Method Article
A Quantitative Detection Method for MicroRNAs in the Kidney of an Ischemic Kidney Injury Mouse Model
In This Article
Summary
This article presents a protocol for detecting microRNA expression in the kidneys of an acute kidney injury mouse model using quantitative real-time reverse-transcription polymerase chain reaction. This protocol emphasizes an ischemic kidney injury mouse model and the careful extraction of microRNA samples.
Abstract
MicroRNAs (miRNAs) are involved in various disease states and are effective biomarkers for the early diagnosis of diseases and treatment in mice. However, standard protocols for the purification of miRNAs and detection of their expression in the kidneys of acute kidney injury (AKI) mice have not been well established. This study developed an effective and simple protocol to purify and quantify miRNAs in the kidneys of an AKI mouse model induced by renal ischemia-reperfusion using quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR). This protocol comprises five steps: 1) induction of AKI by renal ischemia-reperfusion, 2) harvesting of kidneys, 3) purification of total RNA, including miRNAs, from kidneys, 4) cDNA synthesis by reverse transcription of miRNA, and 5) qRT-PCR to detect miRNA expression. Using this protocol, the renal ischemia-reperfusion injury model can be generated with mild to severe forms of AKI. Additionally, if the procedure is followed properly, a consistent AKI model with minimal individual differences can be obtained. This qRT-PCR assay shows a very wide dynamic range and enables the discrimination of mature miRNAs, which can be accurately quantified with high specificity. This protocol can be used to study the miRNA expression profile in AKI kidneys.
Introduction
Ischemia-reperfusion injury (IRI) of the kidney represents one of the major risk factors for Acute kidney injury (AKI) development1. AKI plays a significant role in patient prognosis, but specific therapies and early diagnostic biomarkers have not been established.
MicroRNAs (miRNAs) are short, non-coding RNAs with approximately 18–25 bases. miRNAs are stable in body fluids, and their sequences are highly conserved among animals2. MiRNAs regulate the expression of multiple proteins through thousands of targets, thereby influencing diverse signaling pathways2,3,4,5,6,7,8. In recent years, it has been reported that miRNAs are involved in various disease states and are effective biomarkers for the early diagnosis of several diseases.
The overall goal of this protocol is to successfully purify and detect miRNAs in the kidneys of an AKI mouse model induced by IRI. This IRI model is a widely used model of AKI and renal fibrosis. The advantages of the IRI model include being able to visually confirm whether ischemia-reperfusion has been achieved and determine the exact time of AKI onset. However, a clamp duration that is long enough to cause broad tubular damage is associated with a high mortality rate, whereas a short clamp duration does not cause tubular damage, which results in a large variation in tubular damage progression in this experimental model. Compared with the bilateral clamping model, the right nephrectomy tissue harvested in the unilateral renal IRI model acts as a control and ensures renal failure.
The kidney sample is mashed using a glass homogenizer, wherein, proteins and nucleic acids are separated, and DNA and RNA are separated9. Total RNA, including miRNA, is purified from the kidney sample using a silica-membrane-based spin column9. Subsequently, cDNA synthesis (reverse transcription) is performed from total RNA using poly(A) polymerase and oligo-dT primers10. Finally, the miRNA expression is determined by qRT-PCR using an intercalating dye10. qRT-PCR can more accurately quantify gene expression compared with PCR of amplification volumes at endpoints. qRT-PCR measures high concentrations and is characterized by a wide dynamic range, which allows accurate quantification that depends on the number of cycles. Previous studies reported that simple processes could be used to effectively purify and detect miRNAs in tissues8,9,10. The methods described in this protocol for cDNA synthesis (reverse transcription) and the detection of miRNA expression by qRT-PCR using an intercalating dye have been reported to show high accuracy and sensitivity10.
Additionally, this protocol is simple and achieves consistent results between laboratories. Therefore, this protocol is useful in studies that require highly accurate and sensitive miRNA detection in mouse AKI kidneys.
Protocol
All animal experimental protocols were approved by the animal ethics committee of Jichi Medical University and performed in accordance with the Use and Care of Experimental Animals guidelines from the Jichi Medical University Guide for Laboratory Animals.
1. IRI model
NOTE: Carefully monitor for hypothermia, intestinal moisturization, and depth of anesthesia throughout the procedure.
- Prepare the following items: a 50 mL centrifuge tube containing cotton drenched in isoflurane, a heated surgical pad, a Petri dish with phosphate-buffered saline (PBS), surgical scissors, and forceps. Maintain the PBS at 37 °C.
NOTE: This IRI model was generated using male C57BL/6 mice (9 weeks; 20–25 g) housed in a room with controlled temperature, humidity, and a 12 h light-dark cycle. - Anesthetize the animal using isoflurane: induction concentration 3%–5%, maintenance concentration 2%–3%, and concentration during clamping 1%–1.5%. Confirm the depth of anesthesia by the loss of reflexes. Inhalation anesthesia has the advantage that the time can be adjusted. The procedure might take a significant amount of time because of the exfoliation of the tissue surrounding the kidney. However, the disadvantage of inhalation anesthesia is that the body temperature tends to fall. Throughout the procedure, avoid unnecessary anesthesia.
- Mount the anesthetized mouse on a heated surgical pad on its back, remove the hair surrounding the incision area, and spray the abdominal skin of the mouse with 70% ethanol.
- Make a midline incision in the abdominal skin, muscle, and peritoneal membrane using scissors and forceps.
- Using a cotton swab moistened with PBS, gently push the bowel toward the left side of the extra-abdominal cavity, exposing the right kidney and ureter. The long procedure time is likely to cause postoperative intestinal injury. Thus, it is better to operate without removing the intestines from the abdominal cavity when a visual field can be obtained. Avoid drying out the intestines. Dry swabs or gauze can cause intestinal damage.
- Lift the right ureter with angled forceps. Ligate the right ureter twice using 4/0 silk-braided sutures. Cut between the sutures, leaving the suture that is closer to the kidney longer.
- Carefully hold the remaining suture without pulling too much, and bluntly dissect the connective tissue and fat along the kidney. Performing surgery from behind reduces the risk of liver damage.
- Identify a blood vessel, other than the renal arteries and veins, that supplies blood to the right kidney, and then ligate or clip it. When the right kidney is sufficiently detached, ligate the right renal artery and vein using a 4/0 silk-braided suture. Instead of suturing, use a hemostatic clip.
- Using a cotton swab moistened with PBS, gently push the intestine towards the right side of the extra-abdominal cavity to expose the left kidney, and then cover the intestine with a moistened drape to prevent it from drying out. Dissect the connective tissue and fat around the left kidney.
- Identify a blood vessel, other than the renal arteries and veins, that supplies blood to the left kidney, and then ligate or clip it. Clamp the renal artery and renal vein to induce left kidney ischemia. Position the hemostatic clip approximately 1 cm from the kidney to avoid damage to the renal parenchyma. Successful ischemia can be visually confirmed by a gradual uniform darkening of the kidney.
- Return the intestines to the abdominal cavity, taking care to avoid intestinal twisting; temporarily close the abdominal skin and drape to maintain a moist environment. Reduce the concentration of inhalation anesthesia as much as possible and try to keep the animal warm. The clamping time is 25–45 min. A longer clamping time induces more severe renal damage but also increases long-term mortality. Adjust by analyzing the pathology results.
- Remove the clamp after the period of ischemia has concluded. Ensure that blood flow reperfusion and kidney color improve. Confirm that the bowel is not twisted before closing the abdominal skin. Instill PBS intraperitoneally as a volume supplement before the abdomen is closed in two layers. Close the abdominal skin using 4-0 nylon sutures. For longer renal removal periods, the possibility of intestinal adhesions may be reduced by separately closing the abdominal wall and peritoneum.
- To minimize the risk of post-operative infection, apply an antiseptic (e.g., iodine/alcohol solution) to the surgical area.
2. Kidney sample collection
NOTE: This video has a 45 min clamping time, collecting the kidneys 24 h later.
- Anesthetize the animal with 5% isoflurane and confirm the depth of anesthesia by the loss of reflexes.
- Make a midline incision in the abdominal skin, muscle, and peritoneal membrane using scissors and forceps. Collect the blood from the inferior vena cava punctured by a 27 G needle.
- Make a midline incision in the chest wall and insert a 23 G needle into the left ventricle. Confirm backflow. Make an incision in the liver and inject 20 mL of PBS. As the blood drains, the liver changes color from red to pink, confirming that sufficient blood has been drained.
- Remove the left kidney using forceps and scissors and wash it with PBS in a Petri dish. Remove the renal fascia using forceps, being careful not to dry out the kidney.
- Cut the kidney in half and use one half for histopathology and the other half for the next step. Avoid the renal pelvis, and harvest 30 mg of the kidney. Store this at −80 °C before use.
3. Purifying miRNAs from kidney samples
NOTE: Here, kidney samples weighing 30 mg are homogenized using a glass homogenizer and a biopolymer-shredding system in a microcentrifuge spin column. Subsequently, miRNA from the kidney sample is isolated using a silica-membrane-based spin column.
- Prepare the following items: 1.5 or 2.0 mL microcentrifuge tubes, 100% ethanol, chloroform, a glass homogenizer, ice, a biopolymer-shredding system in a microcentrifuge spin column9, silica-membrane-based spin columns9, phenol/guanidine-based lysis reagent, wash buffer containing guanidine and ethanol (wash buffer 1), and wash buffer containing ethanol (wash buffer 2).
- Place a 30 mg kidney sample into a glass homogenizer and add 700 µL of phenol/guanidine-based lysis reagent. Perform this step on ice because the tissue is denatured by heat.
- Homogenize the kidney sample by slowly pressing the pestle onto the sample by twisting it. Repeat this process several dozen times on ice until the kidney sample has completely dissolved into the phenol/guanidine-based lysis reagent. When miRNA is not obtained in subsequent measurements, this is likely because of insufficient dissolving.
- For further homogenization, transfer the homogenized lysate to the biopolymer-shredding system in a microcentrifuge spin column placed in a 2.0 mL collection tube. Centrifuge this at 14,000 × g for 3 min at room temperature.
- Transfer the homogenized lysate to a new microcentrifuge tube.
- Add 140 µL of chloroform to the homogenized lysate and cap the tube securely. Mix the tube by inversion for 15 s.
- Incubate the samples for 2–3 min at room temperature. Then, centrifuge them at 12,000 × g for 15 min at 4 °C.
- Transfer the supernatant (normally 300 µL) to a new microcentrifuge tube without disturbing the precipitate and add 1.5 volumes (normally 450 µL) of 100% ethanol. Mix the sample by vortexing for 5 s.
- Pipette up to 700 µL of the sample into a silica-membrane-based spin column placed in a 2.0 mL collection tube. Close the column cap, and centrifuge at 15,000 × g for 15 s. Following centrifugation, discard the flow-through in the collection tube.
- Add 700 µL of wash buffer 1 to the silica-membrane-based spin column to thoroughly wash the sample. Close the cap of the column, and centrifuge it at 15,000 × g for 15 s. Following centrifugation, discard the flow-through in the collection tube.
- Add 500 µL of wash buffer 2 to the silica-membrane-based spin column to remove any traces of salt. Close the cap of the column, and centrifuge at 15,000 × g for 15 s. Following centrifugation, discard the flow-through in the collection tube.
- Repeat step 3.11.
- Centrifuge the silica-membrane-based spin column again at 15,000 × g for 1 min. Following centrifugation, discard the flow-through in the collection tube.
- Place the silica-membrane-based spin column in a new 1.5 mL collection tube. Transfer 25 µL of RNase-free water into the column, and close the column cap. Leave the sample at room temperature for 5 min, and then centrifuge it at 15,000 × g for 1 min.
- Transfer the 25 µL eluate containing miRNAs to a new microcentrifuge tube. Because miRNA is degraded by repeated dissolution, it is recommended to dispense the sample into 2 or 3 tubes. Store these at −80 °C before use.
4. Reverse transcription of miRNA
NOTE: Here, 1.0 µg of isolated RNA is reverse-transcribed using reverse transcriptase, poly(A) polymerase, and oligo-dT primers.
- Prepare the following items: 1.5 mL microcentrifuge tubes; 8-well strip tubes; RNase-free water; ice; a 10x Nucleic Acid Mix containing deoxynucleotides, ribonucleotide triphosphates, and oligo-dT primers; a poly(A) polymerase and reverse transcriptase mix; and activating buffer, including Mg2+, deoxyribonucleotides, oligo-dT primers, and random primers10.
- Prepare a master mix solution containing 2.0 µL of 10x Nucleic Acid Mix, 2.0 µL of poly(A) polymerase and reverse transcriptase mix, and 4.0 µL of 5x activating buffer, including Mg2+, deoxyribonucleotides, oligo-dT primers, and random primers, for a total of 8.0 µL per tube.
- Dispense 8.0 µL of the master mix solution into each tube.
- Add 1.0 µg of isolated miRNAs purified from the kidney sample to each tube.
- Add RNase-free water to each tube to a total of 20 µL. Mix thoroughly by pipetting, and centrifuge at 1,500 x g for 15 s.
- Incubate the samples for 60 min at 37 °C, followed immediately by incubation for 5 min at 95 °C. This step can be performed in a thermal cycler.
- Transfer the cDNA to a new microcentrifuge tube and dilute 10 times (1:10) with RNase-free water.
- Store the diluted cDNA short term on ice and long term at −80 °C before use.
5. qRT-PCR of miRNA
NOTE: qRT-PCR of miRNA is performed using an intercalating dye. Here, primers for U6 small nuclear 2 (RNA-2), miRNA-17-5p, miRNA-18a-5p, miRNA-21a-5p, miRNA-132-3p, miRNA-212-3p, miRNA-223-3p, and miRNA-574-5p were used.
- Prepare the following items: 1.5 mL microcentrifuge tubes, 96-well reaction plates for qRT-PCR, adhesive film for the 96-well reaction plate, miRNA-specific primers, PCR Master Mix comprising Taq DNA polymerase, PCR buffer, deoxynucleotides, and universal primers10, and a real-time PCR Instrument.
- Prepare a master mix containing 12.5 µL of 2x PCR Master Mix, 2.5 µL of each miRNA primer (5 µM) dissolved in nuclease-free water, and 1.25 µL of 10x universal primers for each well.
- Dispense 22.5 µL of the master mix into each well of the 96-well plate.
- Add 2.5 µL of template cDNA to each well.
- Seal the plate with the adhesive film for the 96-well reaction plate. Then, centrifuge the plate at 1,000 × g for 30 s.
- Using the real-time PCR instrument and software, run the PCR cycling program as follows.
- Place the plate in the real-time PCR instrument. Assign a name to the sample and target miRNA in each well using the Real-Time PCR software.
- Input the following PCR cycling conditions in the real-time PCR software according to the instructions: preincubation at 95 °C for 15 min, followed by 40 cycles of denaturation at 94 °C for 15 s, annealing at 55 °C for 30 s, and extension at 70 °C for 30 s.
- Analyze the qRT-PCR data using the software of the real-time PCR instrument. Check for non-specific reactions. Analyze the Dissociation curve or the Melt curve to confirm that amplification other than the intended one has not occurred.
- Normalize the expression level of target miRNAs to RNU-6 as an endogenous control. Calculate the relative expression level of target miRNAs using the 2−ΔΔCT method11.
Results
Here, the miRNA expression profile in AKI mice was investigated. The miRNA expression profile has been investigated in various organs and tissues in mice. MiRNAs are important post-transcriptional regulators and are now being extensively studied in the characterization of a variety of diseases, including AKI. MiRNAs have the potential to help elucidate pathological conditions and be applied to the treatment of AKI12. The major causes of AKI are IRI, nephrotoxic insult, and sepsis. IRI of the kidney represents one of the major risk factors for the development of AKI. Numerous miRNAs have been reported to be involved in AKI13.
This study investigated miRNAs that are commonly altered in sepsis and IRI models using the protocol described here. Seventeen miRNAs shown to be upregulated in the kidneys of AKI mice by microarray analyses were selected, and seven of these miRNAs reported to be expressed in humans were investigated. The levels of six miRNAs (miRNA-17-5p, miRNA-18a-5p, miRNA-21a-5p, miRNA-132-3p, miRNA-212-3p, and miRNA-223-3p) were found to be significantly increased in the kidneys of IRI mice compared with those in mock mice using qRT-PCR following the protocol presented in this manuscript (Figure 1).
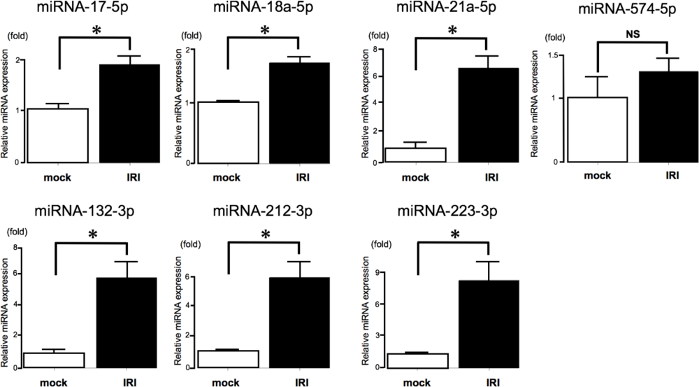
Figure 1: MiRNA expression in the kidney of AKI mice determined by qRT-PCR.
MiRNAs in the kidneys of mice 24 h post-IRI. qRT-PCR determination of the expression of seven miRNAs (miRNA-17-5p, miRNA-18a-5p, miRNA-21a-5p, miRNA-132-3p, miRNA-212-3p, miRNA-223-3p, and miRNA-574-5p) in mock mice (n = 5) and IRI mice (n = 5). Six miRNAs (miRNA-17-5p14, miRNA-18a-5p15, miRNA-21a-5p16, miRNA-132-3p17, miRNA-212-3p18, and miRNA-223-3p19) were shown to be upregulated by qRT-PCR. qRT-PCR analysis showed that miRNA-574-5p expression was unchanged. Values are the mean ± standard error (error bars). Analysis of variance and Student’s t-tests were used to compare the differences among groups. Differences with a p-value < 0.05 were considered significant. Abbreviations: qRT-PCR, quantitative real-time reverse-transcription polymerase chain reaction; miRNAs, microRNAs; NS, not significant; *, p < 0.05. Please click here to view a larger version of this figure.

Figure 2: Serum blood urea nitrogen (BUN) and creatinine (CRE) levels 24 h post-IRI in mice (n = 5) and mock mice (n = 5). Please click here to view a larger version of this figure.
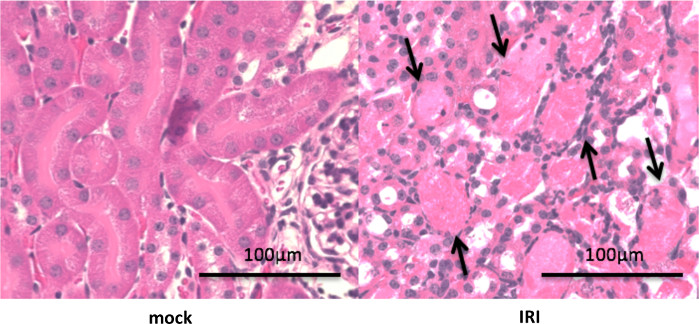
Figure 3: Hematoxylin and Eosin (H&E) staining of tissues after 24 h in an IRI model with a clamp time of 45 min. Male C57/B6 mice aged 9 weeks underwent a right nephrectomy, and the left renal pedicle was clamped for 45 min. Mice were sacrificed at 24 h following renal IRI. Representative photomicrographs (Magnification: 400×) of H&E-stained control and injured kidney sections obtained using an optical microscope. IRI induced acute tubular necrosis (black arrow). Please click here to view a larger version of this figure.
Discussion
Using the protocol presented in this manuscript, miRNAs from the kidney of IRI mice were successfully purified and detected using qRT-PCR. Critical points of the IRI-inducing procedure in the protocol include careful monitoring of body temperature and anesthesia concentrations, which are known to affect AKI20. The strength of this protocol is that it allows visual confirmation of whether ischemia-reperfusion has been achieved. However, there are some limitations to this IRI model. Prolonging the clamp time to induce severe ischemia increases mortality. Additionally, when renal ischemia is not achieved using the left renal artery clamp, it is because blood vessels to the kidney other than the renal artery and vein are not ligated/clipped. There are alternative methods to induce AKI, including drug injection (folic acid, aristolochic acid, cisplatin, gentamycin, glycerol, L-mimosine, CoCL2, kanamycin, and furosemide). In contrast to drug-induced AKI models, the time at which AKI is induced in the present model was accurately determined, with minimal individual differences in the AKI stage. In contrast to models using bilateral renal ischemia, IRI can be induced reliably using the protocol described here. In the present model, it is easy to administer drugs before and after IRI, including the application of miRNAs or related compounds that regulate genes or proteins, and the removed right kidney tissue can be used as a control.
There are three critical points regarding miRNA purification in this protocol. First, it is necessary to extract total RNA on ice because miRNA is pyrolyzed. It is also important to ensure that the kidney sample is properly homogenized and completely dissolved in the lysis reagent, or there will be a difference in the amount of miRNA collected. Second, confirming the stability of the expression levels of endogenous control, miRNAs is essential to accurately assess the level of miRNA expression required. It may be necessary to change the endogenous control depending on the condition, so make sure to determine whether there is a difference in the endogenous control between the control and IRI groups. RNU-6 was used here. When the endogenous controls are significantly different between the compared groups, interpretation of the results will be complicated. In that case, ensure that there are no differences in the conditions of the laboratory animals, animal feed, temperatures between samples, and extraction times. Third, the reliability of qRT-PCR data analysis depends on the quality of the purified miRNAs. If the amount of RNA contained in a sample is different, then the final results will be significantly different. When preparing cDNA, adjust the samples to ensure that the amount of RNA set between samples is the same. It is also necessary to carefully avoid technical errors caused by pipetting. Additionally, if significant miRNA amplification using qRT-PCR is not achieved, then consider increasing the concentration of template cDNA, concentration of each miRNA-specific primer, and number of qRT-PCR cycles.
This qRT-PCR protocol is an intercalator assay that detects any amplified double-stranded DNA, including non-specific reaction products. Although it has a high detection ability, and there is no need to prepare a special fluorescent-labeled probe for each target, it has the disadvantage that the intercalating dye itself may act as an inhibitor or bind to non-specific DNA sequences and give rise to false positives. This is because all double-stranded DNA present in the reaction solution is detected, so non-specific amplification and target amplification are indistinguishable and detected as the same amplification. Therefore, it is necessary to confirm that there are no non-specific reactions after PCR.
First, analyze the Dissociation curve or the Melt curve, which are incorporated as a program in the real-time PCR device. The temperature at which double-stranded DNA dissociates into single strands is the melting temperature (Tm) value. The Tm value depends on sequence characteristics, such as the length of the amplification product and GC content. Therefore, if there is another amplification product, the Tm value will be different, and the change in the fluorescence value will be observed twice. If there is one peak Tm value, there is one amplification product, and if there are multiple peaks, there are multiple amplification products. If no amplification is achieved, then modify the conditions.
One parameter that can be changed is the primer concentration. Generally, a higher primer concentration is more likely to cause non-specific amplification. Therefore, lowering the primer concentration may improve non-specific amplification. Generally, the higher the primer concentration, the higher is the PCR efficiency. Therefore, it is recommended to first try using a high concentration, and then lower the concentration if the assay does not work.
Second, non-specific amplification tends to improve with increasing annealing/extension temperatures. Increase the annealing/extension temperature by 1 °C to determine whether the non-specific amplification disappears.
Third, the number of PCR cycles can be increased or decreased as needed. If only trace amounts of the miRNAs are detected, qRT-PCR may be difficult to use. When gene amplification is observed after 30 cycles, it is challenging to determine whether this is due to non-specific amplification, contamination, or whether a very small amount of specific nucleic acid was detected. The Melt curve is used as a reference, but the possibility of contamination cannot be excluded. In this case, consider using digital-PCR.
There are several alternative methods for determining the level of miRNA expression, such as northern blotting, ribonuclease protection, in situ hybridization, and microarrays, but qRT-PCR is faster and easier to perform than other RNA quantification methods. Furthermore, qPCR detection methods are more sensitive and specific than other assays. Microarrays can simultaneously measure the expression of multiple miRNAs, and the results are strongly correlated with the data obtained by qRT-PCR21. However, no methodology has been established to validate the comparison of microarray data between research groups22.
The present protocol has the following limitations. Prolonging the clamp time to induce severe ischemia increases mortality. It is unsuitable for evaluating severe AKI models. Therefore, set the clamp time for each pathological tissue.
In conclusion, this article describes a protocol for the purification and detection of miRNA expression in IRI mouse kidneys using qRT-PCR.
Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgements
We thank Nam Nguyen, PhD for editing a draft of this manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| Bent Tip Tapered Tweezers without Hook | Natsume Seisakusho | MA-47 | |
| Buffer RLT | Qiagen | 79216 | wash buffer 1 |
| Buffer RWT | Qiagen | 1067933 | wash buffer 2 |
| C57B6 mice | SLC | not assign | |
| forcep with Teeth | Natsume Seisakusho | MA-49 | |
| forcep without Teeth | Natsume Seisakusho | MA-48-1 | |
| Hemostatic clips | Natsume Seisakusho | KN−353 | |
| MicroAmp Optical 96 well reaction plate for qRT-PCR | Thermo Fisher Scientific | 4316813 | 96-well reaction plate |
| MicroAmp Optical Adhesive Film | Thermo Fisher Scientific | 4311971 | adhesive film for 96-well reaction plate |
| miRNA-132-3p primer | Qiagen | MS00003458 | 5'UAACAGUCUACAGCCAUGGUCG |
| miRNA-17-5p primer | Qiagen | MS00029274 | 5'CAAAGUGCUUACAGUGCAGGUAG |
| miRNA-18a-5p primer | Qiagen | MS00031514 | 5'UAAGGUGCAUCUAGUGCAGAUAG |
| miRNA-212-3p primer | Qiagen | MS00003815 | 5'UAACAGUCUCCAGUCACGGCC |
| miRNA-21a-5p primer | Qiagen | MS00009079 | 5'UAGCUUAUCAGACUGAUGUUGA |
| miRNA-223-3p primer | Qiagen | MS00003871 | 5'UGUCAGUUUGUCAAAUACCCCA |
| miRNA-574-5p primer | Qiagen | MS00043617 | 5'UGAGUGUGUGUGUGUGAGUGUGU |
| miRNeasy Mini kit | Qiagen | 217004 | silica-membrane based spin column |
| miScript II RT kit | Qiagen | 218161 | |
| miScript SYBR Green PCR kit | Qiagen | 218073 | |
| QIA shredder | Qiagen | 79654 | biopolymer-shredding system in a micro centrifuge spin-column |
| QIAzol Lysis Reagent | Qiagen | 79306 | phenol/guanidine-based lysis reagent |
| QuantStudio 12K Flex Flex Real-Time PCR system | Thermo Fisher Scientific | 4472380 | real-time PCR instrument |
| QuantStudio 12K Flex Software version 1.2.1. | Thermo Fisher Scientific | 4472380 | real-time PCR instrument software |
| RNU6-2 primer | Qiagen | MS00033740 | not disclosed |
| surgical scissors | Natsume Seisakusho | B-2 | |
| Vascular clip applier | VITALITEC | 1621420 |
References
- Kelly, K. J. Acute renal failure: much more than a kidney disease. Seminas in Nephrology. 26 (2), 105-113 (2006).
- Saikumar, J., Ramachandran, K., Vaidya, V. S. Noninvasive micromarkers. Clinical Chemistry. 60 (9), 1158-1173 (2014).
- Beermann, J., Piccoli, M. T., Viereck, J., Thum, T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiological Reviews. 96 (4), 1297-1325 (2016).
- Yang, G., et al. Discovery and validation of extracellular/circulating microRNAs during idiopathic pulmonary fibrosis disease progression. Gene. 562 (1), 138-144 (2015).
- Zhang, T., et al. Circulating miR-126 is a potential biomarker to predict the onset of type 2 diabetes mellitus in susceptible individuals. Biochemical and Biophysical Research Communications. 463 (1-2), 60-63 (2015).
- Zhong, X., et al. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia. 56 (3), 663-674 (2013).
- Wang, J., et al. Downregulation of urinary cell-free microRNA-214 as a diagnostic and prognostic biomarker in bladder cancer. Journal of Surgical Oncology. 111 (8), 992-999 (2015).
- Morishita, Y., et al. MicroRNA expression profiling in peritoneal fibrosis. Translational Research: The Journal of Laboratory and Clinial Medicine. 169, 47-66 (2016).
- Morse, S. M., Shaw, G., Larner, S. F. Concurrent mRNA and protein extraction from the same experimental sample using a commercially available column-based RNA preparation kit. BioTechniques. 40 (1), 54-58 (2006).
- Mestdagh, P., et al. Evaluation of quantitative miRNA expression platforms in the microRNA quality control (miRQC) study. Nature Methods. 11 (8), 809-815 (2014).
- Bustin, S. A., et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clinical Chemistry. 55 (4), 611-622 (2009).
- Jonas, S., Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nature Reviews. Genetics. 16 (7), 421-433 (2015).
- Ichii, O., Horino, T. MicroRNAs associated with the development of kidney diseases in humans and animals. Journal of Toxicologic Pathology. 31 (1), 23-34 (2018).
- Ma, L., et al. Changes of miRNA-17-5p, miRNA-21 and miRNA-106a level during rat kidney ischemia-reperfusion injury. Zhonghua Yi Xue Za Zhi. 95 (19), 1488-1492 (2015).
- Saikumar, J., et al. Expression, circulation, and excretion profile of microRNA-21, -155, and -18a following acute kidney injury. Toxicological Sciences: an Official Journal of the Society of Toxicology. 129 (2), 256-267 (2012).
- Li, Y. F., et al. MicroRNA-21 in the pathogenesis of acute kidney injury. Protein & Cell. 4 (11), 813-819 (2013).
- Zhou, J., Chen, H., Fan, Y. Systematic analysis of the expression profile of non-coding RNAs involved in ischemia/reperfusion-induced acute kidney injury in mice using RNA sequencing. Oncotarget. 8 (59), 100196-100215 (2017).
- Liu, Y., et al. MicroRNA expression profile by next-generation sequencing in a novel rat model of contrast-induced acute kidney injury. Annals of Translational Medicine. 7 (8), 178 (2019).
- Colbert, J. F., et al. A model-specific role of microRNA-223 as a mediator of kidney injury during experimental sepsis. American Journal of Physiology. Renal Physiology. 313 (2), 553-559 (2017).
- Delbridge, M. S., Shrestha, B. M., Raftery, A. T., El Nahas, A. M., Haylor, J. L. The effect of body temperature in a rat model of renal ischemia-reperfusion injury. Transplantation Proceedings. 39 (10), 2983-2985 (2007).
- Dallas, P. B., et al. Gene expression levels assessed by oligonucleotide microarray analysis and quantitative real-time RT-PCR -- how well do they correlate. BMC Genomics. 6, 59 (2005).
- Rockett, J. C., Hellmann, G. M. Confirming microarray data--is it really necessary. Genomics. 83 (4), 541-549 (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved