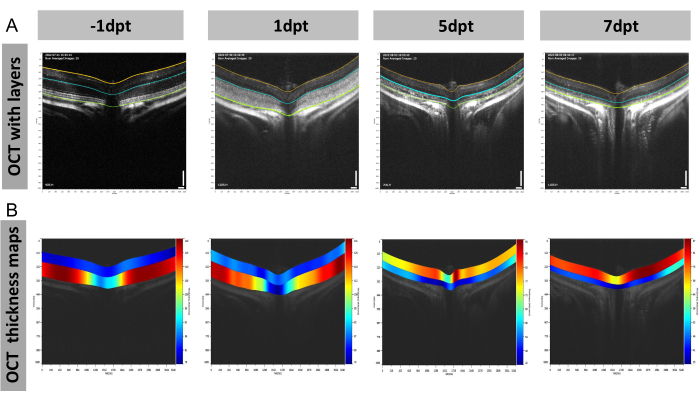
Figure 6: Representative figures of the retinal structural changes in the mouse light-induced retinal degeneration (LIRD) model. (A,B) Representative OCT images with (A) layers and (B) OCT thickness maps at day −1, day 1, day 5, and day 7 after light damage. Abbreviations: dpt = days post-treatment. Scale bar = 100 µm; n = 3. Please click here to view a larger version of this figure.

Figure 7: Representative figures of the retinal structural changes and the signal in the vitreous in the experimental autoimmune uveitis (EAU) rat model. (A,B) Representative fundus and OCT images of the (A) control and (B) EAU model using full-size scanning at 11 days post-treatment. Scale bar = 100 µm; n = 1. Please click here to view a larger version of this figure.
Discussion
This protocol provides instructions for the image collection and thickness measurement of image-guided OCT. By demonstrating the four most popular rodent models of ocular diseases, the researchers found that image-guided OCT provided excellent performance in examining drastic retinal structural alterations. In fact, with high-resolution images, tiny lesions can be found easily in OCT images as well. With the aid of image-guided OCT, a group in the laboratory also found abnormal hyperreflectivity spots within the OPL in a microgravity mouse model and in a Cacna1f mutant mouse model24,25. Beyond that, it is possible to determine the other tiny structural changes from the vitreous to choroid26,27,28,29. In summary, high-resolution OCT can provide an accurate diagnosis of tiny lesions as well as important alterations in the retinal architecture5,6,7,8,9,10.
Compared to non-image-guided commercial OCT and self-built OCT, image-guided OCT has some strengths. For example, it possesses built-in analysis software, which allows users to analyze the thickness of the retinas directly. As OCT is a fundus image-guided machine, position alignment is much easier and simpler, and researchers and students in the laboratory do not spend excessive time learning practical operations. Acquiring high-quality images necessitates the use of appropriate anesthesia and expert techniques. When using image-guided OCT, inadequate anesthesia can lead to subpar image quality. Utilizing Adaptive Optics OCT, however, can competently address issues of inadequate anesthesia and movement during the imaging of mice30,31. In addition, image-guided OCT produces colorful fundus images and high-resolution OCT images simultaneously, which is not a standard configuration for commercial OCT and self-built OCT. This functionality helps users carry out two tasks in an easier manner. Furthermore, image-guided OCT can be combined with an image-guided laser, image-guided ERG, or slit lamp in a single instrument, thus maximizing laboratory space.
Of course, high integration can have both advantages and disadvantages. If the number of users increases to tens or hundreds, the image-guided OCT module should be administered separately. Users must be careful when assembling and disassembling machine elements. Unlike the self-built OCT, the commercial machine shows weaknesses in function development and expansion. Of note, the researchers found that there was a sharp decrease in the quality of the OCT images with image-guided OCT when the refractive medium of the experimental animal eye was bad. Conversely, the commercial OCT showed great tolerance in terms of this issue. Altogether, versatile image-guided OCT helps researchers in the eye field examine rodent retinal structural changes conveniently, reliably, and tractably5,6,7,8,9,10.
Disclosures
None of the authors have any conflicts of interest to disclose.
Acknowledgements
The authors thank the members of the State Key Laboratory of Ophthalmology, Optometry, and Vision Science for their technical support and useful comments regarding the manuscript. This work was supported by grants from the National Natural Science Foundation of China (82101169, 81800857, 81870690), the Zhejiang Provincial Natural Science Foundation of China (LGD22H120001, LTGD23H120001, LTGC23H120001), the Program of Wenzhou Science and Technology Bureau of China (Y20211159), the Guizhou Science and Technology Support Project (Qiankehezhicheng [2020] 4Y146) and the Project of State Key Laboratory of Ophthalmology, Optometry and Vision Science (No. K03-20220205).
Materials
| Name | Company | Catalog Number | Comments |
| BALB/c mouse | Beijing Vital River Laboratory Animal Technology Co., Ltd | Animal model preparations | |
| C57BL/6JNifdc mouse | Beijing Vital River Laboratory Animal Technology Co., Ltd | Animal model preparations | |
| Carbomer Eye Gel | Fabrik GmbH Subsidiary of Bausch & Lomb | Moisten the cornea | |
| Complete Freund’s adjuvant | Sigma | F5881 | EAU experiment |
| Experimental platform | Phoenix Technology Group | Animal model preparations | |
| hIRBP161-180 | Shanghai Sangon Biological Engineering Technology & Services Co., Ltd. | EAU experiment | |
| Ketamine | Ceva Sante Animale | General anesthesia | |
| Laser box | Haag-Streit Group | Merilas 532α | Animal model preparations |
| Lewis rat | Beijing Vital River Laboratory Animal Technology Co., Ltd | Animal model preparations | |
| Mycobacterium Tuberculosis H37RA | Sigma | 344289 | EAU experiment |
| Phoneix Micron IV with image-guided OCT and image-guided laser | Phoenix Technology Group | Animal model preparations | |
| Tissue forceps | Suzhou Mingren Medical Instrument Co., Ltd | MR-F101A-5 | Animal model preparations |
| Tropicamide Phenylephrine Eye Drops | SANTEN OY, Japan | Eye dilatation | |
| Vannas scissors | Suzhou Mingren Medical Instrument Co., Ltd | MR-S121A | Animal model preparations |
| Xylazine | Ceva Sante Animale | General anesthesia |
References
- Cen, L. -. P., et al. Automatic detection of 39 fundus diseases and conditions in retinal photographs using deep neural networks. Nature Communications. 12, 4828 (2021).
- Kashani, A. H., et al. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Progress in Retinal and Eye Research. 60, 66-100 (2017).
- Cheng, D., et al. Inner retinal microvasculature damage correlates with outer retinal disruption during remission in Behçet's posterior uveitis by optical coherence tomography angiography. Investigative Ophthalmology & Visual Science. 59 (3), 1295-1304 (2018).
- Lin, R., et al. Relationship between cone loss and microvasculature change in retinitis pigmentosa. Investigative Ophthalmology & Visual Science. 60 (14), 4520-4531 (2019).
- Dietrich, M., et al. Using optical coherence tomography and optokinetic response as structural and functional visual system readouts in mice and rats. Journal of Visualized Experiments. (143), e58571 (2019).
- Jagodzinska, J., et al. Optical coherence tomography: Imaging mouse retinal ganglion cells in vivo. Journal of Visualized Experiments. (127), e55865 (2017).
- Ye, Q., et al. In vivo methods to assess retinal ganglion cell and optic nerve function and structure in large animals. Journal of Visualized Experiments. (180), e62879 (2022).
- Mai, X., Huang, S., Chen, W., Ng, T. K., Chen, H. Application of optical coherence tomography to a mouse model of retinopathy. Journal of Visualized Experiments. (179), e63421 (2022).
- Allen, R. S., Bales, K., Feola, A., Pardue, M. T. In vivo structural assessments of ocular disease in rodent models using optical coherence tomography. Journal of Visualized Experiments. (161), e61588 (2020).
- Kokona, D., Jovanovic, J., Ebneter, A., Zinkernagel, M. S. In vivo imaging of Cx3cr1gfp/gfp reporter mice with spectral-domain optical coherence tomography and scanning laser ophthalmoscopy. Journal of Visualized Experiments. (129), e55984 (2017).
- Yan, M., Li, J., Yan, L., Li, X., Chen, J. -. G. Transcription factor Foxp1 is essential for the induction of choroidal neovascularization. Eye and Vision. 9 (1), 10 (2022).
- Wolf, A., Herb, M., Schramm, M., Langmann, T. The TSPO-NOX1 axis controls phagocyte-triggered pathological angiogenesis in the eye. Nature Communications. 11, 2709 (2020).
- Li, L., et al. Longitudinal morphological and functional assessment of RGC neurodegeneration after optic nerve crush in mouse. Frontiers in Cellular Neuroscience. 14, 109 (2020).
- Zhang, Y., et al. Elevating growth factor responsiveness and axon regeneration by modulating presynaptic inputs. Neuron. 103 (1), 39-51 (2019).
- Wang, J., et al. Robust myelination of regenerated axons induced by combined manipulations of GPR17 and microglia. Neuron. 108 (5), 876-886 (2020).
- Tian, F., et al. Core transcription programs controlling injury-induced neurodegeneration of retinal ganglion cells. Neuron. 110 (16), 2607-2624 (2022).
- Wu, K. -. C., et al. Deletion of miR-182 leads to retinal dysfunction in mice. Investigative Ophthalmology & Visual Science. 60 (4), 1265-1274 (2019).
- Rattner, A., Nathans, J. The genomic response to retinal disease and injury: Evidence for endothelin signaling from photoreceptors to glia. Journal of Neuroscience. 25 (18), 4540-4549 (2005).
- Rattner, A., Toulabi, L., Williams, J., Yu, H., Nathans, J. The genomic response of the retinal pigment epithelium to light damage and retinal detachment. Journal of Neuroscience. 28 (39), 9880-9889 (2008).
- Hahn, P., et al. Deficiency of Bax and Bak protects photoreceptors from light damage in vivo. Cell Death & Differentiation. 11 (11), 1192-1197 (2004).
- Fan, J., et al. Maturation arrest in early postnatal sensory receptors by deletion of the miR-183/96/182 cluster in mouse. Proceedings of the National Academy of Sciences of the United States of America. 114 (21), 4271-4280 (2017).
- Zhou, J., et al. A combination of inhibiting microglia activity and remodeling gut microenvironment suppresses the development and progression of experimental autoimmune uveitis. Biochemical Pharmacology. 180, 114108 (2020).
- Okunuki, Y., et al. Retinal microglia initiate neuroinflammation in ocular autoimmunity. Proceedings of the National Academy of Sciences of the United States of America. 116 (20), 9989-9998 (2019).
- Dai, X., et al. Rodent retinal microcirculation and visual electrophysiology following simulated microgravity. Experimental Eye Research. 194, 108023 (2020).
- Dai, X., et al. Photoreceptor degeneration in a new Cacna1f mutant mouse model. Experimental Eye Research. 179, 106-114 (2019).
- Liu, Y., et al. Mouse models of X-linked juvenile retinoschisis have an early onset phenotype, the severity of which varies with genotype. Human Molecular Genetics. 28 (18), 3072-3090 (2019).
- Ou, J., et al. Synaptic pathology and therapeutic repair in adult retinoschisis mouse by AAV-RS1 transfer. Journal of Clinic Investigation. 125 (7), 2891-2903 (2015).
- Xiang, L., et al. Depletion of miR-96 delays, but does not arrest, photoreceptor development in mice. Investigative Ophthalmology & Visual Science. 63 (4), 24 (2022).
- Huang, X. -. F., et al. Functional characterization of CEP250 variant identified in nonsyndromic retinitis pigmentosa. Human Mutation. 40 (8), 1039-1045 (2019).
- Jonnal, R. S., et al. A review of adaptive optics optical coherence tomography: Technical advances, scientific applications, and the future. Investigative Ophthalmology & Visual Science. 57 (9), 51 (2016).
- Dong, Z. M., Wollstein, G., Wang, B., Schuman, J. S. Adaptive optics optical coherence tomography in glaucoma. Progress in Retinal and Eye Research. 57, 76-88 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved